Keywords
major bleeding, coronary artery bypass grafting, clopidogrel, ticagrelor
major bleeding, coronary artery bypass grafting, clopidogrel, ticagrelor
In the last two decades, the management of acute coronary syndrome (ACS) has been well defined and periodically updated. Management has developed drastically over this period1. Management options are numerous, and they depend on the facilities of the hospital. Of these treatment options, coronary artery bypass grafting (CABG) is considered the most challenging and the final option when other treatment options, including percutaneous coronary intervention (PCI) and thrombolytic therapy, fail to restore blood flow in the infarct-related artery2. Moreover, the drugs used in ACS patients in all management options are complex, and dual antiplatelet therapy (DAPT) is commonly used. DAPT is globally used to treat patients with ACS. It was first reported in 19963, and was first recommended for treating ACS patients in 2007 in American College of Cardiology (ACC)/American Heart Association (AHA) guidelines4. Since then, DAPT has been widely used in the early management of ACS patients5,6.
Recently, when performing DAPT, whether to use clopidogrel or ticagrelor (the choice between acetylsalicylic acid (ASA) + clopidogrel and ASA + ticagrelor) has remained controversial due to the current assumption that one of the two might provide higher risk of major bleeding7,8. In the Indonesian National Health Insurance drug catalog, in 2018 clopidogrel was withdrawn and substituted with ticagrelor. However, in the drug price list (https://e-katalog.lkpp.go.id/; website in Indonesian), ticagrelor is more expensive than clopidogrel. It is unclear whether the assumptions made about the risk of major bleeding caused by clopidogrel or ticagrelor were supported by the evidence or were possibly the result of conspiracy among pharmaceutical industries to increase their products marketing. Ticagrelor may provide a more potent platelet inhibition effect, therefore reducing the risk of a thrombotic event9. In the context of ACS, the greater effect may be accompanied more complications. Therefore, the benefits of DAPT and the risk of complications (bleeding) should balance. In the case of ACS patients undergoing CABG, to prevent major bleeding, it is recommended that DAPT should be discontinued for at least three and five days before elective CABG for ticagrelor and clopidogrel, respectively10. Furthermore, in the case of emergency or urgent CABG, DAPT should be discontinued prematurely11. The discontinuation of DAPT might increase the risk of a thrombotic event12. However, delay in CABG had also been shown to associate with poor clinical outcome and increased risk of mortality13. Therefore, identifying the appropriate DAPT, whether ticagrelor or clopidogrel, is crucial to prevent the risk of major bleeding. Although 2016 ACC/AHA guidelines recommended ticagrelor over clopidogrel because ticagrelor is considered to have a more potent anti-platelet effect than clopidogrel14, the evidence from previous studies regarding the association between the risk of major bleeding and the use of different DAPT using either clopidogrel or ticagrelor in ACS patients treated with CABG were inconclusive. Therefore, those inconclusive data of previous studies required clarification using a meta-analysis approach.
Therefore, the present study aimed to perform a meta-analysis whether the use of different DAPT (clopidogrel or ticagrelor) might affect the risk of major bleeding or not in ACS patients treated with CABG. Our study outcome could clarify the real effect of the use of DAPT (clopidogrel or ticagrelor) to the risk of major bleeding in ACS patients treated with CABG. Moreover, we also expect that our current meta-analysis might correct previous assumptions concerning the use of different DAPT.
A Meta-analysis was performed during March to October 2019 to assess the association between the incidence of major bleeding and the use of DAPT either clopidogrel or ticagrelor in ACS patients treated with CABG. In effort to attain our goal, potentially relevant papers were identified and collected from PubMed, Embase, Cochrane, and Web of Science to calculate odd ratio (OR) and 95% confidence interval (95%CI) using either fixed or random effect model. A checklist adapted from Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) and the design of our previous meta-analyses15–20 were used to guide the meta-analysis protocols in our present study21. See Reporting guidelines for a completed PRISMA checklist for this study22.
We conducted a systematic search in PubMed, Embase, Cochrane, and Web of Science up to 20 September 2019. The search strategy, conformed to medical subjects heading (MeSH), involved the use of combination the following keywords: ["Major Bleeding"] AND ["Coronary Artery Bypass Grafting" OR "CABG"] AND ["Dual Anti Platelet Therapy" OR "DAPT"], and ["Clopidogrel" OR "Ticagrelor"]. In our searching strategy, language restrictions were not applied. We only used the study with the larger sample size and that was more up-to-date if we found the same data among studies. Moreover, we also searched the potential papers from the reference list of relevant or eligible studies. We also employed the "related article" option in PubMed to broaden our searching strategy. The potentially relevant papers were identified by two independent investigators (Y.P., M.I.). Disagreement between two independent investigators was resolved by discussion and/or by consulting to the senior investigator (J.K.F.).
The inclusion criteria for this study were (1) retrospective studies, (2) prospective studies, (3) randomized controlled trials (RCTs), (4) evaluating the association between the incidence of major bleeding and DAPT either using clopidogrel or ticagrelor in ACS patients treated with CABG, (5) providing sufficient data for calculation of OR with 95% CI. While, articles were excluded if the following criteria were found: (1) irrelevant topic, (2) review, (3) conference presentation, and (4) having low quality (see Quality assessment). For data extraction, information related to (1) name of the first author, (2) year of publication, (3) country of origin, (4) sample sizes of case and controls, and (5) the incidence of major bleeding were extracted from each study. To prevent human errors, data extraction was performed by two independent authors. If discrepancy occurred, a consensus or discussion was established.
The predictor covariate in this study was DAPT either using clopidogrel or ticagrelor. While, the main outcome measure was the incidence of major bleeding in patients receiving both clopidogrel and ticagrelor. The major bleeding included in our analysis was restricted to thrombolysis in myocardial infarction23 and platelet inhibition and outcomes criteria24. Moreover, to confer a comprehensive analysis, we also performed sub-group analysis. Data were classified into the incidence of major bleeding in ACS patients treated with DAPT (clopidogrel or ticagrelor) before and after CABG.
To ensure the quality of each study and to avoid the potential bias in each study, the quality of retrieved studies was controlled and collected by two independent investigators (Y.P., M.I.). The quality and risk of bias of each study was assessed using Methodological Index for Non-Randomized Studies (MINORS) score25. The MINORS score ranged from 0 to 24, and consisted of 12 items. Each item was assessed as 0 if the item was not reported, 1 if the item was inadequate reported, and 2 if the item was adequate reported. Each study was interpreted as having low quality if the score was less than or equal to 12, moderate if the score was less than or equal to 16 and more than 12, and high quality if the score was more than 1625. If disagreement was found between two independent authors, consensus was achieved through discussion between the two investigators. If the disagreement was not resolved, a consultation to senior researcher (JKF) was conducted.
The comparison and effect estimation of major bleeding between DAPT with clopidogrel and ticagrelor were determined using the Z-test. The pooled calculation and effect estimation were described using forest plots. The model of forest plot for describing the comparison and effect estimation was conformed with a Q test. Before analysis using the Z-test, we evaluated heterogeneity and potential publication bias. A Q-test was employed to evaluate heterogeneity. P-value of less than 0.10 was considered to indicate heterogeneity. If we found heterogeneity, a random effect model was used. While, if heterogeneity was not found, a fixed effect model was used. For testing publication bias, an Egger test was used. A P-value of less than 0.05 was considered significantly having publication bias. All analyses in our study were carried out using Review Manager version 5.3 (RevMan Cochrane, London, UK) and Comprehensive Meta-Analysis (CMA, New Jersey, US) version 2.1.
A flowchart of article searches and study selection is shown in Figure 1. Initially, 37 articles were identified from the literature search. However, eight of them were excluded because they did not have relevance to the topic, leaving a total of 29 articles. The full text of these articles was retrieved and reviewed; it was found that 16 studies did not meet the eligibility criteria because they were reviews (n=5), commentaries (n=4), family-based studies (n=3), included the same study data (n=2), and not providing sufficient data for calculation of OR and 95%CI (n=2). Finally, a total of 13 studies were eligible for our meta-analysis. Baseline characteristics of studies included in our analysis are provided in Table 1.
| Author and year | Clopidogrel | Ticagrelor | Setting (before/ after CABG) | Case | Study design | Country | MINORS | ||
|---|---|---|---|---|---|---|---|---|---|
| MB | n | MB | n | ||||||
| Becker et al. 201126 | 476 | 9186 | 446 | 9235 | after CABG | STEMI & NSTEMI | RCT | Mixed | 24 |
| Chang et al. 20192 | 7 | 100 | 7 | 100 | after CABG | NSTEMI | Case - control | Korea | 16 |
| Dery et al. 201427 | 37 | 328 | 6 | 55 | after CABG | ACS | Case - control | Canada | 18 |
| Dinicolantonio et al. 201328 | 654 | 9186 | 619 | 9235 | before CABG | ACS | Case - control | Mixed | 18 |
| Gajayanaet al. 201829 | 44 | 1721 | 7 | 860 | before CABG | ACS | Case - control | USA | 17 |
| Hansson et al. 201430 | 213 | 232 | 166 | 173 | before CABG | ACS | Case - control | Sweden | 24 |
| Hansson et al. 201631 | 95 | 978 | 90 | 1266 | before CABG | ACS | Case - control | Sweden | 19 |
| Held et al. 201132 | 375 | 629 | 362 | 632 | before CABG | ACS | Case - control | US | 24 |
| Holm et al. 201933 | 474 | 1293 | 381 | 1018 | before CABG | ACS | Cohort | Mixed | 24 |
| Kang et al. 201534 | 929 | 9291 | 961 | 9332 | after CABG | ACS | Case - control | Hong Kong | 23 |
| Russo et al. 201835 | 19 | 413 | 5 | 95 | before CABG | ACS | Case - control | Canada | 22 |
| Schaefer et al. 201636 | 0 | 28 | 2 | 28 | before CABG | ACS | Case - control | Germany | 14 |
| Varenhorst et al. 201237 | 62 | 629 | 32 | 632 | before CABG | ACS | RCT | Mixed | 24 |
A total of 13 studies2,26–37, consisting 34,014 patients treated with clopidogrel and 32,661 patients treated with ticagrelor, were included in our study. Of those, the correlation between the use of DAPT (either clopidogrel or ticagrelor) and the risk of major bleeding was found in only three studies29,30,37. A further ten studies failed to clarify the association2,26–28,31–36. Our calculation revealed (Figure 2A) that the incidence of major bleeding was not significantly different between clopidogrel and ticagrelor (OR = 1.10, 95%CI = 0.98–1.24, p = 0.0990). Moreover, in pre-CABG sub-group, we included nine studies28–33,35–37 consisting of 15,109 patients treated with clopidogrel and 13,939 patients treated with ticagrelor. Our results found (Figure 2B) that no significant different of major bleeding incidence was observed between clopidogrel and ticagrelor (OR = 1.19, 95%CI = 0.97–1.45, p = 0.0910). While, in the post-CABG sub-group, a total of four papers2,26,27,34 consisting of 18,905 patients treated with clopidogrel and 18,722 patients treated with ticagrelor was enrolled for our analysis. Our pooled data (Figure 2C) confirmed no significant different in major bleeding incidence between clopidogrel and ticagrelor (OR = 1.00, 95%CI = 0.93–1.08, p = 0.9230). The summary of correlation and effect estimation between the risk of major bleeding and the use of different DAPT is provided in Table 2.
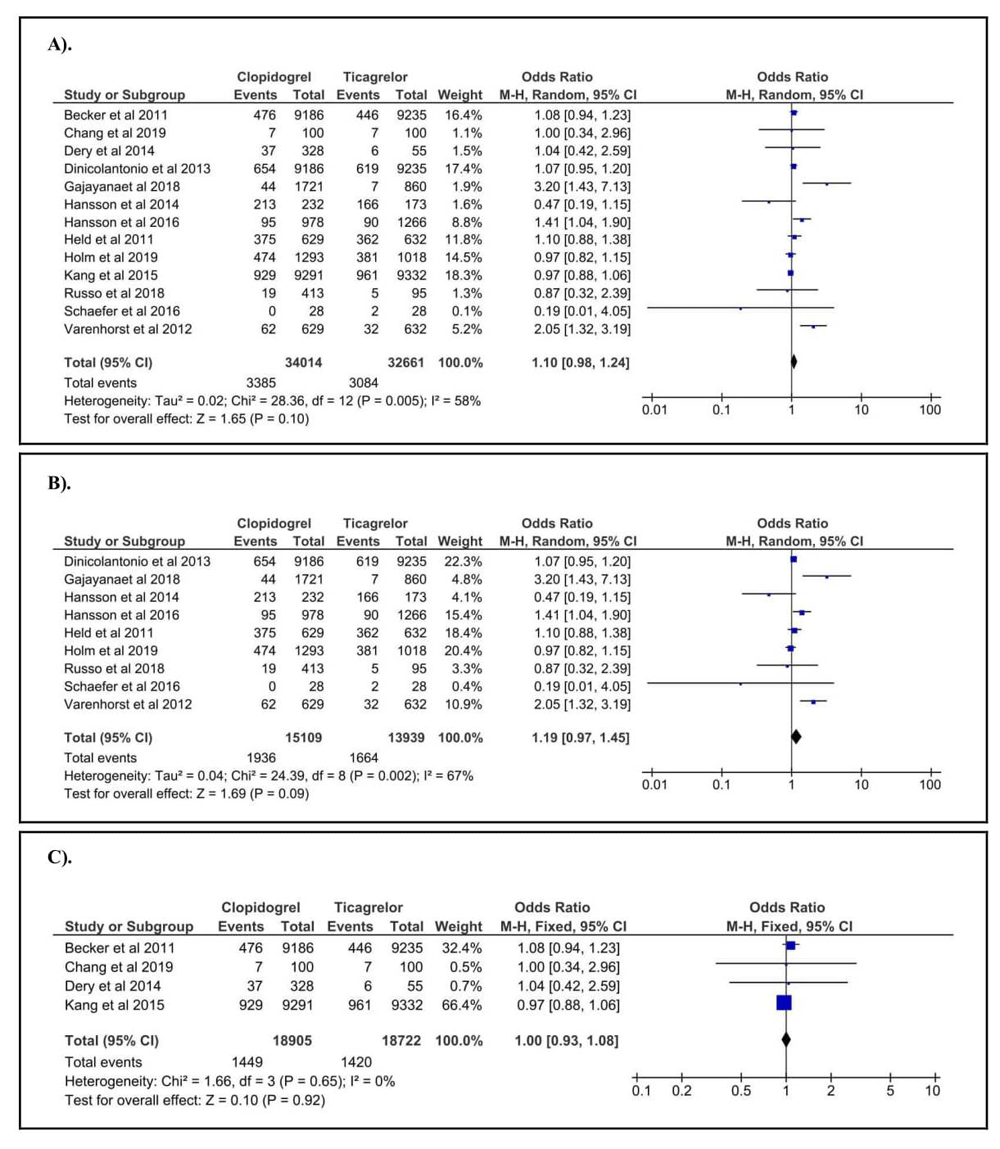
(A) Overall analysis. (B) Pre-coronary artery bypass grafting (CABG) sub-group. (C) Post-CABG sub-group.
Evidence of heterogeneity was assessed using the Q-test. Our analysis found that evidence of heterogeneity (p <0.10) was observed in overall analysis and pre-CABG sub-group. Therefore, random effect model was applied to determine the correlation and effect estimation. While, for post-CABG sub-group, we used fixed effect model to assess the correlation and effect estimation because we did not find the evidence of heterogeneity. Furthermore, potential publication bias was assessed using an Egger test. Our analysis confirmed that potential publication bias was found in post-CABG sub-group (p <0.05). In overall analysis and pre-CABG sub-group, we found no publication bias. The summary of study heterogeneity and potential publication is described in Table 2.
In our present meta-analysis, we included 13 papers consisting of 34,014 and 32,661 patients treated with clopidogrel and ticagrelor following CABG, respectively. Our current findings confirmed that neither clopidogrel nor ticagrelor was associated with risk of major bleeding among ACS patients treated with CABG. To our knowledge, no previous meta-analysis has reported the comparison between clopidogrel and ticagrelor in the context of CABG. Therefore, we were unable to perform a direct comparison. However, in other case settings, meta-analyses have been conducted in the case of PCI and thrombolytic for treating ACS patients. In the case of PCI for treating ACS patients, the reports from previous meta-analyses remained conflicting. A study conducted by Fan et al.38 found that clopidogrel was associated with increased risk of major bleeding compared to ticagrelor. On the other hand, Guan et al.39 revealed that ticagrelor was proven to correlate with increased risk of major bleeding compared to clopidogrel. A meta-analysis conducted by Westman et al.40 involved 15 papers, consisting of 26,093 patients treated with clopidogrel and 7,192 patients treated with ticagrelor. The authors revealed that although ticagrelor was associated with increased risk of minor bleeding compared to clopidogrel, the incidence of major bleeding was not significantly different between ticagrelor and clopidogrel. Moreover, in the case of fibrinolytic, a meta-analysis involving three RCTs showed that neither ticagrelor nor clopidogrel was correlated with the risk of major bleeding41. Furthermore, in the case of ACS, a meta-analysis involving 10 studies revealed that the risk of bleeding was not significantly different between patients receiving clopidogrel and ticagrelor42. Therefore, it makes sense that in our current meta-analysis, no association was observed between the use of DAPT either clopidogrel or ticagrelor and the risk of major bleeding.
To provide a comprehensive analysis, we also performed subgroup analysis, divided into pre- and post-CABG. Our findings in sub-group analysis were consistent with our main findings, we emphasized that the incidence of major bleeding either in pre- and post-CABG was not significantly different between clopidogrel and ticagrelor. To our knowledge, until now the major bleeding effect of clopidogrel and ticagrelor in the setting of before and after CABG has not been well defined. Besides the existence of no previous meta-analysis concerning this subject, reports in other case settings did not assess this effect in the pre- or post-intervention context of. Hence, the possible direct and indirect explanations was difficult to clarify. To date, the major bleeding effect of DAPT therapy before and after CABG remained conflicting. A previous study revealed that discontinuation of DAPT therapy 24–72 hours before emergency CABG was proven to increase the risk of major bleeding35. Moreover, Deo et al.43 also reported that increased risk of major bleeding was observed in post CABG patients treated with ASA and clopidogrel. However, a study by Solo et al.44 might support our findings. They evaluated the incidence of major bleeding between ASA and clopidogrel and ASA and ticagrelor. Although statistical analysis was not directly performed, they confirmed that the risk of major bleeding in post CABG patients among different anti-platelets had no strong evidence. Therefore, due to inconclusive reports regarding the risk of major bleeding and DAPT therapy before and after CABG, further studies are required to clarify our current findings.
The theory underlying the risk of major bleeding due to clopidogrel or ticagrelor is not well defined. However, some theories have been proposed. To stimulate inhibition of platelet aggregation, both clopidogrel and ticagrelor are P2Y12 antagonists. However, associated with the risk of major bleeding, each agent has a different mechanism. Clopidogrel is known to irreversibly induce bleeding by inhibiting P2Y12 receptors, and may cause persistent blockade of the adenosine diphosphate (ADP) binding site. Those inhibitory effects may persist until the platelets are renewed in 7–10 days45. Therefore, as it has a longer inhibitory effect than ticagrelor, those treated with clopidogrel may be more vulnerable to risk of bleeding than ticagrelor42. Ticagrelor is a reversible P2Y12 receptor antagonist. It works directly on P2Y12 receptors, and therefore may produce rapid inhibition effects and provide rapid recovery of platelet function46. It has been already reported that ticagrelor has faster onset and offset than clopidogrel47. As a result, when each drug is stopped, the effect of ticagrelor may disappear faster than clopidogrel. In animal subjects, a study proposed that clopidogrel was found to have 3.5-fold associated with higher bleeding risk compared to ticagrelor48. Clopidogrel is metabolized by cytochrome P2C19 enzyme49, and recent gene-disease interaction studies reported that cytochrome P2C19 CYP2C19*20 C-889T>G (SNP rs11568732) was associated with the risk of bleeding in ACS patients treated with clopidogrel50–52. Therefore, theoretically, the risk of major bleeding with clopidogrel should be higher than with ticagrelor. However, the evidence from previous large-scale studies, including our present meta-analysis, are conflicting and have not clarified the association. Hence, because it was not supported by the evidence, in our opinion, the risk of major bleeding due to different DAPT, for this time being, might be considered as a hypothesis. In the near future, we expected that more complex study designs might be applied to elucidate the real association between the risk of major bleeding and the use of different DAPT.
To the best of our knowledge, our present study was the first meta-analysis assessing the association between the risk of major bleeding and the use of different DAPT in ACS patients treated with CABG. Our current meta-analysis might clarify the inconclusive findings of previous studies regarding this topic, and we emphasized that clopidogrel, compared to ticagrelor, or vice versa, was not associated with the risk of major bleeding in ACS patients treated with CABG. In the last decade, the use of DAPT, either clopidogrel or ticagrelor, has brought about a dilemma for physicians due to the assumption that one of them was considered to trigger the risk of major bleeding. This dilemma was worsened owing to drug marketing competition among pharmaceutical industries to recommend ticagrelor over clopidogrel. However, our present study indicates that the dilemma was not supported by evidence, and therefore the dilemma might be considered as "the ocean without the waves". The present meta-analysis emphasizes the safety of DAPT administration, either clopidogrel or ticagrelor, in the context of the risk of major bleeding, and hence we expect that our present meta-analysis might reduce the dilemma regarding the risk of major bleeding due to the use of DAPT either clopidogrel or ticagrelor among physicians. The management of ACS patients using CABG has developed in the last decade, and therefore the use of DAPT in CABG management should conform with the adequate evidence. Furthermore, we hope that our current meta-analysis might be involved in the future revision of CABG management for treating patients with ACS.
In our present study, several crucial limitations were observed. First, some factors that might contribute to the risk of major bleeding, such as coagulation factors, history of stroke, chronic kidney disease, hyperglycemia, and anemia53, were not included and controlled for. Second, our current findings should be interpreted with caution due to relatively small sample size. Third, more than a half of our included studies were cross-sectional studies. Therefore, further studies with higher study design are required to obtain better evidence. Fourth, human factors (skills) were not involved in the analysis. Fifth, other drugs that might govern the risk of bleeding were not analyzed.
Our meta-analysis reveals that the use of different DAPT either clopidogrel or ticagrelor is not associated with the risk of major bleeding in ACS patients treated with CABG. Our sub-group analysis also fails to confirm this association both in pre- and post-CABG sub-groups. Our findings may provide the clarification of previous conflicting studies in the context of the risk of major bleeding and the use of different DAPT in ACS patients treated with CABG. We also expect that our findings may contribute to the future recommendation of the use of DAPT among ACS patients treated with CABG.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for ‘Comparison of major bleeding in patients with acute coronary syndrome that underwent coronary artery bypass grafting treated with clopidogrel or ticagrelor: a systematic review and meta-analysis’. https://doi.org/10.6084/m9.figshare.11688525.v122.
We thank MIT-Indonesia Research Alliance (MIRA), Lembaga Pengelola Dana Pendidikan (LPDP), and DSKF publishing campus for supporting our project.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Interventional cardiology
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Drug interaction studies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 07 Sep 20 |
read | ||
|
Version 1 10 Feb 20 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)