Introduction
Viruses from the family Filoviridae are negative-stranded RNA viruses having a filamentous shape1. The first member of this family (Marburg) was discovered in 19672, while the Ebola virus was first discovered in 19763. Public attention has been drawn to this rare, but deadly disease4 ever since the current outbreak in West African countries threatened to rapidly deteriorate into a full blown epidemic5,6. Both these viruses cause haemorrhagic fever by quickly suppressing innate antiviral immune responses7. However, quite surprisingly, the Reston Ebola (REBOV) strain, first identified in monkeys and imported into the United States in Reston from the Philippines8, is non-pathogenic in humans9,10.
Previously, we have characterized α helical (AH) structures in Ebola proteins using PAGAL11, and demonstrated that the AHs with characteristically unique feature values are involved in critical interactions with the host proteins12. We show that the AH from Ebola virus membrane fusion subunit GP213, which is disrupted by a neutralizing antibody derived from a human survivor of the 1995 Kikwit outbreak14, has a very large hydrophobic moment compared to other AHs in Ebola proteins12. Similarly, another AH with the highest proportion of negatively charged residues is the binding site of the human karyopherin (KPNA) to the Zaire Ebola (ZEBOV) virus VP24 (ezVP24) protein15.
In spite of sharing a common ancestry16, Marburg and Ebola have different antigenicity of the virion glycoprotein17. Furthermore, the mechanism of immunosuppression is different in these viruses18. These differences are probably the reason for the lesser mortality observed in Marburg outbreaks. In Ebola, the crucial role of host immune system evasion is accomplished by two proteins: VP35 and VP2419. Ebola VP24 inhibits interferon (IFN) signaling by hindering the nuclear accumulation of tyrosine phosphorylated STAT1 by binding KPNA20,21. In contrast, the Marburg virus abrogates the host immune response by inhibiting IFN induced tyrosine phosphorylation of STAT1 and STAT218 via the moonlighting matrix protein, VP4022. Specifically, ezVP24 binds KPNA via two AHs (α5 and α6)15. In Marburg VP24 (mVP24), α5 has distinctively different properties (not easily identified by a sequence or structural alignment), while α6 is just a small turn23. This rationalizes why mVP24 is not immunosuppressive.
We investigated these AHs in VP24 from the REBOV strain (erVP24). While α5 in erVP24 was similar to that in ezVP24, α6 in erVP24 was found to have different properties, caused by the presence of a serine in the place of arginine (S140R). We modelled the apo erVP24 (PDBid:4D9OA) using the ezVP24 in complex with KPNA as a template (PDBid:4U2X) by SWISS-MODEL24, and then docked KPNA to this structure using DOCLASP25. The docked structure helped in visualizing the ability of Arg140 in ezVP24 to make the correct electrostatic interaction with two glutamic acids, one of them residing on α5 in VP24, and the other in KPNA. The effect of single mutations in modulating virulence has been well established26–28. However, our methodology provides a more rational way of finding such critical residues. The possibility of a REBOV mutant gaining immunosuppressive capabilities is particularly disconcerting ever since the isolation of the REBOV strains from pigs29–31. We also highlight the possibility of using α5 and α6 from VP24 as epitopes for generating antibodies32, or designing compounds and peptides to inhibit protein-protein interaction33.
Materials and methods
AHs in proteins were identified using DSSP34. These AHs were then analyzed using PAGAL11. Briefly, the Edmundson wheel is computed by considering a wheel with centre (0,0), radius 5, first residue coordinate (0,5) and advancing each subsequent residue by 100 degrees on the circle, as 3.6 turns of the AH makes one full circle. We compute the hydrophobic moment by connecting the center to the coordinate of the residue and give it a magnitude obtained from the hydrophobic scale (in our case, this scale is obtained from35). These vectors are then added to obtain the final hydrophobic moment. The color coding for the Edmundson wheel is as follows: all hydrophobic residues are colored red, while hydrophilic residues are colored in blue: dark blue for positively charged residues, medium blue for negatively charged residues and light blue for amides.
The protein structures used in the current work are all identified using the PDBid, and are available at www.rcsb.org. We used the SWISS-MODEL program to model the erVP24 (PDBid:4D9OA) structure using the ezVP24 (PDBid:4U2XA) in complex with KPNA as template. See 4D9OA4U2XA.pdb in Dataset 1. Note the residue numbering is not conserved by SWISS-MODEL. For example, Glu113 in PDBid:4D9OA corresponds to Glu97 in PDBid:4D9OA4U2XA. We used DOCLASP25 to dock KPNA to the modelled structure of erVP24 (See Pymol script ‘dockingKPNAtoRestonVP24.p1m’ in Dataset 1). ‘4U2XA.4U2XD.maxdist.out.sort’ in Dataset 1 lists the closest atoms of the residues of VP24 (PDBid:4U2XA) that make contact with human karyopherin (PDBid:4U2XD), sorted based on distances.
All protein structures were rendered by PyMol (http://www.pymol.org/). The sequence alignment was done using ClustalW36. The alignment images were generated using SeaView37. Protein structures have been superimposed using MUSTANG38.
Results and discussion
Dataset 1.Docking human karyopherin to the Reston Ebola VP24 using Zaire Ebola VP24 as template using DOCLASP.
4D9OA4U2XA.pdb: PDB structures for the VP24 protein in Reston Ebola virus (PDBid:4D9OA) with human karyopherin (KPNA; PDBid:4U2XD) docked, based on the Zaire Ebola virus (PDBid:4U2XA) as template.dockingKPNAtoRestonVP24.p1m: PyMol script used to dock KPNA to the modelled structure of Reston Ebola virus.4U2XA.4U2XD.maxdist.out.sort: list of closest atoms of the residues of VP24 (PDBid:4U2XA) that make contact with KPNA (PDBid:4U2XD), sorted based on distances.Difference in α5 in Ebola and Marburg: explaining why Marburg VP24 is not immunosuppressive
ezVP24 has a 39.6% identity (73.8% similar) with mVP24 (Figure 1a), and there is significant structural homology among VP24 proteins from different strains of Ebola and Marburg (Figure 1b). Yet, the mechanism of immune response suppression is different in these viruses from the Filoviridae family18. ‘Reasons why Marburg virus VP24 is not immunosuppressive remain elusive’23. Therefore, we sought to investigate the differences in residues involved in binding KPNA in the ezVP24 and mVP24.
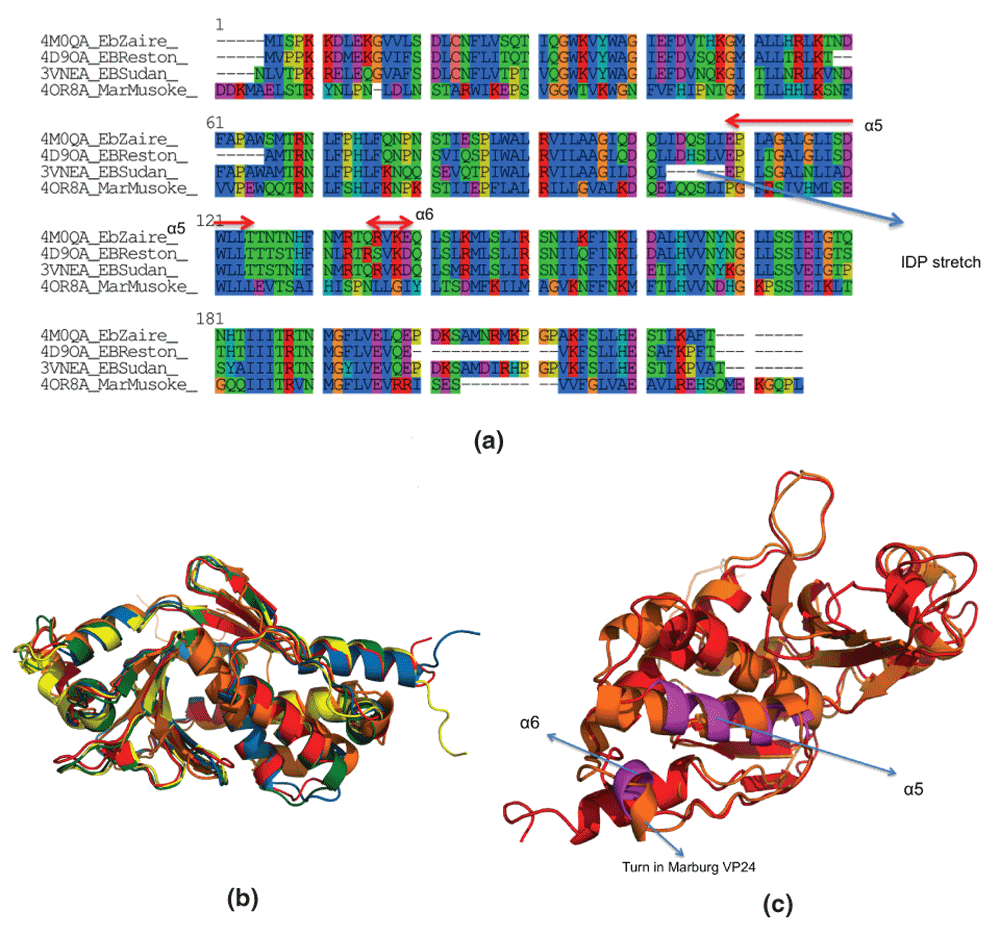
Figure 1. Sequence and structural homology between VP24 proteins from different strains of Ebola and Marburg.
(a) EbZaire: Zaire Ebola, EBSudan: Sudan Ebola, EBReston: Reston Ebola, Mar-Musoke: Marburg Musoke. Multiple sequence alignment was done using ClustalW. (b) Structural alignment of PDBid:4M0QA (Ebola Zaire Apo, in red), PDBid:4U2XA (Ebola Zaire complexed, in green), PDBid:4D9OA (Ebola Reston Apo, in blue), PDBid:3VNEA (Ebola Sudan Apo, in yellow) and PDBid:4OR8A (Marburg Musoke Apo, in orange). Structural alignment was done using MUSTANG38. (c) Helices involved in binding human karyopherin (α5 and α6 in magenta). Note, that the α5 is not a helix in Marburg VP24 (PDBid,4OR8A, in orange), but just a small turn.
ezVP24 binds KPNA via two AHs (α5 and α6), residues on loops and a Lys on a β-sheet (Table 1). In mVP24, α5 has different properties (Figure 2a,b and Table 2), while α6 is just a small turn (Figure 1c). These differences in the properties of AHs involved in binding KPNA in eVP24 to those in mVP24 strongly indicates that mVP24 is not immunosuppressive, as is widely accepted18 (at least, it does not have the same mechanism).
Table 1. Residues in Ebola Zaire VP24 (ezVP24,PDBid:4U2XA) that make contact with human karyopherin (PDBid:4U2XD).
One or more atoms from these residues are within 4 Å of residues from human karyopherin.
| Residues in ezVP24 (PDBid:4U2XA) | Secondary
structure |
|---|
| GLU/113,GLY/117,LEU/121,ASP/124,TRP/125 | α5 |
| THR/129,THR/131, PHE/134,ASN/135,MET/136,ARG/137,THR/138 | loops |
| GLN/139,ARG/140,VAL/141 |
α6 |
| GLN/184,ASN/185,HIS/186,LEU/201,GLN/202,GLU/203,PRO/204,ASP/205 | loops |
| LYS/218 | β9 |
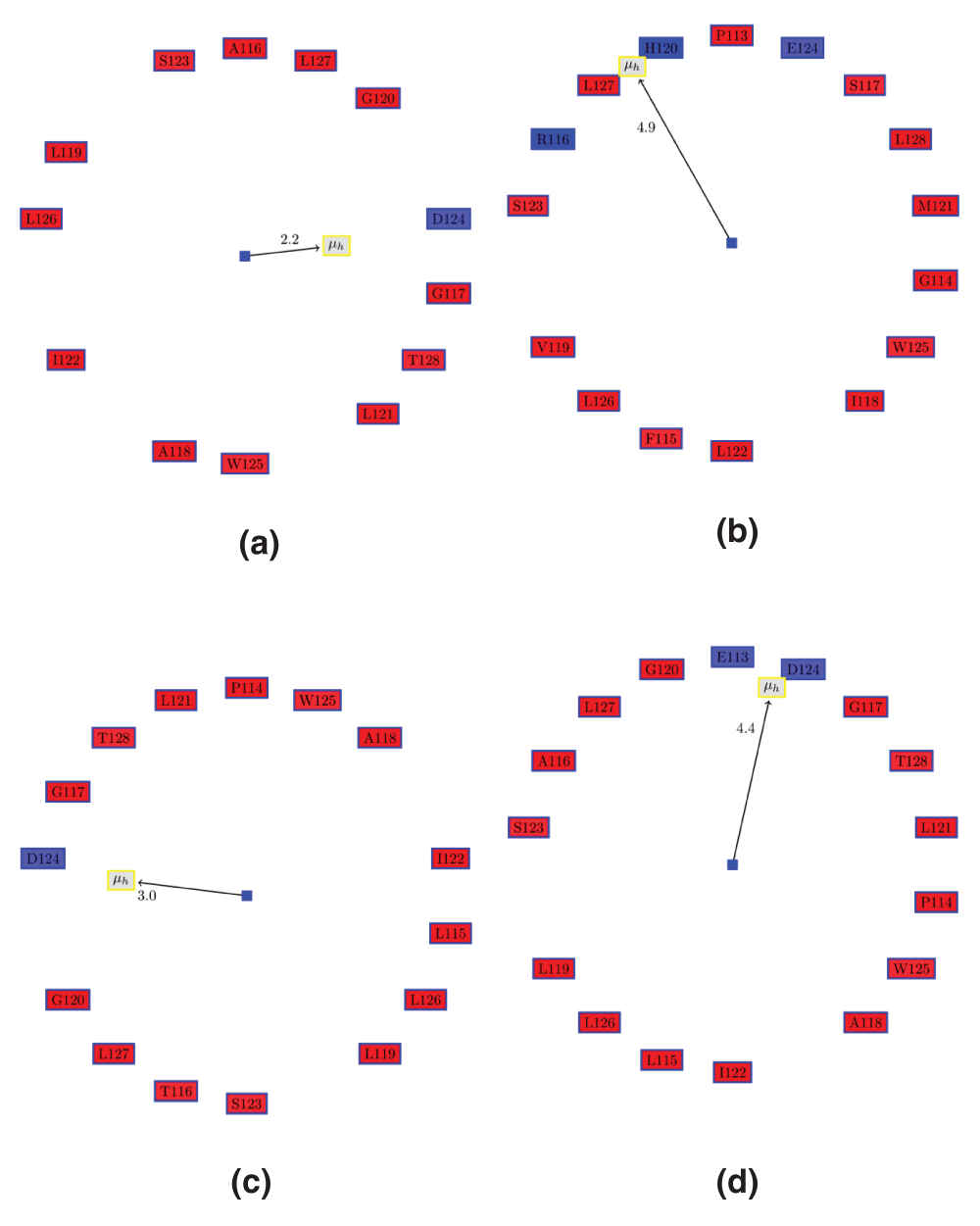
Figure 2. Edmundson wheel for α5 of VP24 in ZEBOV strain (eZVP24), Marburg (mVP24) and REBOV (erVP24) viruses.
The color coding for the Edmundson wheel is as follows: all hydrophobic residues are colored red, while hydrophilic residues are colored in blue: dark blue for positively charged residues, medium blue for negatively charged residues and light blue for amides. (a) Apo ezVP24 (PDBid:4M0QA). (b) Apo mVP24 (PDBid:3VNEA). It can be seen that mVP24 has two positively charged residues in the AH, unlike eZVP24. (c) ezVP24 (PDBid:4U2XA) in complex with human karyopherin (PDBid:4U2XD). Note, that Glu113 and Pro114 are now part of the AH, in contrast to the apo AH in (a). (d) Apo erVP24 (PDBid:4D9OA).
Table 2. Properties of α5 in VP24 proteins from different strains of Ebola and Marburg.
It can be seen that the Marburg VP24 (mVP24) protein has a distinctly different charge residue composition in the helix. This strongly indicates that mVP24 might not bind human karyopherin, which is the mechanism of immunosuppression by the Ebola VP24 proteins. HM: Hydrophobic moment, RPNR: Ratio of the positive to the negative residues, Len: length of the helix, NCH: number of charged residues.
| PDB.Helix | Description | Len | HM | RPNR | NCH |
|---|
| 4M0QA.α5 | Ebola Zaire Apo | 13 | 2.2 | 0 | 1 |
| 4U2XA.α5 | Ebola Zaire in complex with KPNA | 16 | 4.4 | 0 | 2 |
| 4D9OA.α5 | Ebola Reston Apo | 15 | 3 | 0 | 1 |
| 3VNEA.α5 | Ebola Sudan Apo | 14 | 4.1 | 0 | 1 |
| 4OR8A.α5 | Marburg Apo | 16 | 4.9 | 0.7 | 3 |
S140R substitution in α6 may explain why Ebola Reston strain is non-pathogenic in humans
The REBOV strain ‘does not represent an immediate public health menace on the scale of the African Ebola virus’9, possibly due to the generation of antibodies against this strain39. Also, gene expression of infected cells that ZEBOV and Marburg viruses showed fewer activated IFN-inducible genes relative to REBOV40. Thus, most likely, the REBOV strain does not have the same immunosuppressive capabilities of the ZEBOV or Sudan strain. While α5 of erVP24 has properties similar to ezVP24 (Figure 2c), α6 in REBOV VP24 (erVP24) is clearly different (hydrophobic moment, residue composition) in REBOV (Figure 3). For example, Arg140 in ezVP24 is replaced with Ser140 in erVP24.
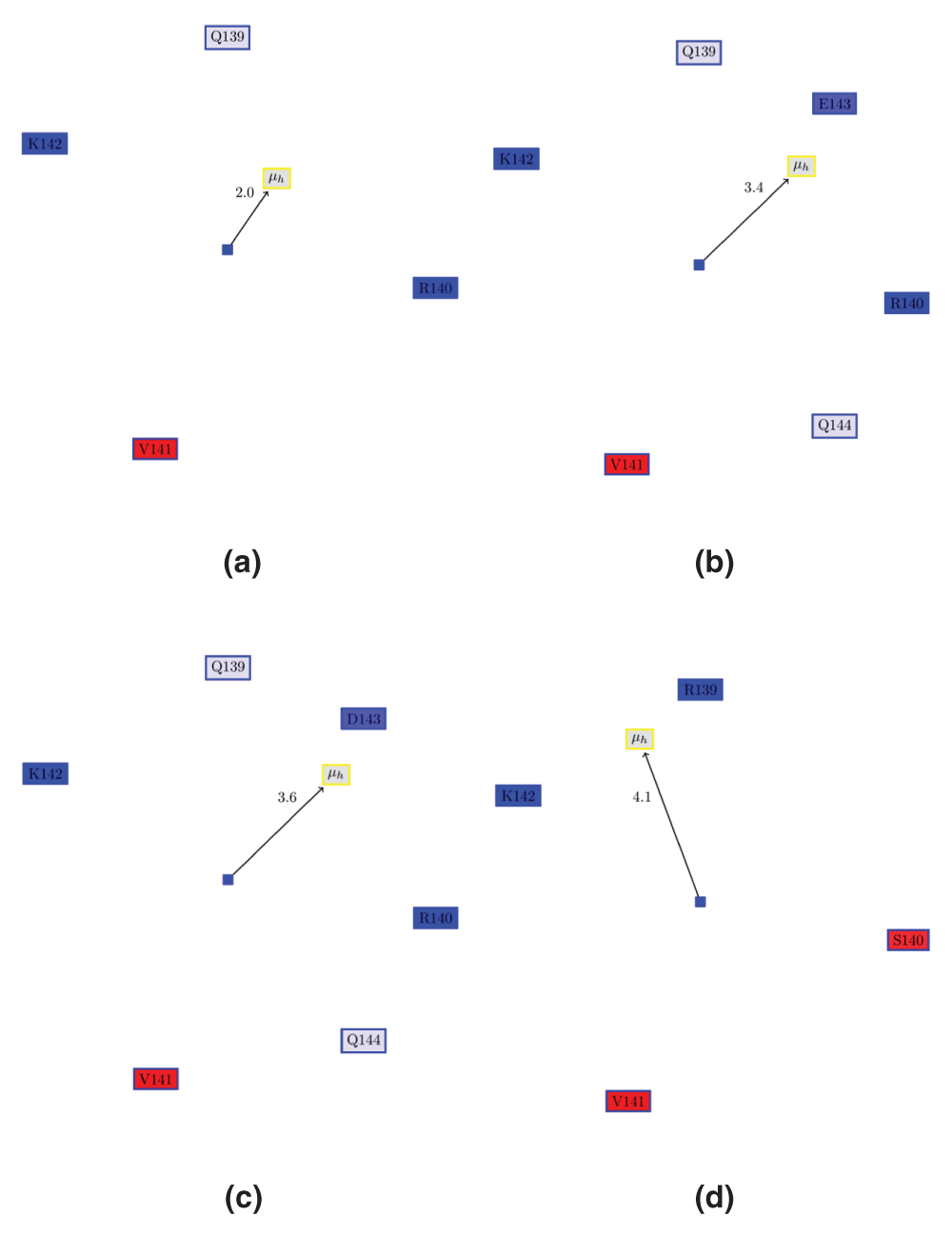
Figure 3. Edmundson wheel for α6 of VP24 in ezVP24, esVP24 and erVP24 viruses.
(a) apo ezVP24 (PDBid:4M0QA). (b) ezVP24 in complex with humans karyopherin (PDBid:4U2X). Note, that the AH is extended by two residues (E143 and Q144) as compared to the apo protein. However, the hydrophobic moment remains the same. (c) α6 of esVP24 (PDBid:3VNEA). (d) α6 of erVP24 (PDBid:3VNEA). It can be seen REBOV VP24 has a different hydrophobic moment than the other, since Ser140 is place of Arg140.
In order to better visualize this difference, and to quantify it, we docked KPNA to erVP24. First, we modelled the apo erVP24 (PDBid:4D9OA) using the ezVP24 complexed with KPNA (PDBid:4U2X) using SWISS-MODEL24. Subsequently, KPNA was docked to this protein using DOCLASP25.
Figure 4 shows the ezVP24 and erVP24 docked to KPNA. In ezVP2, KPNA binding is primarily facilitated by electrostatic attraction between the negatively charged Asp124 in α5 and Lys481 in KPNA (at 3.9 Å)12, and a hydrogen bond between Arg140 (α6) and Glu475 of KPNA (among other hydrogen bonds, Table 3). Also, the ezVP24 itself is stabilized by an electrostatic bond between the negatively charged Glu113/OE1 (α5) and the positively charged Arg140/NH1 (α6) at 3.4 Å. Note, that this pair is at distance of 12.8 Å in the apo ezVP24 (PDBid:4M0QA). This 8 Å conformational change in these AHs emphasizes the role of plasiticity in binding KPNA. In contrast, in the erVP24, the distance between Glu113/OE1 and Ser140/OG changes from 14 Å in the apo enzyme to 6.2 Å in the docked model. Also, Ser140/OG atom is not positively charged unlike Arg140/NH1. Further, the possibility of Ser140/OG making a hydrogen bond with Glu475 of KPNA is remote, since they are 6.7 Å apart. Thus, we conclude that the mutation R140S is likely to be responsible for the non-pathogenic nature of REBOV, since this mutation renders erVP24 incapable of binding KPNA.
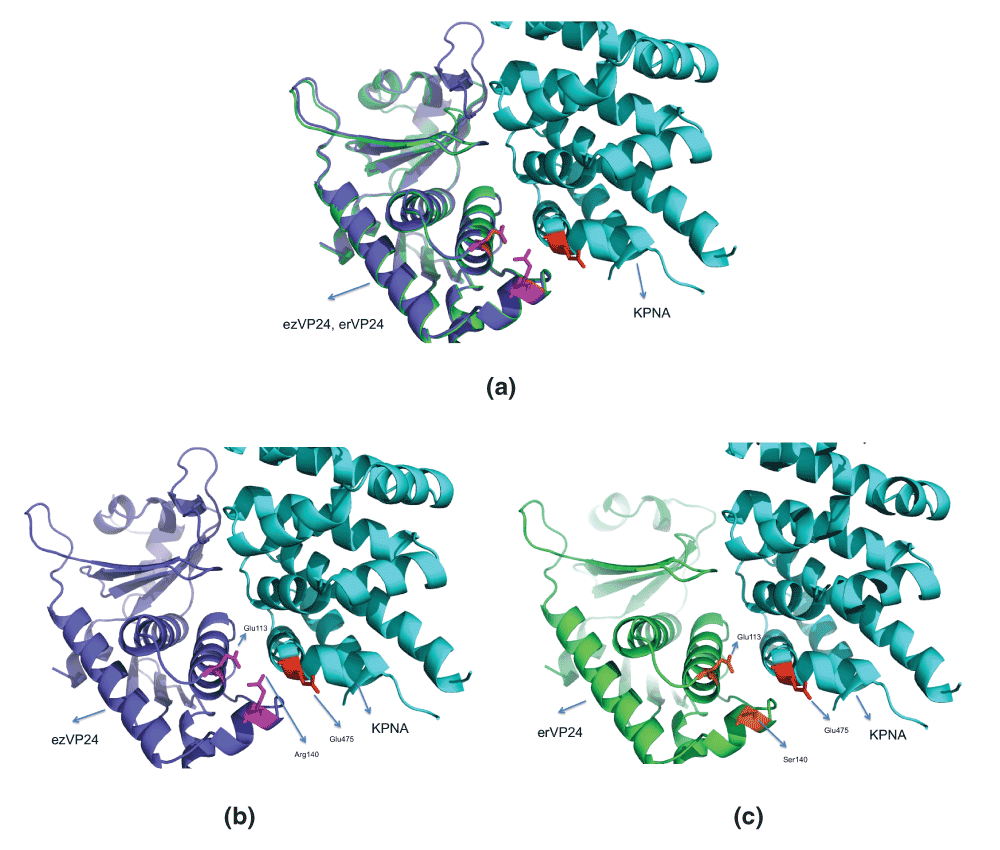
Figure 4. Docking human karyopherin (KPNA) to erVP24.
The erVP24 was modelled using SWISS-MODEL24 using ezVP24 structure complexed with KPNA (PDBid:4U2XA) (See 4D9OA4U2XA.pdb in Dataset 1). The docking was done using DOCLASP25, which superimposes the proteins as well. (a) Superimposition of modelled erVP24 and ezVP24, with bound KPNA. (b) Electrostatic attraction between the negatively charged Glu113/OE1 (α5) and the positively charged Arg140/NH1 (α6) at 3.4 Å, and a hydrogen bond between Arg140 (α6) and Glu475 of KPNA stabilizes the binding. (c) Ser140 replaces Arg140 in erVP24, and fails to make any of the above interactions.
Table 3. Atoms from ZEBOV VP24 (ezVP24) that are closest to the human karyopherin (KPNA) in PDBid:4U2X.
The complete sorted list can be found in ‘4U2XA.4U2XD.maxdist.out.sort’ in Dataset 1. Note, that there is a hydrogen bond between Arg140/NH2 and Glu475/O.
| ezVP24 atom | KPNA atom | Distance (Å) |
|---|
| THR/138/OG1 | ASP/480/OD2 | 2.7 |
| ASN/185/ND2 | ASP/431/O | 2.7 |
| ASN/185/OD1 | ARG/398/NH1 | 2.8 |
| THR/138/N | ASP/480/OD2 | 2.9 |
| ARG/140/NH2 | GLU/475/O | 3.0 |
Role of intrinsically disordered stretches in VP24
It is interesting to note that the apo α5 (PDBid:4M0QA) is extended by two residues towards the N-terminal (Figure 2c, Glu113 and Pro114) in the ezVP24 complex with KPNA (PDBid:4U2XA). Notably, Pro and Glu are the two most disorder promoting residues41. The peptide stretch preceding Glu113 in the Sudan Ebola VP24 (PDBid:3VNEA) is also disordered, and residues in that stretch are unassigned in the crystal structure (Figure 1a). Quite interestingly, the α6 (Figure 3a) is also extended by two residues (towards the C-terminal) in the ezVP24 complex (Figure 3d). As mentioned earlier, this stretch is not a helix in mVP24. In the apo Sudan Ebola VP24, α6 (Figure 3c) is similar to the ezVP24 complex (Figure 3b), and is already extended. This is probably due to the fact that Glu is replaced by Asp, which is not disorder generating. Also, the hydrophobic moment of all three AHs have (almost) the same direction and magnitude (Figure 3a–c). These observations emphasizes the role of intrinsically disordered regions in viral functionality42,43.
Conclusions
The ability of a single mutation to significantly alter the immunosuppressive properties of the Ebola proteins is well established26,27,44. Sequence based methods (whole genome profiling) are typically used to identify these critical mutations26. Structural studies provide an alternate, and possibly more rational, method to identify such mutations. For example, while double (and not single) mutations are required in VP35 to inhibit protein kinase R activation, it is difficult to rationalize this based on sequence data only28. In the current work, we build on previous work that has characterized AH structures in the Ebola proteome to rationalize the lack of immunosuppressive properties in the mVP24. ezVP24 binds to KNPA via two AHs (α5 and α5), loops and a residue on a β-sheet. We attribute the lack of immunosuppressive properties of mVP24 to its inability to bind KPNA, which emanates from different characteristics of α5 of mVP24 compared to ezVP24. Subsequently, we demonstrate that a single mutation in α6 in the erVP24 might endow it with immunosuppressive properties. We corroborate this conclusion by modelling the apo structure of the erVP24 based on the structure of ezVP24 in complex with KPNA using SWISS-MODEL24, and docking KPNA to the modelled structure using DOCLASP25. The REBOV strain, first identified in monkeys and imported into the United States from the Philippines8, has never caused disease in humans9,10. However, the isolation of the REBOV strains from pigs in Philippines29,30, and recently in China31, highlights the significance of finding preventive therapies in the probable scenario a mutant REBOV for VP24 with immunosuppressive capabilities gets transferred to human handlers. Such a difference does not exist in the VP35 protein, where REBOV VP35 has been used as a model to show how they could silence and sequester double-stranded RNA, which are key events in immunosuppression45. We also reiterate the potential of using these AHs from VP24 as epitopes46,47 for generating antibodies32,48,49, or innovating drugs to inhibit protein-protein interaction33,50–54. The presence of two intrinsically disordered residues proximal to these AHs in the apo structure that gain a AH structure upon binding should encourage antibody search to use both apo and complexed AHs. It is certainly worth investigating whether supplementing ZMapp, a cocktail of three antibodies has shown reversion of advanced Ebola symptoms in non-human primates55, with more antibodies would prove more effective.
Data availability
F1000Research: Dataset 1. Docking human karyopherin to the Reston Ebola VP24 using Zaire Ebola VP24 as template using DOCLASP, 10.5256/f1000research.5666.d3806956
Author contributions
SC wrote the computer programs. All authors analyzed the data, and contributed equally to the writing and subsequent refinement of the manuscript.
Competing interests
No competing interests were disclosed.
Grant information
AMD wishes to acknowledge grant support from the California Department of Food and Agriculture PD/GWSS Board. BJ acknowledges financial support from Tata Institute of Fundamental Research (Department of Atomic Energy). Additionally, BJR is thankful to the Department of Science and Technology for the JC Bose Award Grant. BA acknowledges financial support from the Science Institute of the University of Iceland.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Faculty Opinions recommendedReferences
- 1.
Dolnik O, Kolesnikova L, Becker S:
Filoviruses: Interactions with the host cell.
Cell Mol Life Sci.
2008; 65(5): 756–776. PubMed Abstract
| Publisher Full Text
- 2.
Kissling RE, Robinson RQ, Murphy FA, et al.:
Agent of disease contracted from green monkeys.
Science.
1968; 160(3830): 888–890. PubMed Abstract
| Publisher Full Text
- 3.
Pattyn S, van der Groen G, Courteille G, et al.:
Isolation of Marburg-like virus from a case of haemorrhagic fever in Zaire.
Lancet.
1977; 1(8011): 573–574. PubMed Abstract
| Publisher Full Text
- 4.
Colebunders R, Borchert M:
Ebola haemorrhagic fever--a review.
J Infect.
2000; 40(1): 16–20. PubMed Abstract
| Publisher Full Text
- 5.
Piot P:
Ebola’s perfect storm.
Science.
2014; 345(6202): 1221. PubMed Abstract
| Publisher Full Text
- 6.
Piot P, Muyembe JJ, Edmunds WJ:
Ebola in west Africa: from disease outbreak to humanitarian crisis.
Lancet Infect Dis.
2014; 14(11): 1034–1035. PubMed Abstract
| Publisher Full Text
- 7.
Daugherty MD, Malik HS:
How a virus blocks a cellular emergency access lane to the nucleus, STAT!
Cell Host Microbe.
2014; 16(2): 150–152. PubMed Abstract
| Publisher Full Text
- 8.
Jahrling PB, Geisbert TW, Dalgard DW, et al.:
Preliminary report: isolation of Ebola virus from monkeys imported to USA.
Lancet.
1990; 335(8688): 502–505. PubMed Abstract
| Publisher Full Text
- 9.
Miranda ME, White ME, Dayrit MM, et al.:
Seroepidemiological study of filovirus related to Ebola in the Philippines.
Lancet.
1991; 337(8738): 425–426. PubMed Abstract
| Publisher Full Text
- 10.
Miranda ME, Miranda NL:
Reston ebolavirus in humans and animals in the Philippines: a review.
J Infect Dis.
2011; 204(Suppl 3): S757–S760. PubMed Abstract
| Publisher Full Text
- 11.
Chakraborty S, Rao B, Dandekar A:
PAGAL - Properties and corresponding graphics of alpha helical structures in proteins [v2; ref status: indexed, http://f1000r.es/4e7].
F1000Res.
2014; 3: 206. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 12.
Chakraborty S, Rao B, Asgeirsson B, et al.:
Characterizing alpha helical properties of Ebola viral proteins as potential targets for inhibition of alpha-helix mediated protein-protein interactions [v1; ref status: awaiting peer review, http://f1000r.es/4lg].
F1000Res.
2014; 3: 251. Publisher Full Text
- 13.
Weissenhorn W, Carfi A, Lee KH, et al.:
Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain.
Mol cell.
1998; 2(5): 605–616. PubMed Abstract
| Publisher Full Text
- 14.
Lee JE, Fusco ML, Hessell AJ, et al.:
Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor.
Nature.
2008; 454(7201): 177–182. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 15.
Xu W, Edwards MR, Borek DM, et al.:
Ebola virus VP24 targets a unique NLS binding site on karyopherin alpha 5 to selectively compete with nuclear import of phosphorylated STAT1.
Cell Host Microbe.
2014; 16(2): 187–200. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 16.
Suzuki Y, Gojobori T:
The origin and evolution of Ebola and Marburg viruses.
Mol Biol Evol.
1997; 14(8): 800–806. PubMed Abstract
| Publisher Full Text
- 17.
Feldmann H, Nichol ST, Klenk HD, et al.:
Characterization of filoviruses based on difierences in structure and antigenicity of the virion glycoprotein.
Virology.
1994; 199(2): 469–473. PubMed Abstract
| Publisher Full Text
- 18.
Valmas C, Grosch MN, Schumann M, et al.:
Marburg virus evades interferon responses by a mechanism distinct from Ebola virus.
PLoS Pathog.
2010; 6(1): e1000721. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 19.
Basler CF, Amarasinghe GK:
Evasion of interferon responses by Ebola and Marburg viruses.
J Interferon Cytokine Res.
2009; 29(9): 511–520. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 20.
Reid SP, Leund LW, Hartman AL, et al.:
Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation.
J Virol.
2006; 80(11): 5156–5167. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 21.
Zhang AP, Bornholdt ZA, Liu T, et al.:
The Ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold.
PLoS Pathog.
2012; 8(2): e1002550. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 22.
Radzimanowski J, Effantin G, Weissenhorn W:
Conformational plasticity of the Ebola virus matrix protein.
Protein Sci.
2014; 23(11): 1519–27. PubMed Abstract
| Publisher Full Text
- 23.
Zhang AP, Bornholdt ZA, Abelson DM, et al.:
Crystal structure of Marburg virus VP24.
J Virol.
2014; 88(10): 5859–5863. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 24.
Arnold K, Bordoli L, Kopp J, et al.:
The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling.
Bioinformatics.
2006; 22(2): 195–201. PubMed Abstract
| Publisher Full Text
- 25.
Chakraborty S:
DOCLASP - Docking ligands to target proteins using spatial and electrostatic congruence extracted from a known holoenzyme and applying simple geometrical transformations [v1; ref status: awaiting peer review, http://f1000r.es/48g].
F1000Res.
2014; 3: 262. Publisher Full Text
- 26.
Hartman AL, Ling L, Nichol ST, et al.:
Whole-genome expression profiling reveals that inhibition of host innate immune response pathways by Ebola virus can be reversed by a single amino acid change in the VP35 protein.
J Virol.
2008; 82(11): 5348–5358. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 27.
Yen B, Mulder LC, Martinez O, et al.:
Molecular Basis for Ebolavirus VP35 Suppression of Human Dendritic Cell Maturation.
J Virol.
2014; 88(21): 12500–12510. PubMed Abstract
| Publisher Full Text
- 28.
Schumann M, Gantke T, Muhlberger E:
Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain.
J Virol.
2009; 83(17): 8993–8997. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 29.
Marsh GA, Haining J, Robinson R, et al.:
Ebola Reston virus infection of pigs: clinical significance and transmission potential.
J Infect Dis.
2011; 204(Suppl 3): S804–S809. PubMed Abstract
| Publisher Full Text
- 30.
Barrette RW, Metwally SA, Rowland JM, et al.:
Discovery of swine as a host for the Reston ebolavirus.
Science.
2009; 325(5937): 204–206. PubMed Abstract
| Publisher Full Text
- 31.
Pan Y, Zhang W, Cui L, et al.:
Reston virus in domestic pigs in China.
Arch Virol.
2014; 159(5): 1129–1132. PubMed Abstract
| Publisher Full Text
- 32.
Wilson JA, Bray M, Bakken R, et al.:
Vaccine potential of Ebola virus VP24, VP30, VP35, and VP40 proteins.
Virology.
2001; 286(2): 384–390. PubMed Abstract
| Publisher Full Text
- 33.
Azzarito V, Long K, Murphy NS, et al.:
Inhibition of α-helix-mediated protein-protein interactions using designed molecules.
Nat Chem.
2013; 5(3): 161–173. PubMed Abstract
| Publisher Full Text
- 34.
Joosten RP, te Beek TA, Krieger E, et al.:
A series of PDB related databases for everyday needs.
Nucleic Acids Res.
2011; 39(Database issue): D411–419. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 35.
Jones MK, Anantharamaiah GM, Segrest JP:
Computer programs to identify and classify amphipathic alpha helical domains.
J Lipid Res.
1992; 33(2): 287–296. PubMed Abstract
- 36.
Larkin MA, Blackshields G, Brown NP, et al.:
Clustal W and Clustal X version 2.0.
Bioinformatics.
2007; 23(21): 2947–2948. PubMed Abstract
| Publisher Full Text
- 37.
Gouy M, Guindon S, Gascuel O:
SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building.
Mol Biol Evol.
2010; 27(2): 221–224. PubMed Abstract
| Publisher Full Text
- 38.
Konagurthu AS, Whisstock JC, Stuckey PJ, et al.:
MUSTANG: a multiple structural alignment algorithm.
Proteins.
2006; 64(3): 559–574. PubMed Abstract
| Publisher Full Text
- 39.
Ksiazek TG, West CP, Rollin PE, et al.:
ELISA for the detection of antibodies to Ebola viruses.
J Infect Dis.
1999; 179(Suppl 1): S192–S198. PubMed Abstract
| Publisher Full Text
- 40.
Kash JC, Muhlberger E, Carter V, et al.:
Global suppression of the host antiviral response by Ebola- and Marburgviruses: increased antagonism of the type I interferon response is associated with enhanced virulence.
J Virol.
2006; 80(6): 3009–3020. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 41.
Uversky VN:
The alphabet of intrinsic disorder. II. Various roles of glutamic acid in ordered and intrinsically disordered proteins.
Intrinsically Disord Proteins.
2013; 1(1): 18–40. Publisher Full Text
- 42.
Goh GK, Dunker AK, Uversky VN:
Protein intrinsic disorder and influenza virulence: the 1918 H1N1 and H5N1 viruses.
Virol J.
2009; 6: 69. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 43.
Xue B, Blocquel D, Habchi J, et al.:
Structural disorder in viral proteins.
Chem Rev.
2014; 114(13): 6880–911. PubMed Abstract
| Publisher Full Text
- 44.
Ebihara H, Takada A, Kobasa D, et al.:
Molecular determinants of Ebola virus virulence in mice.
PLoS Pathog.
2006; 2(7): e73. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 45.
Kimberlin CR, Bornholdt ZA, Li S, et al.:
Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression.
Proc Natl Acad Sci U S A.
2010; 107(1): 314–319. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 46.
Takada A, Feldmann H, Stroeher U, et al.:
Identification of protective epitopes on Ebola virus glycoprotein at the single amino acid level by using recombinant vesicular stomatitis viruses.
J Virol.
2003; 77(2): 1069–1074. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 47.
Wilson JA, Hevey M, Bakken R, et al.:
Epitopes involved in antibody-mediated protection from Ebola virus.
Science.
2000; 287(5458): 1664–1666. PubMed Abstract
| Publisher Full Text
- 48.
Takada A, Ebihara H, Jones S, et al.:
Protective eficacy of neutralizing antibodies against Ebola virus infection.
Vaccine.
2007; 25(6): 993–999. PubMed Abstract
| Publisher Full Text
- 49.
Qiu X, Alimonti JB, Melito PL, et al.:
Characterization of Zaire Ebolavirus glycoprotein-specific monoclonal antibodies.
Clin Immunol.
2011; 141(2): 218–227. PubMed Abstract
| Publisher Full Text
- 50.
Wells JA, McClendon CL:
Reaching for high-hanging fruit in drug discovery at protein-protein interfaces.
Nature.
2007; 450(7172): 1001–1009. PubMed Abstract
| Publisher Full Text
- 51.
Chapman RN, Dimartino G, Arora PS:
A highly stable short alpha-helix constrained by a main-chain hydrogen-bond surrogate.
J Am Chem Soc.
2004; 126(39): 12252–12253. PubMed Abstract
| Publisher Full Text
- 52.
Bird GH, Madani N, Perry AF, et al.:
Hydrocarbon double-stapling remedies the proteolytic instability of a lengthy peptide therapeutic.
Proc Natl Acad Sci U S A.
2010; 107(32): 14093–14098. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 53.
Bird GH, Boyapalle S, Wong T, et al.:
Mucosal delivery of a double-stapled RSV peptide prevents nasopulmonary infection.
J Clin Invest.
2014; 124(5): 2113–24. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 54.
Harrison RS, Shepherd NE, Hoang HN, et al.:
Downsizing human, bacterial, and viral proteins to short water-stable alpha helices that maintain biological potency.
Proc Natl Acad Sci U S A.
2010; 107(26): 11686–11691. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 55.
Qiu X, Wong G, Audet J, et al.:
Reversion of advanced Ebola virus disease in nonhuman primates with ZMapp.
Nature.
2014; 514(7520): 47–53. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 56.
Chakraborty S, Rao B, Asgeirsson B, et al.:
Dataset 1 in: Correlating the ability of VP24 protein from Ebola and Marburg viruses to bind human karyopherin to their immune suppression mechanism and pathogenicity using computational methods.
F1000Research.
2014. Data Source




Comments on this article Comments (0)