Keywords
antibiotic, antiviral, COVID-19, immunomodulators, levofloxacin, remdesivir.
This article is included in the Research Synergy Foundation gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
antibiotic, antiviral, COVID-19, immunomodulators, levofloxacin, remdesivir.
The version 2 has revised abstract to state comparison statements in replacement of association statements. As a consequence of 300 word limit, two sentences in version 1 were deleted in the abstract. On the result, the Table 3 has revised to show all p-values and to remove mechanical ventilator data from the column into statement on the result text. A new author was also added, who performed statistical analysis for this version.
See the authors' detailed response to the review by Harapan Harapan
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) had caused at least 240 million cases of coronavirus disease 2019 (COVID-19) and more than 4.8 million deaths worldwide until 18 October 2021.1 At that time, Indonesia has reported 4.2 million confirmed COVID-19 cases with over 140 thousand deaths.2 The management of COVID-19 using repurposed drugs has been authorized since the beginning of the pandemic3 to rapidly control the mortality and morbidity of this new disease in the absence of evidence of clinical trial results.
Exploration on the effectiveness of repurposed drugs for COVID-19 in the first year of pandemic remains inconclusive. For example, a clinical trial on the most promising antiviral remdesivir showed its benefit on the clinical improvement at day 15.4 However, the interim report of the World Health Organization’s (WHO) Solidarity trial showed the lack of benefit of remdesivir on the reduction of mortality and hospitalization duration.5 Similarly, the Indonesian national guideline for COVID-196,7 recommends the antiviral favipiravir. However, this drug is not part of the recommendation from the WHO guideline based on the evidence from clinical trials evaluating remdesivir and favipiravir for COVID-19 therapy.8 The latest version of the WHO guidelines for COVID-19 therapy provides strong recommendation for systemic corticosteroid in severe and critical COVID-19, and conditional recommendation against remdesivir in hospitalized COVID-19.9 Corticosteroid and tocilizumab are drugs modulating the immune response that plays a critical role in the pathogenesis of severe COVID-19. As pneumonia is the main clinical manifestation of COVID-19, the use of antibiotics for COVID-19 patients regardless of the evidence of bacterial infection is concerning.10
The report on the pharmacological therapy of COVID-19 in Indonesia is not as extensive as that in other Asian countries with high cases of COVID-19, such as China and India. This retrospective study aimed to investigate the clinical use of antiviral, antibiotic and immunomodulatory agents in hospitalized COVID-19 patients during the first year of the pandemic. The treatment using these drugs were considered common based on the pathogenesis of infection and inflammation on COVID-19 disease. These drugs are also listed in the Indonesian national guideline for COVID-19.6,7 We also evaluated the length of stay (LOS) and the outcome following hospitalization to understand the benefit of pharmacological therapy of antiviral, antibiotic and immunomodulators.
This study was conducted in two main hospitals affiliated with the Faculty of Medicine Universitas Islam Bandung, Indonesia. One of the hospitals was a private hospital located in the Eastern of Bandung City, whereas another hospital was a referral government hospital for the West Java Province area located in Bandung Suburb. We performed a retrospective study using medical records of patients who were hospitalized during the first year of the COVID-19 pandemic from July 2020 to March 2021. The inclusion criteria for the subjects of this study were ≥ 18 years old, had confirmed COVID-19 and had peripheral oxygen saturation (SpO2) at admission. The minimum sample size of 172 patients was calculated to compare two independent means11 using the standard deviation of LOS from a previous study.12 The sample was collected based on a non-probability sampling procedure resulting in 249 patients. A confirmed COVID-19 patient was proven by a laboratory result for positive detection of the nucleic acid of SARS-CoV-2 virus using a reverse transcription-quantitative polymerase chain reaction (RT-qPCR) test from the nasopharyngeal and oropharyngeal sample. The severity of COVID-19 disease was categorized as severe (SpO2 < 90%) and non-severe (SpO2 ≥ 90%).
The main antiviral drugs evaluated in this study were remdesivir, favipiravir, and oseltamivir. Azithromycin, levofloxacin, and ceftriaxone were the main antibiotics assessed. Immuno-modulators included methylprednisolone, dexamethasone, and tocilizumab. We evaluated the LOS and death as two main outcomes of therapy. An additional outcome evaluated was the use of a mechanical ventilator indicated by intensive care unit (ICU) admission. As the mortality in hospitalized COVID-19 was also associated with sociodemographic characteristics and laboratory result at admission,13 we also addressed these potential confounder factors.
The data on medical records were collected based on three relevant timelines. Baseline data at admission time were demographic characteristics (age, sex), comorbid disease (diabetes mellitus, hypertension, asthma, other conditions), SpO2 measurement, and hematology results. Data during the hospitalization period were pharmacological therapy using antiviral, antibiotics, and immunomodulatory drugs. The outcome of therapy was evaluated at the end of hospitalization. To validate the evaluation of therapy, the subjects were excluded if the duration of hospitalization was shorter than three days regardless of the causes. This period is suggested for clinical evaluation of community-acquired pneumonia (CAP) in adults.14 This approach is to minimize the bias on data collection for evaluating the outcome of therapy.
The numeric data was first analyzed for normality using the Kolmogorov-Smirnov test for further relevant parametric or non-parametric analysis. Statistically significant difference of SpO2 or LOS between two groups of therapies was analyzed by Mann-Whitney test, unless otherwise stated, whereas for more than two groups Kruskal-Wallis with Dunn’s multiple comparison test was used. The association of pharmacological therapy with the death was analyzed using Fisher’s exact test. The screening for potential confounding factors that are comorbidity, age and laboratory results was analyzed by Fisher’s exact, student t-test and Mann-Whitney, respectively. The difference was considered statistically significant if the p-value was less than 0.05. A multivariable logistic regression model was constructed for all variables reached p-value <0.25 in the screening to determine the odd ratio and identify confounding factors for the outcome of death. A confounding factor was defined as a variable that changed the odds ratio of the pharmacological therapy >10% after the adjustment for the relevant variable. The statistical analysis and data display was performed using GraphPad Prism V.8 software (La Jolla, CA). The logistic regression was performed using SPSS version 23 (Armonk, NY: IBM Corp).
The demographic and clinical characteristics of 249 patients are shown in Table 1.36 Most of the patients were aged 40-60 years (54.2%) and categorized as having non-severe COVID-19 (74.7%). Slightly more patients were male (55.4%) than female (44.6%). Almost half of patients had comorbid conditions (43.3%) with hypertension (12.5%) and diabetes mellitus (9.2%) as the two most frequent single comorbidities.
Pharmacotherapy of main drugs in the management of hospitalized confirmed COVID-19 patients is shown in Table 2. All patients received antiviral drugs and almost all patients were treated with antibiotic agents. Patients who were treated using antiviral drugs were more likely to receive a single (55.4%) or double combination (33.7%) of drugs. Remdesivir was the most frequent drug used in antiviral regimens for single drug (36.3%), and double combination with faviriavir (22.1%) or with oseltamivir (6%). A triple combination of these drugs was prescribed to a few patients (2.4%). Remdesivir was used via the intravenous (IV) route with a loading dose of 200 mg and maintenance dose of 100 mg once daily. Favipiravir was used orally or IV with a loading dose 1600 mg and maintenance dose of 600 mg twice daily. Oseltamivir was given orally with the dose 75 mg twice daily. The same dose for these drugs was used in different regimens of the drug combination. The duration of antiviral therapy varied from 1 day to 21 days at the most prolonged period. The mean duration of remdesivir and favipiravir was about 7 days in various regimens. The mean duration of oseltamivir as a single drug therapy was 7 days, however the duration was shorter in the combination regimens.
| Drugs | Number (%) (N=249) | Route | Doses (mg) | Freq/day | Duration of therapy (day) |
|---|---|---|---|---|---|
| Mean (min-max) | |||||
| Antiviral | |||||
| Single drug | 138(55.4) | ||||
| Remdesivir (RDV) | 91 (36.3) | IV | 100a | 1 | 7 (1-13) |
| Favipiravir (FAV) | 32 (12.9) | Oral/IV | 600b | 2 | 8 (1-14) |
| Oseltamivir (OST) | 15 (6) | Oral | 75 | 2 | 7 (1-14) |
| Double combination | 84 (33.7) | ||||
| RDV + FAV | 55 (22.1) | IV+Oral/IV | 100a + 600b | 1 + 2 | 7(1-14) + 6(1-21) |
| RDV + OST | 15 (6) | IV + Oral | 100a + 75 | 1 + 2 | 8(5-11) + 1(1-2) |
| FAV + OST | 14 (5.6) | Oral + Oral | 600b + 75 | 2 + 2 | 5 (1-6) + 2 (1-7) |
| Triple combination | |||||
| RDV + FAV + OST | 6 (2.4) | IV+Oral+ Oral | 100a + 600b + 75 | 7(4-12) + 5(2-6) + 3(1-7) | |
| Others | 15 (6) | ||||
| Lopinavir/Ritonavir | 7 (2.8) | 400/100 | 2 | 6 (3-10) | |
| Isoprinosine | 14 (5.6) | Oral | 1000-3000 | 4 | 11 (7-16) |
| Antibiotics | |||||
| Single drug | 20 (8) | ||||
| Azithromycin (AZI) | 9 (3.6) | Oral | 500 | 1 | 10.8 (8-15) |
| Levofloxacin (LVX) | 9 (3.6) | IV | 500/750 | 1 | 5.6 (1-12) |
| Ceftriaxone (CRO) | 2 (0.8) | IV | 1000-3000 | sdc/1 | 5.5 (1-10) |
| Double combination | 218(86.9) | ||||
| AZI + LVX | 41 (16.5) | Oral+Oral/IV | 500 + 750 | 1 + 1 | 10.4 (5-16) + 9 (1-14) |
| AZI + CRO | 1 (0.4) | Oral + IV | 500 + 1000 | 1 + 3 | 19 + 8 |
| LVX + CRO | 179 (71.9) | IV +IV | 500/750 + 2000 | 1 + sdc/1 | 6.1 (1-21) + 4.2 (1-19) |
| Triple combination | |||||
| AZI+ LVX + CRO | 4 (1.6) | Oral+ IV + IV | 500 + 750 + 1000 | 1 + 1 + 3 | 12(5-21) + 7.3(1-12) + 8.3(6-10) |
| Others | 1 (0.4) | ||||
| Amoxicillin | 1 (0.4) | Oral | 500 | 3 | 5 |
| No Antibiotics | 3 (1.2) | NA | NA | NA | NA |
| Immuno-modulator | |||||
| Single drug | 91(36.3) | ||||
| Dexamethasone (DEX) | 29 (11.6) | IV | 1-6 | 1-3 | 2.2 (1-10) |
| Methylprednisolone (MP) | 60 (24.1) | IV/oral | 6.25/125 | 1/3 | 2.2 (1-13) |
| Tocilizumab (TCZ) | 2 (0.8) | IV | 200 | 1 | 1 |
| Double combination | 5 (2) | ||||
| MP + DEX | 2 (0.8) | Oral/IV + IV | 4/62.5 + 5 | 2/1 + 5 | 4.3 (1-11) + 2 (1-4) |
| MP + TCZ | 3 (1.2) | IV + IV | 6.25/125+200 | 1 | 1 (1)+4.7(1-9) |
| Triple combination | |||||
| MP + DEX + TCZ | 1 (0.4) | IV + IV + IV | 125 +5 + 400 | 1+2+1 | 1 + 2 + 2 |
| No immuno-modulator | 152(52.9) | NA | NA | NA | NA |
Antibiotic therapy occurred in most patients (86.9%) using a regimen consisting of two drugs. A combination of levofloxacin and ceftriaxone was the main regimen given to 71.9% of patients (Table 1). Levofloxacin and ceftriaxone were delivered intravenously once daily, and ceftriaxone was often used as a single dose. Levofloxacin had a standard dose of 500 mg with some patients receiving 750 mg, whereas ceftriaxone had the common dose of 2000 mg. There were 16.5% of patients receiving a combination of levofloxacin and azithromycin in which the oral route favoured. Azithromycin 500 mg once daily was the main oral antibiotic for COVID-19 in various regimens and had the longest mean of duration therapy in the single (10.9 days), double (10.7 days) and triple (12 days) combinations. Thus, the main antibiotic therapy was the combination of IV levofloxacin and ceftriaxone, while azithromycin was the main antibiotic for oral delivery.
Fewer than half (47.1%) of patients were treated with immunomodulatory drugs which were mainly used in a single regimen (36.3%) (Table 1). Methylprednisolone was the most frequent (24.1%) immunomodulatory drug used, followed by dexamethasone (11.6%). The mean duration of immunomodulatory drugs (2-4 days) was generally shorter than that of the previous antiviral and antibiotic therapy.
Peripheral oxygen saturation is one of the indications of drug therapy in the Indonesian guideline of COVID-19 management. We evaluated whether different combinations of antiviral and antibiotic drugs or immunomodulatory therapy were associated with the baseline of SpO2 recorded at admission (Figure 1). The level of SpO2 in the group of patients receiving the double combination of remedisivir and favipiravir or other antivirals (lopinavir/ritonavir and isoprinosine) was significantly lower than that when receiving oseltamivir alone (p = 0.01 or p = 0.034, respectively) (Figure 1A). Among the antibiotic drug regimens, Figure 1B shows that patients receiving the combination of levofloxacin and ceftriaxone had higher levels of SpO2 than those receiving combination of azithromycin and levofloxacin (p = 0.019). However, few patients with a very low level of SpO2 were found in both of groups as shown by plots below 80% of SpO2 in Figure 1B. In contrast, the treatment of immunomodulatory drugs was very likely indicated by the level of SpO2 as shown by Figure 1C that the patients receiving immune-modulators were strongly associated with the lower level of SpO2 (p = 0.001) compared to those not treated with immune-modulators (Figure 1C).
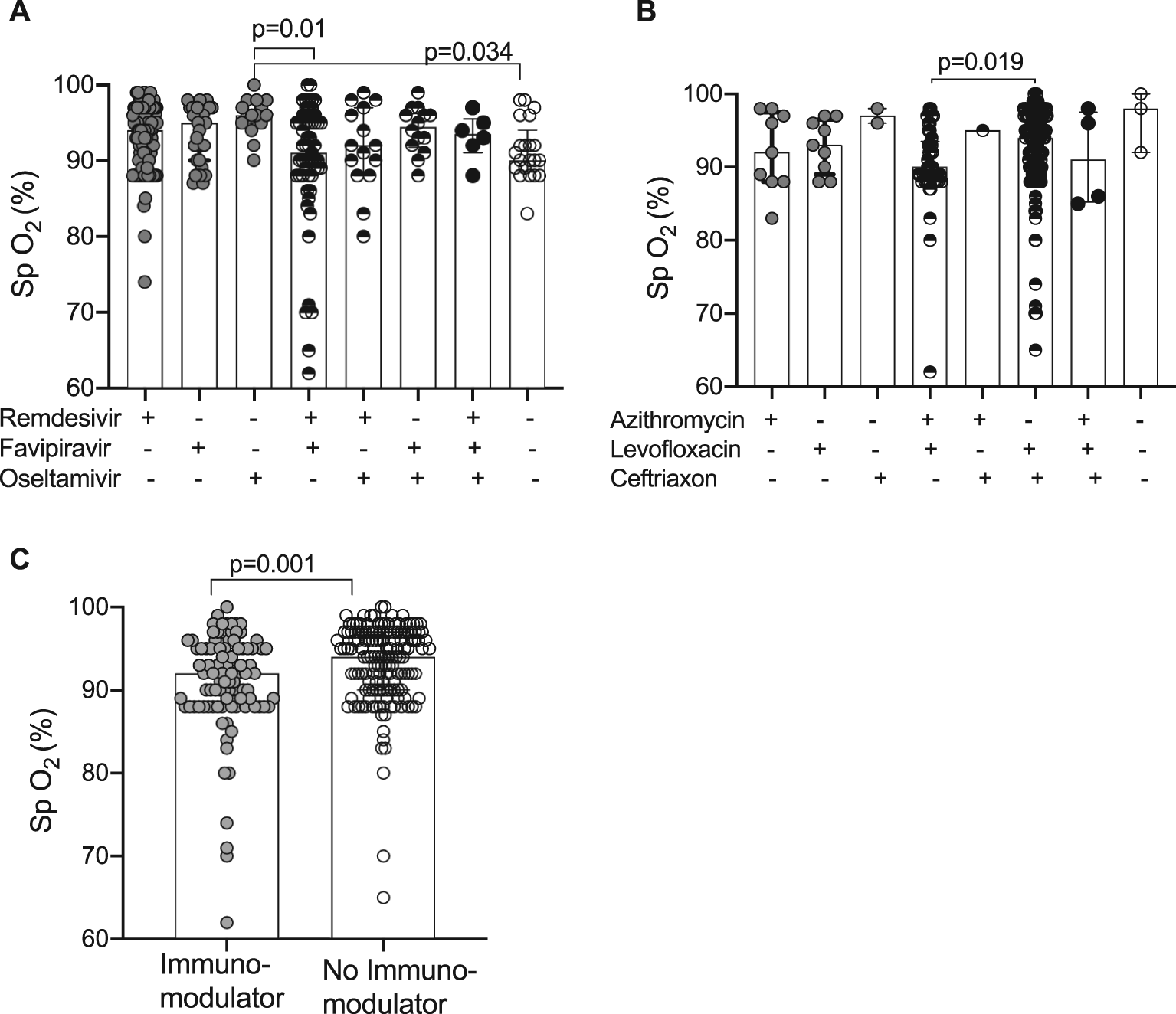
(A) Baseline SpO2 and the antiviral therapy using remdesivir, favipiravir, and oseltamivir in the various drug combinations. (B) Initial SpO2 and antibiotic combination of relevant azithromycin, levofloxacin, and ceftriaxone. (C) Immunomodulatory therapy using methylprednisolone, dexamethasone, and tocilizumab. Data are presented as median and interquartile range (IQR). Statistical differences were analyzed by Kruskal-Wallis and Dunn’s multiple comparisons (A, B) or Mann-Whitney test (C).
The efficacy of pharmacological therapy in hospitalized COVID-19 patients was evaluated by the LOS and the death as the main outcome, and the use of mechanical ventilator as the additional outcome. Out of 249 patients in this study, there were 22 deaths (8.8%) (Table 3). Three patients required mechanical ventilators and all of these patients used immunomodulatory drugs. The median of LOS from all patients was 7 days with interquartile range (IQR) 5-10 days. Patients who were treated with remdesivir or the combination of favipiravir and oseltamivir had a shorter LOS compared to those who were treated with the other antiviral drugs (p = 0.03 and p = 0.01, respectively). However, treatment with the combination of remdesivir and favipiravir was associated with the deaths (p < 0.0001).
Patients who were treated with the combination of levofloxacin and ceftriaxone had significantly shorter LOS (p < 0.0001) compared to those receiving the combination of azithromycin and levofloxacin. However, the combination of levofloxacin and ceftriaxone was also associated with the outcome of death (p = 0.0018). The use of immunomodulatory drugs was associated with a longer LOS (p = 0.0043) and death (p < 0.0001) compared to the patients who were untreated with immunomodulatory drugs. Thus, regimen therapies associated with shorter LOS were remdesivir alone, the combination of favipiravir and oseltamivir, and the combination of levofloxacin and ceftriaxone. However, the outcome of death was associated with the combination of remdesivir and favipiravir, the combination of levofloxacin and ceftriaxone, and the immunomodulatory drugs.
Table 4 showed the baseline characteristics of patients that were statistically associated with death. Compared to patients who were discharged alive, death patients had older age (p = 0.0115), comorbid conditions (0.0058), lower lymphocyte number (p = 0.0061) and higher neutrophil to lymphocyte ratio (NLR) (p = 0.004). To note, the group of discharged alive had no data of hematology (lymphocyte, thrombocyte, and NLR) for seven patients.15 This screening suggests baseline clinical characteristics were potential confounding factors for the outcome of death following hospitalization.
SD = standard deviation; SpO2 = peripheral oxygen saturation; IQR = interquartile range.
| Characteristics | Death (n = 22) | Discharged alive (n = 227) | p-value |
|---|---|---|---|
| Age (year) mean (SD) | 59.4 (10.2) | 51.7 (13.8) | 0.0115*,a |
| Sex, n (%) | 0.115b | ||
| Female | 6 (5.4) | 105 (94.6) | |
| Male | 16 (11.6) | 122 (8.4) | |
| Comorbid condition, n (%) | 0.0058**,b | ||
| No comorbid | 6 (4.3) | 135 (95.7) | |
| Comorbid | 16 (14.8) | 92 (85.2) | |
| SpO2 (%), median (IQR) | 90.5 (88-93.5) | 94 (90-97) | 0.0285*,c |
| Lymphocyte (109/L), median (IQR) | 1.1 (0.7-1.2) | 1.3 (0.9-1.8) | 0.0061**,c |
| Thrombocyte (109/L), median (IQR) | 218 (144-261) | 226 (178-289) | 0.4083c |
| Neutrophil to Lymphocyte ratio (NLR) median (IQR) | 6.2 (3.9-10.3) | 3.7 (2.4-6.3) | 0.004**,c |
To confirm the confounding factors of pharmacological therapy, we performed multivariable analysis for the outcome of death but not for the LOS, because the data of LOS was not normally distributed. The stratification analysis based on the severity of the disease showed that it was not an interaction variable. Patients treated with the combination of remdesivir and favipiravir or treated with immunomodulatory drugs had increased risk of death (odds ratio [OR] 4.1; 95% confidence interval [CI] 1.4-12.1 or OR 6.3; 95% CI 1.7-23.5, respectively) before adjustment. This remained significant after adjustment for age, sex, gender, comorbid, and lymphocyte count (OR 4.1; 95% CI 1.4-12.2 or OR 6.2; 95%CI 1.7-23.3, respectively) (Table 5). However, all the clinical characteristics at admission, except for the NLR, were confounding factors for the risk of death following therapy using combination of remdesivir and favipiravir or immunomodulatory drugs.
Our study evaluated the clinical use of antiviral, antibiotic and immunomodulatory drugs for hospitalized patients confirmed with COVID-19. We found that antivirals and antibiotics were the standard treatment delivered to almost all the patients, whereas the immunomodulator treatment was an additional therapy. We assessed the clinical use of these drugs with SpO2 for the indication of therapy, and with LOS and death for the outcome of therapy.
The results showed that remdesivir is the primary drug composing various antiviral regimens. Remdesivir was recommended conditionally for COVID-19 patients who required oxygen supplementation16 and severe COVID-19 patients defined by a low level of SpO2.8 However, treatment with remdesivir alone in our study did not correlate significantly with the level of SpO2 compared to favipiravir or oseltamivir. Patients with more severe COVID-19 were more likely to receive the combination of remdesivir and favipiravir or other antiviral drugs (lopinavir/ritonavir or isoprinosine). On the other hand, oseltamivir seems to be the first antiviral choice for the non-severe COVID-19. The initial SpO2 was not the main indication for the choice of the rest antiviral regimens.
Remdesivir in this study was used intravenously with a loading dose of 200 mg followed by 100 mg/day for a mean duration of 7 days. This dose provides an effective conversion of its metabolite into intracellular adenosine triphosphate analogue that selectively inhibits viral RNA polymerase.17 The same dose of remdesivir was used in other studies,5,18,19 however, some studies used remdesivir for 5 days18,20 or 10 days.18,19 The 5-day remdesivir was associated with better outcome at day 11,18 but another study found no differences.20 A meta-analysis revealed that the 5-day remdesivir provided similar benefit but fewer adverse events than the 10 day.21 Favipiravir in our study was given with a loading dose of 1200 mg and a daily dose of 600 mg. This dose was half of that used in several studies on COVID-19,16 but the same dose as a recent study evaluating favipiravir in recurrent COVID-19.22 Favipiravir (Avigan®) selectively inhibits viral RNA polymerases of influenza viruses and has a broad-spectrum antiviral activity for neglected and emerging RNA viruses.23,24 Treatment with favipiravir alone was considered safe and effective to shorten viral shedding in recurrent positive COVID-19 patients.22 Few studies showed the benefit of combined therapy of favipiravir with methylprednisolone.16 Combination of remdesivir and favipiravir was the most common antiviral regimen in our study, however, this was not available on the list of drug therapy used in clinical trials of COVID-19.16,25 Therefore, the safety and the efficacy of the combination of remdesivir and favipiravir is unknown. Indeed, clinical judgement to guide management decisions is part of WHO’s conditional recommendation.9 Further studies should evaluate clinical symptoms for comprehensive evidence for clinical judgement.
The empiric antimicrobial therapy for COVID-19 patients in our study was very high (98.2%). This proportion is higher than that reported in the United Kingdom (UK) (85.2%),26 Netherland (60.1%),10 and Surabaya Indonesia (75.3%).27 In fact, microbiological testing showed that the bacterial infection and co-infection among hospitalized COVID-19 patients was infrequent.10 Several studies reported 1.25 % of 925 patients,10 19.7% of 218 patients,27 1107 of 48902 patients were confirmed bacterial co-infection. In COVID-19 patients in the UK, the most frequent etiology of respiratory co-infection was Staphylococcus aureus and Haemophilus influenza, of secondary respiratory infection was Enterobacteriaceae and S aureus, and in bloodstream infection was Escherichia coli and S aureus.26 In the Indonesian setting, Gram-negative was the common causative agents of bacterial infection in COVID-19 patients.27 These findings suggested that the choice for empirical antimicrobial should be treated for Gram-negative bacteria and S aureus until the results of culture available.
Our study showed that IV levofloxacin (500/750 mg), IV ceftriaxone (200 mg), and oral azithromycin (500 mg) were the common antibiotic therapy used in COVID-19 patients. The combination of intravenous levofloxacin and ceftriaxone was the most frequent antibiotic regimen prescribed. The choice of levofloxacin for COVID-19 patients is probably because fluoroquinolone antibiotic has a broad-spectrum activity. Fluoroquinolone antibiotic is used in severe CAP, and has the potency of antiviral and immune-modulator.28 Ceftriaxone is a broad-spectrum beta-lactam antibiotic that has been one of the choices for treating community-onset pneumonia (COP). The dose of 1000 mg has a similar cure rate to 2000 mg.29 Azithromycin is a macrolide antibiotic that is effective against Gram-positive, Gram-negative, and atypical bacteria. It has primarily been used as a treatment for upper and lower respiratory infection with its potential application for COVID-19 due to its effect of antiviral and immune-modulator.30 A systematic review and meta-analysis study reported the prevalence of antibiotic use in COVID-19 patients was 24.5% azithromycin, 10% fluoroquinolone, and 9% ceftriaxone.31 However, the study found the lack of data on the specific indication and specific name antibacterial agents. The standard broad-spectrum antibiotics in our study were not supported by the type of bacteria found in most studies.26,27,31 However, our study has added value in providing the name of antibacterial agents used.
The latest version of living WHO guideline recommends both corticosteroid and IL-6 receptor blocker (tocilizumab) for severe and critical COVID-19 patients.9 Our study found that patients who were treated with immunomodulatory drugs (methylprednisolone, dexamethasone, and tocilizumab) had a significantly lower level of SpO2 compared to those who were untreated with these drugs (p = 0.001). Systemic corticosteroid such as 6 mg of oral or intravenous dexamethasone or 50 mg of intravenous hydrocortisone was strongly recommended for severe and critical COVID-19, but not for the non-severe COVID-19 because of the low certainty evidence on the increased risk of death.32 Similarly, the RECOVERY clinical trial showed the efficacy of dexamethasone on reducing the incidence of death was for the severe and critical, but not on the non-severe COVID-19.33 Interestingly, in our study, the use of immunomodulators was associated with prolonged LOS (p = 0.0043) and a higher number of deaths (p < 0.0001) compared to the non-immunomodulatory drug user. Thus, the treatment of more severe COVID-19 patients with immunomodulatory drugs did not improve the outcome.
Our study evaluated the LOS and death as the outcome for clinical use of antiviral, antibiotic, and immunomodulatory therapy. Our study found that remdesivir alone was associated with shorter LOS than therapy using other antiviral drugs (lopinavir/ritonavir or isoprinosine). This is in line with a recent review updating the results of randomized clinical trials (RCT) on antiviral agents which showed that remdesivir could increase clinical improvement but lacked benefit on preventing death.25 A study in Surabaya reported that COVID-19 patients confirmed with bacterial infection had longer LOS and higher mortality than those without bacterial infection.27 However, the study did not evaluate the outcome of antibiotic therapy as we did. Multivariable analysis in our study found concerning safety issues for two regimen therapies. Patients who were treated with the combination of remdesivir and favipiravir had a four times higher risk of death compared to those receiving antiviral drugs other than this regimen. Similarly, patients treated with immunomodulatory drugs had a six times higher risk of death compared to those untreated with immune-modulators. However, several baseline clinical characteristics including age, gender, sex, comorbid condition, SpO2 level, and lymphocyte contributed to this risk and became confounding factors. It has been known from the beginning of COVID-19 pandemic that older age and comorbid disease, in particular hypertension and diabetes mellitus, were associated with higher death rate.34 Lower lymphocyte count was also associated with the severity of COVID-19.27,35 Further confirmation studies should control the baseline clinical characteristics of COVID-19 patients to validate the increased risk of death following the treatment with the combination of remdesivir and favipiravir or immunomodulatory drugs.
This study has several limitations. The non-probability sampling may affect the ability to generalize of the result to a broader population. Our study did not include data on the clinical symptoms of COVID-19 patients and the microbiological testing that would provide more comprehensive clinical judgment for antiviral and antibiotic therapy. However, to our understanding, this is among the first retrospective study reporting the details on the specific name of drugs and drug combination of antiviral and antibiotic for COVID-19 patients. Further prospective study to ensure the drug interaction and safety profile of combination drug regimens is warranted.
Clinical use of antiviral drugs and antibiotics in our study were very likely the standard therapy applied to almost all hospitalized COVID-19 patients. On the other hand, the treatment of COVID-19 patients using the immunomodulatory drugs was an additional therapy. The most common antiviral regimen was the combination of remdesivir and favipiravir, whereas the most frequent antibiotic regimen was the combination of levofloxacin and ceftriaxone. Treatment of hospitalized COVID-19 with remdesivir alone, the combination of favipiravir and oseltamivir, and the combination of levofloxacin and ceftriaxone was associated with short LOS. There was increased risk of death in patients treated with the combination of remdesivir and favipiravir and the immunomodulatory drugs. However, clinical characteristics at admission including age, sex, comorbid condition, SpO2 level, and lymphocyte count contributed to this risk.
Figshare: Clinical use of antiviral, antibiotic and immunomodulatory drugs in hospitalized COVID-19 patients: a retrospective study in Bandung Indonesia.
https://doi.org/10.6084/m9.figshare.16530615.v1.36
This project contains the following underlying data:
‐ Clinical characteristics, therapy of antiviral, antibiotic and immunomodulatory drugs, hematology results for all patients
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We would like to thank Adzan Fitri and health workers at the hospitals Guntur Sepatapati, Diah Zakiah Ismawati and Andri Muhammad Ramdani for supporting this study on data collection. All these people had given a written permission to be named in this section of article.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Drug discovery
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer biology, neurology, immunology
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Virology and Public Health
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 28 Jul 23 |
read | read | |
|
Version 1 27 Oct 21 |
read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)