Keywords
Aquaculture, fatty acids profile, coconut water, palm sap sugar, giant gourami, growth performance, feed efficiency
This article is included in the Agriculture, Food and Nutrition gateway.
Giant gourami (Osphronemus goramy Lacepede, 1801) is a popular freshwater species in Indonesia, but there is limited information on feed used for its cultivation. Therefore, this study aims to determine effect of feed enriched with fermented coconut water and palm sap sugar solution on growth, feed efficiency, and carcass composition of juvenile sago gurami.
A total of 2 litres coconut water and 1litres palm sap sugar solution (10%) were formulated. Each formulated product was then mixed with 6 g Aspergillus niger (P1), 6 g Rhizopus oligosporus (P2), and 6 g Saccharomyces cerevisiae (P3) to obtain the supplements. Subsequently, supplementation was carried out on commercial feed at a dose of 300 ml/kg of feed. Samples supplemented with P1, P2, and P3 were designated P1, P2, and P3 diets, while feed added to freshwater was considered P4 (placebo). Gurami sago juvenile (initial weight 50±2.5 g and length 13.2±0.4 cm) were then placed in triplicate nets (2×1×1 m) in a freshwater concrete pond with a stocking density of 30 fish/net.
The different products formulations had a significant effect (p<0.05) on growth performance. The weight gain in P1, P2, P3, and P4 diets were 167.24%, 193.99%, 134,22%, and 115.98%, respectively. For feed conversion efficiency, the values obtained were 0.65, 0.73, 0.65, and 0.64 in P1, P2, P3, and P4, respectively. Furthermore, supplementing commercial feed with varying products formulation had a significant impact (p<0.05) on the fatty acid composition and carcass body of gurami sago. Lipid content in fish carcass fed P1, P2, P3, and P4 were 2.90%, 4.42%, 2.98%, and 2.76%, respectively.
Based on the results, P2 contained a higher concentration of fatty acids compared to other diets, leading to increased body weight, feed efficiency, and carcass fatty acid composition in sago gurami reared in freshwater concrete ponds.
Aquaculture, fatty acids profile, coconut water, palm sap sugar, giant gourami, growth performance, feed efficiency
The author has made several significant changes to this paper. These changes include refinements to the title, methods and abstract results. In the introduction section, a crucial statement added is the supplementation of commercial feed with a formulated product consisting of coconut water, palm sugar, and various fermented fungi (Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae). This formulation has not been tested on fish before. In the methods section, the author has included information that the floating commercial feed used has a diameter of 2 mm. The samples are then gradually provided to ensure complete consumption by the experimental fish, preventing washout and dissolution of the fermentation products sprayed into the rearing water during feeding. Different formula additions to the experimental feed are performed daily before being given to the experimental fish. To analyze and approximate carcass composition, fish samples are euthanized by injecting their brains with a No. 7.5 G x 1-inch livestock syringe to examine the entire carcass. The discussion section provides information on the relative functions of coconut water and palm sugar and their influence on feed nutrition. In the conclusion section, it is added that the P2 (presumably the formulated product) improves fish growth, feed efficiency, and carcass fatty acids. With these changes, the paper becomes more comprehensive and provides more transparent and detailed information.
See the authors' detailed response to the review by Norazmi-Lokman Nor Hakim
See the authors' detailed response to the review by Nurul Huda
Giant gourami (Osphronemus goramy Lacepede, 1801) is one of the most important freshwater fish species in Indonesia due to its high market value1–4. However, its contribution to the total production of freshwater aquaculture is lower compared to tilapia, African catfish, and Pangasius catfish5. This has led to the active engagement of the Ministry of Maritime Affairs and Fisheries of the Republic of Indonesia in encouraging fish farmers to increase the annual production of giant gurami from various local strains, including Tambago, Palapa, Bastar, Galunggung, Blusafir, and Sago4,6–8.
Several global initiatives have been implemented to enhance production of giant gurami using aquaculture activities, such as adjusting feeding rates in floating cages9, changing stocking density in concrete freshwater ponds4, and diversifying aquaculture systems, including earthen freshwater ponds, concrete freshwater ponds, and floating cages7,8,10. Although these aquaculture operations use commercial pellet fish food, feed conversion ratio (FCR) remains elevated, ranging between 1.43 and 1.65. This condition often leads to a low feed efficiency ratio, where 30 to 40% of the meal consumed is released as a waste load to water bodies4,8,11, thereby causing significant concerns due to the high cost of fish feed12–18.
Various strategies have been developed to improve aquafeed nutrition, including enrichment with fish oil9,19, soybean oil20, iodine + selenium21, EPA + DHA22, and probiotics23. The enrichment process is often carried out to increase the amino acids, fatty acids, minerals, and vitamin content, which cannot be produced by the animals in sufficient quantities to meet their needs17,24,25.
At present, it is essential to evaluate the supplementation of feed with natural ingredients that are cost-effective and sustainable from plant resources, without compromising growth of cultured fish and aquafeed quality. Among these natural ingredients are coconut water and palm sap sugar, which both contain health-friendly nutrients, such as minerals, amino acids, enzymes, organic acids, fatty acids, vitamins, and phenolic compounds21,25–28. Coconut water has been successfully used to treat various diseases in humans, including throat infections, tapeworms, gonorrhea, digestive problems, influenza, lice, giardia, bronchitis, and cholera29–31. Furthermore, palm sap sugar has also been reported to possess health benefits due to its low glycemic index as well as antioxidants, vitamins, and minerals content32–34. Previous reports have showed the ability of fungus to improve aquafeed's nutritional value15,35,36. The supplementation of commercial feed with formulated products consisting of coconut water, palm sugar, and fermented various fungus (Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae) has never been tested on fish. Therefore, this study aims to determine effect of feed enriched with fermented coconut water products on juvenile sago gurami's growth and carcass composition (Osphronemus goramy Lacepède, 1801). The health lipid index of the samples was also evaluated by assessing various types of fatty acids in carcass. In this study, it was hypothesized that the use of various formulated products as supplements could improve the nutritional quality of feed and carcass, feed efficiency, and juvenile growth rate.
This study was carried out by Hafrijal Syandri and colleagues under the project entitled Optimization of New Formula Products Based on Local Materials To Strengthen Food Independence In The Aquaculture Sector In The New Normal Era of Coronavirus Disease (Covid-19). Furthermore, the Ministry of Education, Culture, Research, and Technology of the Republic of Indonesia funded this study, with grant number:170/E4.1/AK.04.PT/2021. The procedures were approved by the Ethics Committee for Research and Community Service at Bung Hatta University (110/LPPM/Hatta/III-2021, in line with the ARRIVE guidelines. Approval was also given to collect and rear juvenile gurami sago in the Aquaculture Laboratory, Faculty of Fisheries, and Marine Science Universitas Bung Hatta. All efforts had been made to relieve the suffering of experimental animals, showing gurami sago didn't suffer and were still in good condition during their return to the pond after the procedures. For euthanized samples, the process was carried out by piercing part of the fish's brain. Gurami sago fish were not classified as a protected animal according to Indonesian legislation.
A total of 300 g of palm sap sugar (Arenga pinnate M.), purchased from local farmers, was cooked in 3,000 ml freshwater at 60 °C for 15 minutes and then cooled for 20 minutes in an open space. Subsequently, 3 liters of the sample was mixed with 6 liters of mature coconut water (Cocos nucifera L.). The total products formulated was 9 liters, which was divided into three containers of 3 liters. The first, second, and third parts were added with 6 g of Aspergillus niger (P1), 6 g of Rhizopus oligosporus (P2), and 6 g of Saccharomyces cerevisiae (P3), respectively. Each portion (3,000 ml) was fermented for 48 hours in a plastic jerry can with a capacity of 5 liters. The aeration process was then carried out continuously using Aerasi Fujimac MAC-40K-40L/min made in Japan.
The floating commercial feed used had a diameter of 2 mm, with a proximate composition (dry weight %) of 10.66% moisture content, 30.10% crude protein, 4.09% crude fat, and 45.35% carbohydrate total, 2.5% ash, and 9.18% crude fiber. Furthermore, it was supplemented with products P1, P2, and P3, up to 300 ml/1 kg. Commercial feed with only freshwater (P4) was used as a control in the procedures. All products were each sprayed evenly to 1 kg of the commercial feed, and dried in open air for 30 minutes. The samples were then given gradually to ensure complete consumption by the experimental fish. This was carried out to prevent the leaching and dissolution of sprayed fermentation products into the rearing water during feeding. The addition of different formulas to the experimental diet was carried out every day before being given to the experimental fish.
A total of 360 juvenile gurami sago of local strains were obtained from the Aquaculture Laboratory Faculty of Fisheries and Marine Science at Universitas Bung Hatta Padang of Indonesia. The exclusion criteria were samples in poor health or did not eat the commercial food initially provided. Furthermore, the samples were acclimatized for 30 days before the experiment, which commenced in January 2021. Juvenile fish were placed in the concrete freshwater pond 24-m3 (6×4×1 m) with a capacity of 5,600 L. During the acclimation, juvenile was fed commercial feed with 30.10% crude protein content, 4.09% crude fat, 2.5% crude ash, and 9.18% crude fiber. Feeding was carried out three times daily (09.00 AM, 1.00 PM, and 5.00 PM), and the fish were fed the equivalent of 3% of their body weight per day.
The 360 juvenile gurami sago had an average weight and length of 50±2.5 g and 13.2±0.4 cm and had not been previously in earlier studies. The weight of the sample was measured using AD-600i scales with 0.001 g accuracy (ACIS model number AD-600i, China) and its use was approved by the Indonesian Directorate of Metrology. Meanwhile, body length was measured using a meter ruler with 1 mm accuracy. The fish were distributed in 12 nets framed with size 2-m3 (2×1×1 m) PVC pipe (1200 L capacity) placed inside two freshwater concrete ponds of size 18-m3 (6×2×1.5 m). The experimental groups were P1, P2, and P3 along with one control group (P4) with three replications each (12 total). Each net contained 30 fish, and the samples were randomized to groups using a lottery method by Hafrijal Syandri and Azrita undefined. Water temperature varied between 27 °C and 30 °C (mean 28.5 °C), while the dissolved oxygen (DO) level was 5.8 to 6.2 mg L-1 (mean 5.72), with pH of 6.5-6.8 (mean 6.67). The parameters of temperature, DO level, and pH were recorded weekly throughout the experiment. Water samples were collected at 10.00 AM at a depth of 20 cm from each concrete pond for the determination of these parameters. Water temperature, oxygen, and pH were measured using a thermometer (Celcius scale), oxygen meter (YSI model 52, Yellow Spring Instrument Co, Yellow Spring, OH, USA), and pH meter (Digital Mini- pH meter, 0-14pH, IQ Scientific, Cemo- Science Thailand).
Fish were given feed of 2 mm floating type pellets supplemented with products formulation P1, P2, P3, and control (P4) three times a day at 9.00 AM, 1.00 PM, and 5.00 PM. Furthermore, the samples were hand-fed at a 3% body weight rate per day until the end of the study, which was after rearing them for 90 days from February to April 2021. Each experimental fish received well-fermented pellets until full consumption. The samples were collected every 30 days to evaluate length and weight by fasting them for 24 hours before sampling to empty the intestinal contents. A total of 9 fish per net were randomly sampled every 30 days and euthanized with tricaine methanesulfonate (MS 222, Sigma Aldrich Co, USA MO, 50 mg L-1), followed by measurement of length and weight. The amount of MS 222 used was 2,000 mg at each measurement of the sample (0, 30, 60, and 90 days).
This study used standard methods from the Association of Authorized Analytical Chemists37 to analyze the proximate composition of experimental diets and fish carcass. Furthermore, one fish (mean weight 100 g) from each experiment replicates (P1, P2, P3), and control (P4) were collected from the rearing nets and then euthanized by injecting their brains with a No. livestock iron syringe 7.5 G x 1 inch to examine the whole-body carcass. Samples of the diet and wet fish were dried at 105 °C for two hours. The crude protein was analyzed using an automatically processed Kjeldahl (Buchi 430/323) using a Kjeltec methods (6.25), automatic Kjeldahl system (Buchi/430/323) model 1625, Moline IL USA. The fat content was assessed using a Soxhlet Apparatus with the Soxhlet system 1046 (Foss, Hoganas Sweden). The ash content was analyzed using a muffle furnace (600 °C for four hours), and the total carbohydrates was calculated by subtracting the sum of % crude protein, % crude fat, % crude ash, and % moisture contents from 100%37. The proximate composition of diets and carcass was calculated by P.T. Saraswanti Indo Genetech Bogor Indonesia (SIG Laboratory, Accredited Testing Laboratory-LP-184-IDN).
Diets and carcass were analyzed with a fatty acid composition using the gas chromatography-mass spectrometry (GC-MS) method. The total lipid extraction was carried out using the method proposed by Folch et al. (1957) with few modifications, as described by Rajion, 198538 using a chloroform: methanol (2.1. v/v) solvent system. Transmethylation was performed using 14% methanolic boron trifluoride. Furthermore, diets and carcass was analyzed for fatty acid composition by PT. Saraswanti Indo Genetech Bogor Indonesia (SIG Laboratory, Accredited Testing Laboratory-LP-184-IDN).
A total of 9 fish sampled from each net were weighed and accounted for separately during the final sampling. Weight gain (WG, %), specific growth rate (SGR, %/day), FCR, and feed conversion efficiency (FCE) were assessed based on formulas9,10,39:
The nutritional quality of lipids AI and TI was calculated based on the equations40.
where
AI = Atherogenic index
TI = Trombogenic index
C12:0 = lauric acid
C14:0 = myristic acid
C16:0 = palmitic acid
C18:0 = stearic acid
ΣMUFA = sum concentrations of all MUFA
Σn-6 = sum concentrations of n-6 PUFA
Σn-3 = sum concentrations of n-3 PUFA
The data obtained in this study were analyzed using the SPSS 16.0 software package (SPSS; Chicago IL). Levine's test was used to determine the homogeneity of data, and one-way ANOVA was used for determining the treatment effect, followed by a post hoc Duncan's multiple range test41. Data were reported as mean value ± standard deviation for each treatment42. Furthermore, Microsoft Office Professional Plus 2019 was used for plotting the figures.
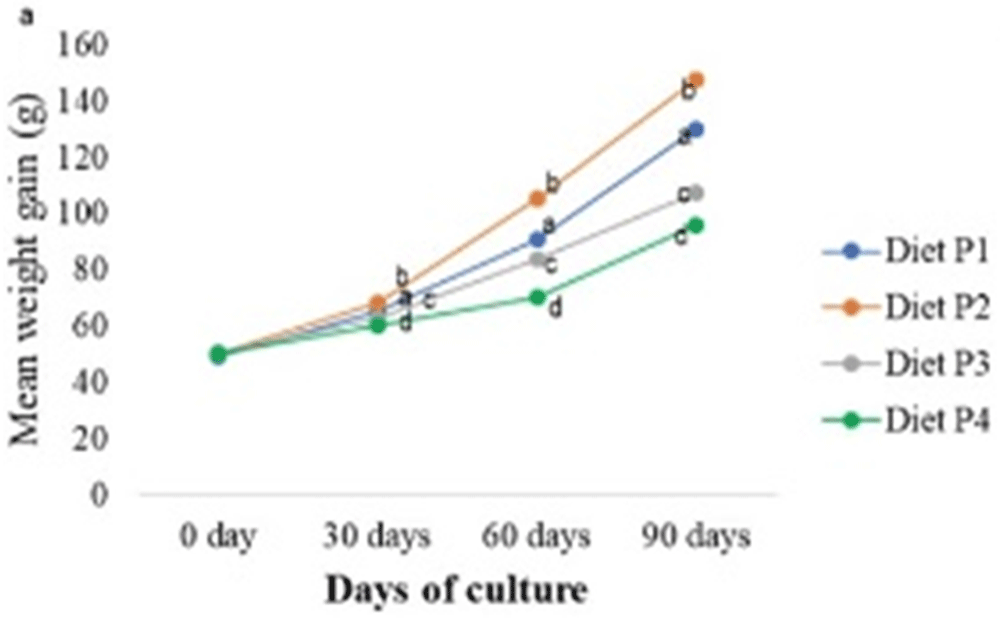
In this study, no samples were excluded from the analysis and procedures. Figure 1 showed growth performance of gurami sago at 30, 60, and 90 days for all groups during the experimental phase. Furthermore, no death was observed with any diets and the survival remained at 100% for all treatments during the 90 days of experimentation. Juvenile gurami sago fed with P2 had significantly higher growth performance with increased weight gain, lower FCR, and improved FCE, compared to others with P1, P3, and P4, as shown in Table 1. The P2 diet also led to a significant increase in fat and carbohydrates but there was no significant difference in protein, as shown in Table 2.
| Diet P1 | Diet P2 | Diet P3 | Diet P4 | P-value | |
|---|---|---|---|---|---|
| Biometric measurements | |||||
| Initial body weight (g) | 49.40±3.80 | 50.26±0.47 | 50.69±2.48 | 50.80±0.68 | |
| Final body weight (g) | 132±2.08b | 147.74±1.02a | 118.74±1.22c | 109.73±1.89d | 0.435 |
| Weight gain (%) | 167.24±13.97b | 193.99±4.46a | 134.22±12.59c | 115.98±2.88d | 0.011 |
| Specific growth rate (%/day) | 1.09±0.10b | 1.15±0.01a | 0.95±0.06c | 0.98±0.05d | 0.033 |
| FCR | 1.50±0.08b | 1.36±0.04a | 1.53±0.03c | 1.55±0.02d | 0.069 |
| FCE | 0.65±0.01b | 0.73±0.02a | 0.65±0.01a | 0.64±0.01a | 0.394 |
| Diet P1 | Diet P2 | Diet P3 | Diet P4 | P-value | |
|---|---|---|---|---|---|
| Proximate composition (g/100 g) | |||||
| Moisture | 62.49±0.36d | 62.77±0.26c | 64.23±0.12b | 65.55±0.39a | 0.265 |
| Protein | 30.29±0.45a | 28.98±0.37a | 29.30±0.16a | 28.69±0.28a | 0.363 |
| Lipid | 2.88±0.02c | 4.67±0.04a | 2.99±0.03b | 2.79±0.03d | 0.505 |
| Carbohydrates total | 1.98±0.07b | 2.04±0.07a | 1.30±0.08c | 1.29±0.06d | 0.834 |
| Crude fibre | 0.68±0.01c | 0.82±0.01b | 0.95±0.04a | 0.37±0.01d | 0.096 |
| Ash | 2.16±0.02a | 1.54±0.01d | 2.11±0.04b | 1.63±0.02c | 0.223 |
The fat content differed significantly among the four diets, with P2 showing significantly higher protein and fat contents compared to P1, P3, and P4. Carbohydrate content was similar in diets P1, P2, and P3 but significantly higher compared to P4. Furthermore, the energy content differed significantly among the four diets, as shown in Table 3.
| Proximate composition (g/100 g) | Diet P1 | Diet P2 | Diet P3 | Diet P4 | Initial composition | P-value |
|---|---|---|---|---|---|---|
| Moisture | 36.95±0.79c | 37.28±0.01b | 35.60±0.16c | 38.46±0.18a | 10.66 | 0.005 |
| Protein | 20.44±0.10a | 21.27±0.12a | 20.28±0.03a | 19.36±0.41d | 30.10 | 0.029 |
| Crude lipids | 3.48±0.04b | 3.65±0.11a | 3.49±0.04c | 3.08±0.05d | 4.09 | 0.067 |
| Carbohydrates | 29.60±0.74a | 28.78±0.30ab | 30.36±0.14ac | 26.21±0.36d | 45.35 | 0.101 |
| Crude fibre | 6.74±0.06c | 6.67±0.06b | 6.38±0.22d | 9.19±0.01a | 9.18 | 0.105 |
| Ash | 2.80±0.07a | 2.36±0.01c | 2.45±0.06b | 2.75±0.30d | 2.50 | 0.007 |
| Energy (kcal/100g) | 234.4±0.29a | 237.49±0.66b | 240.55±0.81c | 230.05±0.26d | 340.99±0.40 |
The four diets showed a high level of saturated fatty acids (SFA), with palmitic acid (C16:0) and stearic acid (C18:0) being the most abundant. The concentration of monosaturated fatty acids (MUFA) was similar in P1, P2, and P3 but very low in P4, with the highest values recorded for oleic acid (C18:1 n-9) in all diets. Myristoleic acid (C14-1 n-9) or heptadecenoic acid (C17:1 n-8) was not detected in P4 and initial feed composition. Regarding polyunsaturated fatty acids (PUFA), P2 had the highest value, with linolenic acid (C18:3 n-3) being dominant. Furthermore, EPA (C20:5 n-3) and DHA (C22:6 n-3) were found in P1, P2, and P3, while these compounds were absent in P4 (Table 4).
| Type FA | Diet P1 | Diet P2 | Diet P3 | Diet P4 | Initial composition | P-value |
|---|---|---|---|---|---|---|
| C12:0 (Lauric acid) | 0.79±0.01a | 0.65±0.01b | 0.57±0.01c | 0.35±0.01d | 0.34 | 1.000 |
| C13:0 (Tridecanoic acid) | n.d | n.d | n.d | n.d | n.d | - |
| C14:0 (Meristic acid) | 0.75±0.01a | 0.74±0.0b | 0.75±0.01ac | 0.51±0.01d | 0.13 | 0.446 |
| C15:0 (pentadecanoic acid) | 0.95±0.01a | 1.01±0.0b | 1.03±0.01c | n.d | n.d | 0.094 |
| C16:0 (Palmitic acid) | 27.4±0.15a | 27.55±0.0b | 23.45±0.01c | 19.07±d | 21.05 | 0.014 |
| C17:0 (Heptadecanoic acid) | 0.22±0.01a | 0.24±0.0b | 0.17±0.0c | 0.12±0.0d | 0.45 | 0.931 |
| C18:0 (Stearic acid) | 5.25±0.01a | 5.27±0.0b | 5.24±0.01c | 4.24±0.01d | 5.31 | 0.532 |
| C20:0 (Arachidic acid) | 0.14±0.0a | 0.53±0.01b | 0.28±0.0c | n.d | n.d | 0.067 |
| C24:0 (Lignoceric acid) | n.d | n.d | n.d | n.d | n.d | |
| ∑ SFA | 35.5±0.17a | 35.99±0.02b | 31.49±0.03c | 24.59±0.04d | 27.28 | 0.117 |
| C14-1 n-9 (Myristoleic acid) | 0.48±0.01a | 0.46±0.01b | 0.38±0.01c | n.d | n.d | 0.167 |
| C16:1 n-7 (Palmitoleic acid) | 2.08±0.01a | 2.89±0.01b | 2.83±0.01c | 1.97±0.01d | 1.99 | 0.940 |
| C17:1 n-8 (heptadecenoic acid) | 0.12±0.01a | 0.13±0.01b | 0.11±0.01c | n.d | n.d | 0.167 |
| C18:1 n-9 (Oleic acid) | 26.88±0.01a | 27.68±0.01b | 23.35±0.02c | 19.76±0.01d | 22.98 | 1.000 |
| C20:1 n=7 (Eicocyanic acid) | 0.15±0.01a | 0.14±0.01b | 0.15±0.00ac | 0.06±0.01d | 0.06 | 0.441 |
| ∑ MUFA | 29.71±0.04a | 31.33±0.04b | 26.82±0.03c | 21.79±0.02d | 25.03 | 0.849 |
| C18:2 n-6 (Linoleic acid) | 27.81±0.01a | 28.86±0.01b | 27.21±0.01c | 22.66±0.01d | 25.21 | 0.702 |
| C18:3 n-3 (Linolenic acid) | 1.07±0.01a | 1.08±0.01b | 1.07±0.01c | n.d | n.d | 0.167 |
| C20:2 n-6 (Eicosadienoic acid) | n.d | n.d | n.d | n.d | n.d | |
| C20:4 n-6 (Arachidonic acid) | 0.21±0.01a | 0.20±0.01b | 0.21±0.01ac | 0.07±0.01d | 0.09 | 0.702 |
| C20:5 n -3 (EPA) | 0.56±0.01a | 0.59±0.34b | 0.62±0.01c | n.d | n.d | 0.001 |
| C22:6 n-3 (DHA) | 2.09±0.01a | 2.52±0.01b | 2.10±0.01c | n.d | n.d | 0.330 |
| ∑ PUFA | 31.74±0.02a | 32.66±0.03b | 31.21±0.05c | 22.78±0.02d | 25.3±0.02 | 0.002 |
| ∑n.dFA | 2.80±0.01 | 0.02±0.01 | 10.48±0.03 | 30.84±0.03 | 22.39±0.01 | |
| ∑FA (%) | 100 | 100 | 100 | 100 | 100 | |
| Σ n-3 | 3.74±0.03a | 4.19±0.02b | 3.82±0.02c | n.d | n.d | 0.066 |
| Σ n-6 | 28.02±0.02a | 29.06±0.01b | 27.42±0.02c | 27.42±0.02d | 22.72±0.01 | 0.382 |
| Σn-6/ Σn-3 | 7.53±0.06a | 6.93±0.03b | 7.17±0.04c | 0.0 | 0.0 | 0.036 |
| PUFA/SFA | 0.85±0.03a | 0.85±0.01b | 1.04±0.08c | 0.92±0.02c | 0.96±0.006 | 0.004 |
| DHA/EPA | 3.66±0.05a | 4.21±0.10b | 3.34±0.02c | 0.0 | 0.0 | 0.030 |
| Lipid content (%) | 3.47±0.01a | 3.68±0.02b | 3.49±0.00c | 3.09±0.00d | 3.12±0.03 | 0.494 |
Gurami fish fed diets P1, P2, P3, and P4 showed high levels of SFA in their body carcass, with palmitic acid (C16:0) and stearic acid (C18:0) being the most abundant. The oleic acid (C18:1 n-9) in all body carcass contained higher values. For samples fed P1 diet, carcass had the highest palmitoleic acid content (C16:1) compared to others. Furthermore, PUFA had the highest value in samples fed P2. The results showed that linolenic acid (C18:2n-6) was dominant among the four carcass. EPA and AA levels were lacking in all four samples, while DHA was high, as shown in Table 5.
| Type FA | Diet P1 | Diet P2 | Diet P3 | Diet P4 | P value |
|---|---|---|---|---|---|
| C12:0 (Lauric acid) | 0.41±0.01a | 0.56±0.01b | 0.60±0.01c | 0.40±0.01d | 0.757 |
| C13:0 (Tridecanoic acid) | n.d | n.d | n.d | n.d | |
| C14:0 (Meristic acid) | 0.85±0.02a | 0.81±0.01b | 0.51±0.02c | 0.49±0.02d | 0.802 |
| C15:0 (pentadecanoic acid) | 0.57±0.01a | 0.88±0.02b | 0.65±0.02c | 0.60±0.02d | 0.802 |
| C16:0 (Palmitic acid) | 22.83±0.02a | 24.03±0.01b | 23.04±0.02c | 20.70±0.02d | 0.482 |
| C17:0 (Heptadecanoic acid) | 0.11±0.01a | 0.14±0.02b | 0.12±0.02c | 0.11±0.02d | 0.786 |
| C18:0 (Stearic acid) | 5.24±0.01a | 5.23±0.01b | 5.26±0.01c | 5.22±0.02d | 0.702 |
| C20:0 (Arachidic acid) | 0.59±0.01a | 0.76±0.01b | 0.48±0.02c | 0.56±0.02d | 0.702 |
| C24:0 (Lignoceric acid) | 0.07±0.01a | 0.09±0.01b | 0.05±0.02c | 0.03±0.01d | 0.757 |
| ∑ SFA | 30.70±0.30a | 32.51±0.01b | 30.72±0.03ac | 30.51±1.64d | 0.612 |
| C14-1 n-9 (Myristoleic acid) | n.d | n.d | n.d | n.d | |
| C16:1 n-7 (Palmitoleic acid) | 2.48±0.01a | 2.42±0.01b | 2.43±0.02c | 2.08±0.02d | 0.797 |
| C17:1 n-8 (heptadecenoic acid) | 0.22±0.01a | 0.68±0.02b | 0.75±0.02c | 0.58±0.02d | 0.797 |
| C18:1 n-9 (Oleic acid) | 26.19±0.01a | 26.46±0.01b | 24.18±0.02c | 21.98±0.02d | 0.702 |
| C20:1 n=7 (Eicocyanic acid) | 0.26±0.01a | 0.39±0.02b | 0.29±0.02c | 0.25±0.02d | 0.786 |
| ∑ MUFA | 29.15±0.0a | 29.95±0.02b | 27.65±0.0c | 24.89±0.03d | 0.098 |
| C18:2 n-6 (Linoleic acid) | 27.39±0.01a | 29.48±0.01b | 24.11±0.02c | 20.15±0.02d | 0.670 |
| C18:3 n-3 (Linolenic acid) | 1.72±0.01a | 1.79±0.01b | 3.19±0.02c | 3.03±0.02d | 0.629 |
| C20:2 n-6 (Eicosadienoic acid) | 0.36±0.0a | 0.42±0.01b | 0.28±0.01c | 0.27±0.01d | 0.532 |
| C20:4 n-6 (Arachidonic acid) | 0.31±0.01a | 0.46±0.02b | 0.38±0.01c | 0.36±0.02d | 1.000 |
| C20:5 n -3 (EPA) | 0.22±0.02a | 0.50±0.01b | 0.25±0.02c | 0.11±0.01ad | 0.983 |
| C22:6 n-3 (DHA) | 2.08±0.01a | 2.11±0.02b | 2.14±0.01c | 2.08±0.01d | 0.546 |
| ∑ PUFA | 32.08±0.02a | 34.76±0.02b | 30.35±0.01c | 26.00±0.02d | 0.467 |
| ∑ ndFA | 8.07±0.0 | 2.79±0.0 | 11.25±00 | 21.01±00 | |
| ∑ FA (%) | 100 | 100 | 100 | 100 | |
| Σ n-3 | 4.02±0.01a | 4.4±0.03b | 5.59±0.3c | 5.22±0.02d | 0.482 |
| Σ n-6 | 28.06±0.96a | 30.36±0.02b | 24.77±0.44c | 20.73±0.03d | 0.001 |
| Σn-6/ Σn-3 | 7.12±0.21a | 6.74±0.31b | 4.43±0.03d | 3.97±0.03d | 0.007 |
| PUFA/SFA | 1.03±0.01a | 1.07±0.01b | 0.99±0.01c | 0.92±0.0d | 0.067 |
| DHA/ EPA | 9.35±0.65a | 4.08±0.11b | 8.63±0.69c | 18.54±2.54d | 0.063 |
| AI | 1.78±0.16a | 1.75±0.0b | 1.87±0.01c | 1.79±0.02d | 0.009 |
| TI | 0.60±0.0a | 0.56±0.05b | 0.43±0.02c | 0.72±0.02d | 0.006 |
| Lipid content (%) | 2.90±0.02a | 4.42±0.01b | 2.98±0.01c | 2.76±0.03d | 0.786 |
a b c d - significant differences in rows
Values are % total fatty acid expressed as mean ± SD. of three separate determinations.
n.d= Unidentified fatty acids, SFA= Saturated fatty acids; MUFA= Monounsaturated fatty acids; PUFA= Polyunsaturated fatty acid; FA= Fatty acids
Initial commercial pellet feed
Commercial feed supplemented with formulated products directly affected growth performance. In this study, the highest increases in final body weight (g), body weight gain (%), and specific growth rate (%/day) occurred in P2 at 147.74±1.02 g, 193.99±4.46%, and 1.15±0.01, respectively. Furthermore, supplementing feed with formula products caused a 30% decrease in feed protein level (initial composition) to 19.36±0.41% and 21.27±0.12% of all the experimental diets. Based on this result, the moisture of feed increased from 10.66% to a range of 35.60%-37.28%. Although the various treatments did not cause a difference in body protein levels, a significant effect was observed on the body weight of livestock. In this context, the experimental phase did not significantly affect carcass protein of the body. The protein content was also not different for each diet, but there was a difference between the final growth and FCR. FCR at diet P2 was 1.36, while a value of 1.55 was observed in P4. The commercial fish feed caused a higher FCR when the samples were cultured in earthen freshwater ponds (FCR = 1.87) compared to concrete freshwater ponds (FCR = 1.45)4,8. The weight gain and growth of the animal decreased with increasing fiber content in the diet43. P4 had a high fiber content because it did not contain formulated products, leading to lower growth and FCE compared to others.
Carbohydrate levels in all experimental diets were lower than the initial composition but had no effect on growth of gurami sago. This species belonged to the group of herbivorous fish10. Several studies had shown that herbivorous and omnivorous species could increase amylase activity higher than carnivores44. However, the poor overall growth was caused by low carbohydrate digestibility45. Previous reports had reported that fish growth and feed efficiency could increase by providing feed supplemented with varying levels of carbohydrates46–48, regardless of the content of protein, fat, crude fiber, and carbohydrates in the experimental diet. This study recommended using products formulated from natural sources of coconut water, palm sugar, and fungus in commercial feed, thereby increasing production value of the net yield and bringing more significant financial benefits.
The aquaculture industry must use rich nutritional feed with high protein, fatty acids, minerals, and vitamin content16,17,49,50. Therefore, increasing feeding nutrition and maximizing digestive enzyme activity in farmed fish could be done by providing raw fermented feed ingredients15,51–53. In this context, the new method used to improve feed quality, feed efficiency, and growth rate in gurami sago was to supplement the meals with formulated P1, P2, and P3 products. Coconut water contains growth factors that stimulate various types of bacteria with the aim of increasing nutrition54, because coconut water contains high amounts of amino acids and nutrients, especially potassium (in the range of 237.41 to 361.20 mg/100 mL), manganese (in the range of 20 to 197 μg/100 mL), and iron (0.51 mg/100 mL)55. On the other hand, palm sugar is also claimed to have health benefits because it has a low glycemic index and contains antioxidants, vitamins and minerals (32–34). This strategy had been successful in increasing the nutrient quality of commercial feed, where the sample supplemented with P2 had the highest total fat content with 35.99% SFA, 31.33% MUFA, and 32.66% PUFA. The results showed that P4 had the lowest total fat content, with 24.59% SFA, 21.79% MUFA, and 22.78% PUFA.
This study showed that the commercial fish feed equipped with P1, P2, and P3 contained more complete fatty acids at 3.47%, 3.68%, 3.49%, and 3.09%, respectively, compared to P4. Based on the results, P4 did not contain linolenic acid (C18:3 n-3), EPA (C20:5n-3), and DHA (C22:6n-3). This showed that commercial feed must be supplied with linolenic acid, EPA, and DHA due to their essential role in meeting physiological needs, production performance, and health22,56–58. In this experiment, EPA + DHA levels were 2.65%, 3.11%, and 2.72% in P1, P2, and P3, while this parameter was not recorded in P4 (control). For Atlantic salmon (Salmo salar L.) juvenile, the EPA and DHA levels in their diet were recommended to range from 0.50 to 1.0%59,60. Despite the absence of EPA and DHA values for P4, freshwater fish, including gurami sago, were estimated to be able to synthesize unsaturated fatty acids (HUFAs), such as C20 and C22, from C18 PUFA in feed through chain elongation and desaturation reactions. Adequate amounts of C18:3-n3 and C18:2n-6 were expected to meet their EFA requirements. The amount of DHA and EPA varied significantly between the P1, P2, and P3 diets, while DHA/EPA ratios increased due to supplementation with formulation products. The differences in the DHA/EPA ratio in each experimental diet were due to differences in the use of fermenters, namely Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae. According to previous studies, the calculated ratio of DHA/EPA in the diet must be precise to develop better feed formulations61. For example, the ratios in the diet for maximum growth of Golden pompano, Trochinotus ovatus juvenile was 1.4617, 1.30 for gilthead seabream, Sparus aurata62, 0.53 for Atlantic salmon, Salmo salar in freshwater60, and 1.02 for Nile tilapia, Oreochromis niloticus63.
This study estimated that a DHA/EPA ratio of 1.70 in the P2 diet could optimally increase growth rate and feed efficiency. However, these parameters depended on physiological, environmental, and farming factors64. The Aspergillus niger, Rhizopus oligosporus, and Saccharomyces cerevisiae in this experiment had also been used in previous studies to ferment feed raw materials, such as corn-cob, soybean meal, and sunflower cake65,66. Furthermore, Rhizopus oligosporus was the main microorganism used in the fermentation process because it produced a wide range of enzymes, such as carbohydrase, proteases, lipases, and phosphatase67–69. Coconut water also played an essential role in enriching the nutritional quality because it contained minerals, amino acids, enzymes, organic acids, fatty acids, vitamins, and a few phenolic compounds26–28. Palm sap sugar had also been shown to have an essential role in increased feed quality due to its minerals, vitamins, and antioxidant content33,34.
Several freshwater fish species had low values of PUFA and higher levels of MUFA and PUFA19,69,70. However, gurami sago had a high level of PUFA and a lower presence of MUFA. The results showed that some of the fatty acids in the animal body were affected by diet. Palmitic acid (C16:0) in SFA group was the most abundant in all treatments but was lower in P4. The highest body carcass levels were obtained in P2 and P3 at 24.03% and 23.04%, respectively. The C:16 and C18:2 n-6 fatty acids accumulated in giant gurami fed high-fat content diets, as showed for common carp (Cyprinus carpio)71 and Atlantic salmon (Salmo solar)72. In experiments carried out with Oreochromis niloticus fed diets, rich in lipids, linolenic and oleic acids accumulated in significant concentrations19,71,73. Other fish species, such as Silver barb (Puntius gonionotous)74 and Asian red-tailed catfish (Hemibagrus wyckioides)75 could synthesize 20C PUFA of n-3 and n-6 series from 18C PUFA by desaturation and elongation. This capacity underscored the essential role of 20C PUFA in this group, serving as potential precursors of prostanoids. The potent ability had also been observed in other freshwater fish, such as the zebrafish (Danio rerio)76.
In this present study, eicosatrienoic acid (C20:2 n-6) was not detected in all experiment diets. However, the fatty acid was present in the body carcass of giant gurami in all treatments ranging from 0.27% to 0.42% of total fatty acids composition. This data showed that it had an essential role in the body of the animal. Gurami sago fish synthesized the compound from other precursors ranging from 0.095% and 0.097% of total body lipids composition. AA, EPA, and DHA fatty acids, which were restricted in the diets, remained in the experimental fish in different proportions. This difference was due to the variation of added products formulation to each diet.
The results showed that the giant gurami sago strain was capable of preserving its normal PUFA levels (C18:2 n-6, C18:3 n-3, C20:2 n-6, C20:4 n-6, C20:5 n -3 and C22:6 n-3), but these fatty acids were at very low or undetectable levels in the diets, such as eicosadienoic acid type. This showed that gurami sago, similar to other freshwater fish species, such as Oreochromis niloticus63 and Cyprinus carpio21, could elongate and desaturate fatty acids from precursors present in the diet, including 18C:0 fatty acids and possibly from linoleic fatty acid in large quantities.
The atherogenic index (AI) ranged from 1.68 and 2.19, and the thrombogenic index (TI) was between 0.43 and 0.72 in all diets. This result was related to a significant discrepancy in SFA values between experimental diets. Several studies had shown that AI and TI indexes were directly related to the levels of C14:0, C16:0, and C18:0, known as thrombogenic promoters19. AI and TI levels in gurami sago fed P1, P2, and P3 diets were lower compared to P4 (control). Several studies had shown that AI and TI indices showed the potential to stimulate platelet aggregation. The lower the value of these indices, the greater the protective potential of coronary artery disease77. The recommended AI and TI values by the Food and Agriculture Organization and the World Health Organization78 for human health ranged from 0.4 to 0.5. Although the AI and TI values of gurami sago were higher than 0.5, its consumption had no negative effect on consumers' health. In aquaculture, AI and TI indices, among others, depended on the levels of fish oil supplement in the diet19, the sources of oils9,57, effect of fish farming activities, and handling methods after harvest79. Several reports had also showed that feed quality, age, gender, species, and environmental conditions had significant effect69,70,80,81.
In conclusion, this study showed that feed products made from natural and sustainable sources of coconut water, palm sap sugar, and fungus combined with commercial feed could enhance nutrient-rich diets in gurami sago. The main factor that improved after the treatment was the fatty acid composition of the PUFA group (i.e., linolenic acid, DHA, and EPA). Furthermore, the increase was related to growth rate, FCR, and FCE. The P2 formulation was optimal for feed quality, such as proximate and fatty acid composition. The results showed that P2 increased fish growth, feed efficiency, and carcass fatty acids. Based on the results, it also contributed to improving the nutritional quality of giant gourami lipids, which was beneficial to consumers' health. These results provided basic knowledge about efforts to improve the quality of nutrient-rich feed and the basis for efficient use of feed in future fish farming operations.
figshare: The use of new products formulated from water coconut, palm sap sugar, and fungus to increase nutritional feed quality, feed efficiency, growth, and carcass of gurami sago (Osphronemus goramy Lacepède, 1801) juvenile. https://doi.org/10.6084/m9.figshare.1664107382
This project contains the following underlying data:
- Table 1a. Raw data growth for 0, 30, 60, 90 days.
- Table 1b. Raw data growth performance, FCR, FCE_90 days_giant gurami
- Table 2. Raw data proximate composition carcass of gurami sago after 90-days
- Table 3. Raw data proximate composition of diets
- Table 4. Raw data Composition of fatty acids and total lipid in the diets enriched
- Table 5. Raw data Composition of fatty acids of carcass
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Fish Nutrition
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Expertise : Marine Biotechnology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: aquaculture
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Food Science.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 29 Dec 23 |
read | read | ||
|
Version 1 08 Nov 21 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)