Keywords
epidemiology, SARS-CoV-2, COVID-19, contact tracing, bidirectional tracing, backward tracing, B.1.1.7, test-trace-isolate
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
This article is included in the Max Planck Society collection.
epidemiology, SARS-CoV-2, COVID-19, contact tracing, bidirectional tracing, backward tracing, B.1.1.7, test-trace-isolate
The frequency of the B.1.1.7 variant of SARS-CoV-2 has grown rapidly from its initial detection in October 2020 to become the dominant strain in southeastern England by the start of 2021. Studies have estimated the new strain is between 40% and 80% more contagious1,2. The rapid exponential growth of B.1.1.7, now found in dozens of countries, risks another and potentially higher wave of COVID-19 cases prior to widespread vaccination. Meanwhile, early reports suggest that current vaccines3 and prior SARS-CoV-2 exposure4 may be less protective against the B.1.351 and P.1 variants now common in South Africa and Brazil.
All three variants share an otherwise rare del11288–11296 mutation in orf1ab that can be detected using a single RT-PCR reaction5; B.1.1.7 can also be distinguished with the TaqPath diagnostic test6, twenty million of which are manufactured weekly7. As such, existing COVID-19 testing infrastructure can be used to track the transmission of the new variants. Samples testing positive by other kits can be re-screened8 without an emergency use authorization.
Test-trace-isolate (TTI) strategies have been widely used to mitigate the spread of SARS-CoV-29. Models by the present authors10 and others11 have found that incorporating backwards tracing to identify infector individuals could dramatically increase the efficacy of tracing programs. However, testing delays, mistrust, and low compliance have undermined the confidence of health authorities in the benefits of TTI12,13. Moreover, efficacy sharply decreases when caseloads are high14, as is true for SARS-CoV-2 – but not yet the variants – in many regions.
Given the current low prevalence of the variants in most jurisdictions and the ability to identify cases of the new variant using existing testing infrastructure, we hypothesized that TTI programs dedicated to controlling them could substantially reduce the harm inflicted prior to widespread vaccination of populations later in 2021, especially if vaccine reformulation is needed. Such programs could be enhanced through incorporation of bidirectional tracing10.
However, the effectiveness of TTI strategies varies widely from region to region due to programmatic and population-level differences in variables such as the proportion of cases who share their contact history with contact tracers; the proportion who comply with quarantine and isolation requests; and the overall rate of tracing success. Given this variation, it is unclear whether tracing programs exhibiting realistic levels of performance could feasibly dampen the spread of the new variants.
To evaluate the potential benefits of applying targeted test-trace-isolate to control variants, we applied a branching-process model of COVID-19 contact tracing10 to estimate the change in the effective reproduction number achievable across a wide range of parameters.
In our branching-process model10, each case generates a number of new cases drawn from a negative binomial distribution according to pre-specified incubation- and generation-time distributions (Table 1). Cases are identified and isolated based on symptoms alone or through contact tracing. Cases either comply with isolation requests or ignore them completely according to some fixed probability of compliance; cases that comply generate no further cases.
| Parameter | Value | Sources and Notes |
|---|---|---|
| % asymptomatic carriers | 40% | 15–19 |
| Relative infectiousness of asymptomatic carriers | 45% | Informed by viral loads and tracing results described in 15,19–23 |
| % environmental transmission | 5% | 24,25 |
| Proportion pre-symptomatic transmission | 38% | Informed by 19,20,22,23,26–31 |
| Generation time skew parameter (α) | 0.397 | Corresponds to pre-symptomatic transmission rate specified above. |
| % of symptomatic cases identified without tracing | 50% | 32 |
| % of cases who comply with isolation | 50%, 70%, 90% | Assumed |
| Test sensitivity | 70% | 33,34 |
| Rbase (before test/trace/isolate) | 1.0 to 2.0 | Assumes a pre-B.1.1.7 R of ~1.01,2. |
| Overdispersion | 0.11 | 35 |
| Number of initial cases | 20 | Assumed |
| Incubation period | 6.0 ± 2.1 days (lognormal distribution) | 1,36,37 |
| Delay from onset to isolation | 3.8 ± 2.4 days (Weibull distribution) | 38 |
| Delay for testing | 1 ± 0.3 days (gamma distribution) | Assumed |
| Delay for manual tracing | 1.5 ± 4.8 days (lognormal distribution); median 0.5 days | Previous reports suggest most contacts can be traced within one day, but some take longer39 |
Successful tracing depends on the identified case sharing their contact history with tracers, and on the contact in question taking place within the time window (measured in days pre-symptom onset for symptomatic cases, and days pre-identification for asymptomatic cases). Environmental transmission is assumed untraceable. Symptomatic cases require a positive test before initiating contact tracing.
Outbreaks were initialized with 20 index cases to minimize stochastic extinction and designated as “controlled” if reaching extinction before reaching 10,000 cumulative cases. Effective reproduction numbers (Reff) were computed as the mean number of child cases produced per case10.
To investigate the potential for TTI to mitigate the spread of variants, we investigated the effective reproduction number achieved across a range of data-sharing and trace-success rates (Figure 1). To account for uncertainty in variant transmissibility, we explored outcomes for reproduction numbers between 1.2 and 2.0; these values assume that non-tracing interventions are already in place.
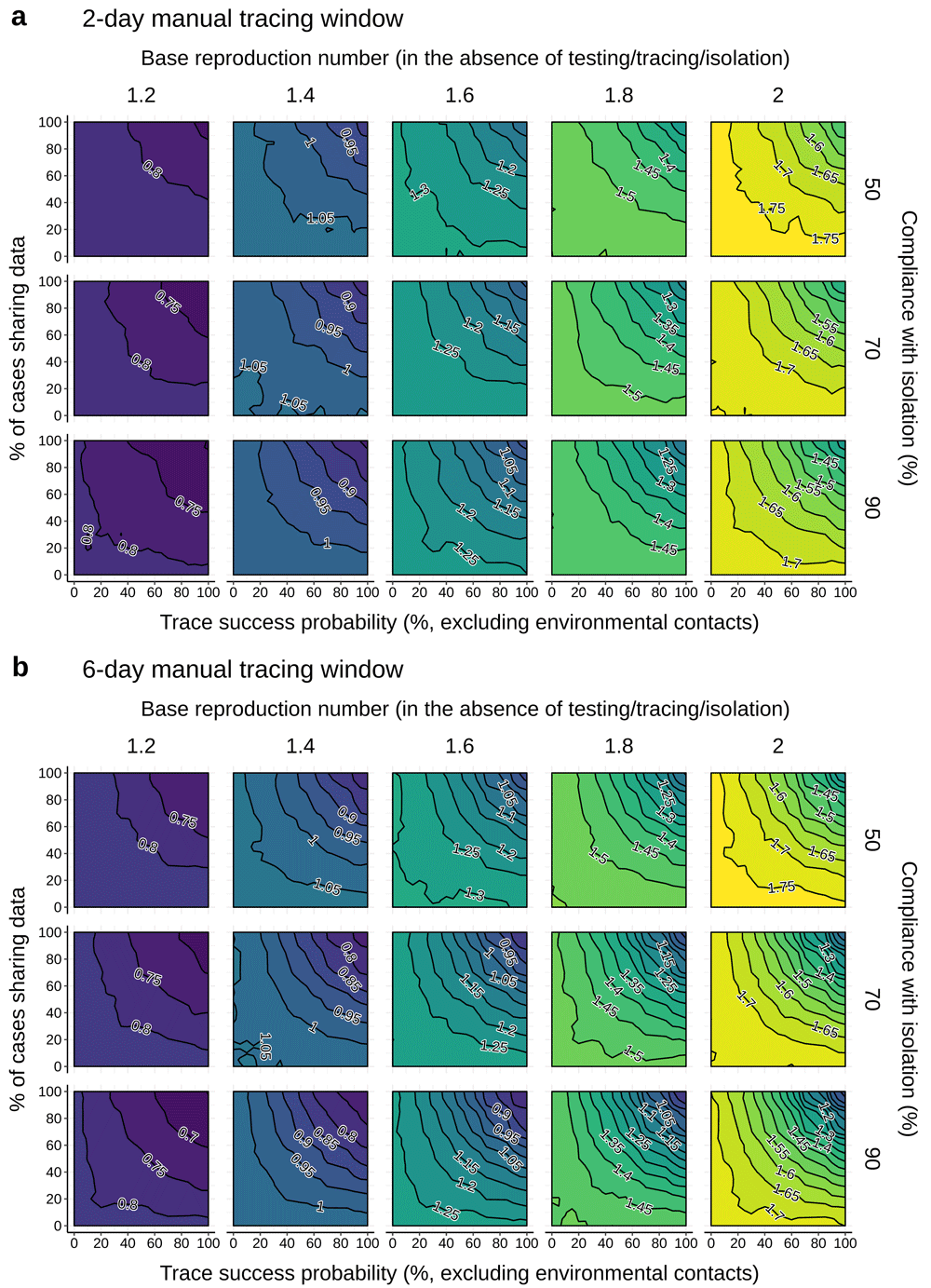
Neighbor-averaged contour plots, showing Reff achieved by bidirectional manual contact tracing with a tracing window of (a) two or (b) six days pre-symptom onset, under different combinations of trace success probability (x-axis), rate of data sharing with manual contact tracers (y-axis), rate of compliance with isolation and quarantine (row) and base reproduction number (columns). Other disease parameters are specified in Table 1. Isolation of symptomatic cases is sufficient to reduce R even when no traces succeed and/or no cases share their data with contact tracers. “Trace success probability” refers to trace attempts that are not otherwise blocked by environmental transmission or refusal to share data.
In the absence of contact tracing, identification and isolation of symptomatic cases alone reduced Reff by 0.2 to 0.3 even when quarantine and isolation compliance was low (Figure 1, top rows). When identification and isolation left Reff substantially greater than 1 (when base R ≥ 1.4), moderate levels of tracing could have substantial effects.
When contacts were traced up to two days prior to symptom onset, roughly 60–70% data sharing and trace success rates were required to achieve an Reff reduction of at least 0.1, relative to isolation alone. If the window was extended to six days pre-onset to enable more effective bidirectional tracing, roughly 45–55% data sharing and trace success was sufficient. Higher levels of data sharing and trace success could achieve substantially larger reductions: in many scenarios, 85% data sharing and trace success reduced Reff by >0.2 in the two-day case and >0.35 in the six-day case.
Due to the exponential growth of uncontrolled epidemics, small reductions in Reff can have a large impact on the total number of downstream cases arising from a given index case over a given timespan. For example, under a simple geometric series approach, reducing Reff by 0.1 from a starting value between 1.2 and 2.0 reduces the total number of child cases after 10 generations by 37–43%; an Reff reduction of 0.2 results in a reduction in child cases of 61–66%. Given an average generation time of six days, 10 generations equates to roughly two months – enough time, given sufficient delay in the spread of the new variant, to vaccinate a substantial fraction of the population.
Our results suggest that regions with even moderately functional contact tracing programs focused on the new variants could substantially slow their spread. Given a two-day window for bidirectionally tracing contacts pre-symptom onset, our model predicts that a program with 70% trace success, 70% data sharing, and 70% compliance with isolation could achieve an Reff reduction of at least 0.1 relative to the no-tracing case. Given a six-day window for efficient bidirectional tracing, regions with just 50% data-sharing, trace success, and isolation compliance could achieve a reduction of 0.1.
Under simple assumptions, such a reduction would reduce the number of child cases produced in two months by roughly 40%, buying time for vaccination to immunize many more people. More effective tracing programs can achieve larger reductions. Higher rates of cooperation might be achieved through home visits by contact tracers; exoneration for anything discovered in the course of contact tracing13; and financial and other support of people in quarantine and isolation40. In principle, concentrating vaccination in communities experiencing out-of-control variant transmission could further impair viral spread and increase the sustainability of TTI for COVID-19 control.
These results assume a high availability of suitable diagnostic tests and a rapid and consistent testing turnaround. They also take no account of any medical, demographic, geospatial or behavioral variation between cases that could influence the spread of the new variants.
Our results suggest that TTI programs could help slow the spread of more transmissible and vaccine-resistant variants in regions where they are currently rare, providing vital time for widespread vaccination. As TTI efficacy is limited at high caseloads14, these findings indicate that tracing programs should immediately prioritize controlling the new variants rather than less transmissible – but currently more widespread – ancestral strains.
All data underlying the results are available as part of the article and no additional source data are required.
Source code available from: https://github.com/willbradshaw/covid-bidirectional-tracing.
Archived source code as at time of publication: http://doi.org/10.5281/zenodo.427955741
License: MIT License
K.M.E. conceived the study. J.H.H. and A.L.L. identified a suitable model framework. W.J.B. designed and programmed the adapted model, advised by the other authors. W.J.B. ran all simulations and generated figures. All authors jointly wrote and edited the manuscript.
We thank Aaron Bucher of the COVID-19 HPC Consortium and Amazon Web Services for granting us extra cloud compute credits.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Smith L, Potts H, Amlȏt R, Fear N, et al.: Adherence to the test, trace and isolate system: results from a time series of 21 nationally representative surveys in the UK (the COVID-19 Rapid Survey of Adherence to Interventions and Responses [CORSAIR] study). medRxiv. 2020. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology. Previous research on contact tracing for SARS-CoV-2.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Adam DC, Wu P, Wong JY, Lau EHY, et al.: Clustering and superspreading potential of SARS-CoV-2 infections in Hong Kong.Nat Med. 26 (11): 1714-1719 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious disease modelling
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Apr 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)