Keywords
non-contact electric fields, ECCT, mammary tumors, CD8+ T cells, CD4/CD8 ratio
This article is included in the Cell & Molecular Biology gateway.
non-contact electric fields, ECCT, mammary tumors, CD8+ T cells, CD4/CD8 ratio
Breast cancer is the most common cancer among women in the world, in which 1 in 8 women in the world is at risk of developing breast cancer1. Furthermore, about 2.1 million new cancer cases in 2018 are breast cancer cases and this type of cancer is the leading cause of death in women with cancer2. Commonly, the treatment of breast cancer is carried out in two ways. The first, termed local therapy, controls or removes the tumor in specific areas, such as through breast-conserving therapy and mastectomy that may be followed by radiotherapy. The second, called systemic therapy, uses the circulatory system in the body to inhibit cancer cells growth that spread in parts of the body. This type of treatment includes chemotherapy, targeted therapy, and endocrine therapy3. However, breast cancer therapy applied so far often causes negative effects, such as the presence of pathophysiological mechanisms that cause chronic pain4, or the mechanism of multidrug resistance (MDR) in breast cancer cells as a reaction to chemotherapy5,6. Moreover, breast cancer therapies may attack certain normal cells or tissues that result in toxicity symptoms7. A novel cancer therapy based on electric field exposure with intermediate frequency and low intensity called Tumor Treating Fields (TTFields) has been developed to prevent such negative effects of cancer therapy and still inhibit cancer growth. No toxic side effects or adverse events were found on animal tumor models nor patients under the exposure to this electric field-based cancer therapy, except for skin toxicity or dermatitis of low severity8,9.
Since skin toxicity or dermatitis arises in electric field therapy due to prolonged direct contact between the electrodes and the skin10, we have developed a type of non-contact electric field therapy using capacitive modality to avoid such injuries. In our previous study in preclinical setting, cancer therapy namely Electro-Capacitive Cancer Therapy (ECCT) using the non-contact electric fields with intermediate frequency (100 kHz) and low intensity (18 peak-to-peak Voltage/Vpp) could reduce the size of breast tumors in mice significantly without causing histological damage to mammary and skin tissues. In addition, lymphocyte and macrophage infiltration was found around the tumor area through the blood vessels11. One of the mechanisms of tumor cells death can be carried out with the act of anti-tumor mechanism of various immune cells, including helper and cytotoxic T cells of lymphocytes, as well as macrophages12. Cytotoxic CD8+ T cells which is activated by helper CD4+ T cells, may enter the tumor microenvironment and induce apoptosis, resulting in shrinkage of the tumor lesions12,13. Macrophages with the M1 phenotype play an important role in the recognition and destruction of cancer cells and their presence usually indicates a favorable prognosis. Whereas macrophages with the M2 phenotype are involved in anti-inflammation, helping the angiogenesis process and the expression of scavenger receptors, and have a significant role in tumor development and metastasis12,13, including breast tumor14,15. One of the marker proteins to observe the infiltration of macrophages in a tissue, including mammary tumor tissue, is the CD68 protein14–16.
Cancer therapy that can re-induce immune response to destroy and clean up tumor cells is a therapy that is being developed because of its positive effects. By activating immune cells, the immune cells may reach the tumor-specific areas, including metastatic area, and specifically attacking only tumor cells, not the normal cells in the vicinity17. Petri et al.18 reported that static electric fields increase the immune response in mice. Moreover, electric fields modulate the activation and polarisation of some immune cells, including T cells19 and macrophages20. Voloshin et al.21 reported that TTFields exposure combined with anti-PD-1 therapy induced immunogenic cell death in lung and colon tumors. In addition, Pratiwi et al.22 reported the increased expression of CD68 and caspase-3 in rat breast tumor tissues under the exposure to 150 kHz non-contact electric fields while reducing the expression of PCNA and ErbB2 proteins. However, the study by Pratiwi et al.22 showed an increase of the tumor size during therapy. Using different intermediate frequency (100 kHz), we examined the effect of non-contact electric fields exposure on tumor growth in mammary tumors-induced rats and observed the activation of immune cells in fighting the tumor cells. We hypothesized that the increase in the size of mammary tumors would not happen under exposure to this non-contact electric fields with the induction of immune cells, especially lymphocytes and macrophages.
This study was conducted at the Integrated Research and Testing Laboratory (LPPT) of Universitas Gadjah Mada (UGM) and at the Animal Structure and Development Laboratory of Faculty of Biology, UGM. LPPT UGM has been awarded ISO/IEC 17025:2000 accreditation for the competence of testing and calibration22. Experimental protocol in this study was performed following approval by the Ethical Clearance Committee of LPPT UGM with ethical clearance number: 00015/4/LPPT/IV/2017. The Ethical Clearance Committee stated that this study has been declared to meet the ethical requirements for research on experimental animals and the Ethical Clearance Committee has the right to conduct monitoring during the research.
A total of 40 5-week-old healthy female Sprague Dawley (SD) rats (Rattus norvegicus, Berkenhout 1769) weighing 50−80 gram were used for this study. SD rats have been used as animal tumor model to study human breast cancer with various induction agents, as well as with DMBA administration23,24, since rats have 98% genetic homology with humans25. The rats were supplied by LPPT UGM laboratory, Yogyakarta, Indonesia, and never used for any other experiment. Rats showing any symptoms of illness or abnormalities were excluded from the experiment. The rats were acclimated for 1 week in polypropylene cages as communal home cages with each size: 50 × 40 cm2 that covered with rice hulls bedding at the bottom. One communal cage consisted of 5 rats. The temperature of the animal room was maintained at between 23–26°C with 81.09% average relative humidity to avoid dehydration during electric fields exposure, lighting condition was light from lamps during the day and total darkness during the night (12L:12D photoperiod). The rats were fed with AD2 pellet animal diet and tap water ad libitum. However, during treatment, cucumber slices were given as a substitute for water, to avoid the animals being shocked with electricity, since the tip of the drink bottle contains metal which can permit electric fields. Cage cleaning was carried out every day by cleaning the animal waste from the cage and changing food and water. Individual marking was conducted by giving picric acid to rat fur, while group marking was conducted by labelling the cage with a paint marker to avoid potential confounders.
The SD rats were divided into four groups, namely control (non-induction and non-therapy/NINT), placebo (non-induction and therapy/NIT), DMBA-induced mammary tumors without therapy (induction and non-therapy/INT), and DMBA-induced mammary tumors with therapy (induction and therapy/IT) groups. The sample size in each group was calculated according to the Federer formula, where 6 biological replicates were used for each group22. The animals were selected randomly and assigned to control and treatment groups. Rats were picked randomly for mammary tumors induction with a single dose of DMBA (20 mg/kg body weight) administration two times per week for 5 weeks. The first 6 rats to develop tumors were assigned to IT group, and then 6 more rats were assigned to INT group. Not all rats grew tumors after DMBA administration. All healthy, normal rats in the placebo (NIT) group were given corn oil as DMBA (7,12-dimethylbenz[a]anthracene) solvent. DMBA compound is a neuroendocrine disruptor that commonly used for carcinogenesis in specific organs, including breast tumors in rats26,27. All administrations were performed by the same researcher (AGF). Each researcher in the team had different task. Besides the researcher who administered DMBA, other researchers measured the size of the nodules, recorded the morphology of mammary tumors, dissected the euthanized rats and analysed the data statistically. One researcher (FA) controlled and monitored all the different steps in the experiment.
Following the development of mammary tumors up to 1.5 cm in length, all rats in the IT group were exposed to intermediate frequency (100 kHz) and low intensity (18 Vpp) non-contact electric fields generated between pairs of capacitive electrodes embedded in individual cages that had been modified to an ECCT device (Figure 1a). The device is called non-contact because its electrodes do not directly attach to the animal skin (Figure 1b). All individual cages were placed on the same shelf at the same height. Non-contact electric fields therapy was performed 10 hours per day for 21 days with 2 hours rest after first 5 hours therapy with the same starting time of therapy each day. The therapy was stopped when the mammary tumors has increased to 2.25 cm2 in size or it has reached the last day of therapy. All rats in placebo (NIT) group were also exposed to non-contact electric fields in the same period, 3 weeks after the last corn oil administration. The duration of electric fields exposure for 10 hours per day was shorter than the previous exposure in our preliminary study11 to minimize any possible side effects due to exposure to electric fields8,10. After treatment, all animals were returned to their communal cages.

(a) ECCT with oscillators connected to electricity, (b) A rat inside an individual cage of ECCT. The dimension of the cage was 23 cm × 18 cm × 19 cm. The arrangement of the electrodes has been described in our previous study11.
During therapy, all tumors were palpated once every 2 days and their size (cm2) was measured with a digital caliper and tabulated. All nodule measurements were conducted by the same researcher (NF). In this study, we did not measure nodule size in volume due to tool limitations. The tumor size data were used to calculate the tumor surface area (TSA) using the formula for circle and ellipse areas, and subsequently the TSA data were used to calculate the tumor growth rates (TGR) of mammary tumors. The TGR was calculated by using specific growth rates (SGR) formula28 with modification as follows: SGR (%/day) =ln (A2/A1)/(t2 – t1), where A1 and A2 are the tumor surface area before (t1) and after therapy (t2), respectively. Besides tumor size, the morphology of mammary tumors was also recorded by the same researcher (JIMP).
After therapy was completed, all rats were euthanized by an overdose of ketamine (150 mg/kg of body weight) for deep anesthesia using intramuscular injection. The rats were pinned ventral side up on a dissected box and dissected with the same surgeon (AGF). Nodules and normal breast tissues were taken and washed using physiological saline. All tissues were fixed using neutral buffered formalin (NBF). Three nodules from three different rats were collected from each INT and IT groups, while one normal breast tissue was collected from each NINT and NIT groups. These six nodules were used for histological examination with immunohistochemical staining (IHC) of the target antigens. While the normal breast tissues were used for comparison of the overall histological structure without IHC.
Histological examination was performed by using paraffin-embedded Hematoxylin and Eosin (H&E)-stained histology slides followed by IHC29 using Starr Trek Universal HRP Detection Kit Biocare. Observations and counting were performed on the mitotic figures on H&E preparations. Six antibodies purchased from Abcam Cambridge, United Kingdom, were used for IHC, namely anti-PCNA polyclonal antibody (Cat# ab18197, RRID:AB_444313), anti-ErbB2 monoclonal antibody (Cat# ab16901), anti-caspase-3 polyclonal antibody (Cat# ab13847), anti-CD68 monoclonal antibody (Cat# ab201340), anti-CD4 polyclonal antibody (Cat# ab203034), and anti-CD8 alpha monoclonal antibody (Cat# ab33786, RRID:AB_726709). The dilution for anti-CD4 and anti-CD8 antibodies was 1:750, where 1 µl of antibody was diluted with 750 µl of BSA-PBST solution (Bovine Serum Albumin 1% - Phosphate Buffered Saline with Tween® 20). While the dilution for anti-PCNA, anti-ErbB2, anti-caspase-3 and anti-CD68 antibodies was 1:500. As much as 100 µl diluted primary antibody was applied on the appropriate slide covered with a parafilm and placed in a humidity chamber. Then, the slide was removed to a refrigerator (4°C) overnight. One to two drops (50 to 100 µl) of biotinylated secondary antibody Trekkie Universal (Biocare Medical, STUHRP700 H L10) were applied on the slides covered with a parafilm and incubated for 30 minutes at room temperature. The concentration of antibodies given was followed the manufacturer’s instructions.
All measured data were analysed with appropriate methods without any exclusion. Semi-quantitative IHC scoring was performed using the percentage of positive area of stained cells (CD4 and CD8-positive T cells; caspase-3, ErbB2, PCNA-positive tumor cells) and the number of stained cells (CD68-positive macrophages). IHC scoring was performed in ImageJ ver.1.51, a freely available software22. The comparison between positive area of stained cells and total area showed the distributions of targeted antigens and cells30. Scoring was carried out in 50 fields per group (INT and IT groups), in which 17–18 random fields were examined in one slide. In addition, CD68+ macrophages counting was performed by using 60 fields per group with 20 random fields examined in a slide. In total, three slides from three different nodules were examined from each group.
All data were tested for normality (Shapiro-Wilk test, α = 0.05). The data of mammary tumors growth rate from 12 animals (6 rats each from IT and INT groups) and the number of mitotic figures were analysed using T-test (α = 0.05) to find statistical significance. The data of PCNA, ErbB2, caspase-3, CD68, CD4, and CD8-positive cells and CD4/CD8 ratio were analysed quantitatively using non-parametric Mann-Whitney test, since the data were not normally distributed. All data were analysed statistically using GraphPad Prism ver.8.4.3 software22 for Windows by the same researcher (NF). Qualitative data (morphology of mammary tumors) were analysed descriptively.
The outcome of this study was the growth rate of mammary tumors under exposure to non-contact electric fields. Comparison of histological characteristics between breast tissue and breast cancer in the animal tumor model, the distribution of molecular markers that described the proliferation and the mechanism of cell death, and the response of immune cells under exposure, as will be explained coherently below.
The growth rate of rat mammary tumors in IT group (-0,043 ± 0,065) was significantly lower (p<0.05) than that in INT group (0,108 ± 0,078) and showing negative tumor growth rate as shown in Figure 2a. There was no tumor growth in the placebo (NIT) group. Mammary tumors morphology in INT and IT groups showed differences, in which mammary tumors in the INT group were generally more compact as previously reported by Pratiwi et al.22 and appear bruised (Figure 2b) due to thrombocytopenia from the malignancy31, whereas mammary tumors in the IT group were generally softer because they contained fluid22 and some were blackened (Figure 2c). The blackened mammary tumors in the IT group were released from the surrounding tissue, as shown in Figure 3a. These tumors initially darkened, then hardened like a scab as occurs in a dry wound, and slowly sloughed off from the surrounding tissue, causing an open wound which then dried up (Figure 3b) and shrank upon exposure to non-contact electric field (Figure 3c).

(a) SGR of mammary tumors in INT and IT groups, (b) morphology of a mammary tumor in INT group with the appearance of bruises, (c) morphology of a mammary tumor in IT group with a blackened condition. Different letters (a, b) on top of the charts indicate significant differences (p<0.05).
The characteristics of normal breast tissue and the pathogenesis of actively dividing cells in DMBA-induced breast cancer in rats can be seen through H&E staining (Figure 4). Figures 4a, b show microscopical sections of breast tissue from rats of NINT and NIT groups, respectively, having normal histological features with the presence of adipose tissue and other connective tissues, and in the absence of actively proliferating cells. In addition, a myoepithelial cell layer was clearly visible in the glandular epithelium32. Conversely, Figure 4c, d show the level of malignancy of breast cancer cells from rats of INT and IT groups, respectively. They show histological feature of papillary carcinoma with the characteristics of malignant epithelial proliferation protruding into the lumen of the glandular duct that made it constricted. However, breast cancer in rats of the IT group tend to be less malignant than that in the INT group, indicated by the clear glandular duct and glandular lobule lumens, and the prominent epithelial structure as well (Figure 4d). In addition, the lumens in IT group still maintained an intact layer of cells (Figure 4d), while most of the lumens in INT group did not (Figure 4c), due to the pressure exerted by the breast cancer cells. Moreover, the region of adipose tissue and other connective tissues were also pressed by the mammary tumor cells (Figure 4d). On the other hand, the tumor tissue itself undergoing necrosis (Figure 4c, d) which was characterized by inflammation33,34 and the necrotic areas with inflammatory environment in the INT group was larger than that in the IT group. Figure 4e and Figure 4f show mitotic figures and apoptotic bodies in INT and IT groups, respectively.

(a) Control (NINT) group, (b) Placebo (NIT) group, (c) Non-therapy (INT) group, (d) Therapy (IT) group, (e) Mitotic figures (indicated by arrows) and apoptotic bodies (in circles) in INT group, and (f) Mitotic figures and apoptotic bodies in IT group. CT= Connective tissue, NA= Necrotic area, DEC= Ductal epithelial cells, MEC= Myoepithelial cells, LU=Lumen, AC= Adipose Cells, BV = Blood Vessel.
Immunohistochemical staining with anti-PCNA, anti-ErbB2 and anti-caspase-3 detected PCNA, ErbB2, and caspase-3-positive cells, respectively, in rat mammary tumors. The expression of PCNA in INT and IT groups were detected in the nucleus of tumor cells (Figure 5a and Figure 5b, respectively), while the expression of ErbB2 in both groups was found on the cell membranes (Figure 5c and Figure 5d, respectively). Caspase-3 was expressed in different locations in mammary tumors of INT and IT groups (Figure 5e and Figure 5f, respectively). The expression of caspase-3 in INT group was found in the cell cytoplasm (Figure 5e), while in IT group, caspase-3 expression was found in the nucleus (Figure 5f) and resulted in chromatin condensation and DNA fragmentation, indicating that apoptosis has reached a late stage35,36. In addition, the expression of caspase-3 in IT group was also found at hollow regions in mammary tumors (Figure 5f), whereas caspase-3 expression in INT group was rarely found in the same region (Figure 5e).

(a) Expression of PCNA in the nucleus of tumor cells in non-therapy (INT) group, (b) Expression of PCNA in the nucleus of tumor cells in therapy (IT) group, (c) Expression of ErbB2 on the cell membranes of tumor cells in INT group, (d) Expression of ErbB2 on the cell membranes of tumor cells in IT group, (e) Expression of caspase-3 in the cytoplasm of tumor cells in INT group, (f) Expression of caspase-3 in the nucleus and Hollow regions (HR) of tumor cells in IT group. Brown color chromogen and arrows indicates positively stained cells.
Breast cancer cells that actively proliferated in rats of the INT group were seen to be relatively greater than those in the IT group, indicated by the higher number of mitotic figures per field in the INT group (10.14±3.80) compared to that in the IT group (5.43±1.81) as shown in Figure 6a. In addition, the percentage of positively stained areas of PCNA in INT group (36.16 ± 5.16%) was significantly higher than that in INT group (23.65 ± 3.84%, Figure 6b). Figure 6c and Figure 6d show that INT group had a greater percentage of positively stained areas of ErbB2 (0.045 ± 0.029%) and caspase-3 (8.90 ± 5.48%) than those in IT group (0.036 ± 0.029 for ErbB2 and 3.01 1.52% for caspase-3). However, the difference of percentage of positively stained areas of caspase-3 between the two groups was considered statistically significant (p<0.05), while the difference in ErbB2 expression was not significant (p>0.05).
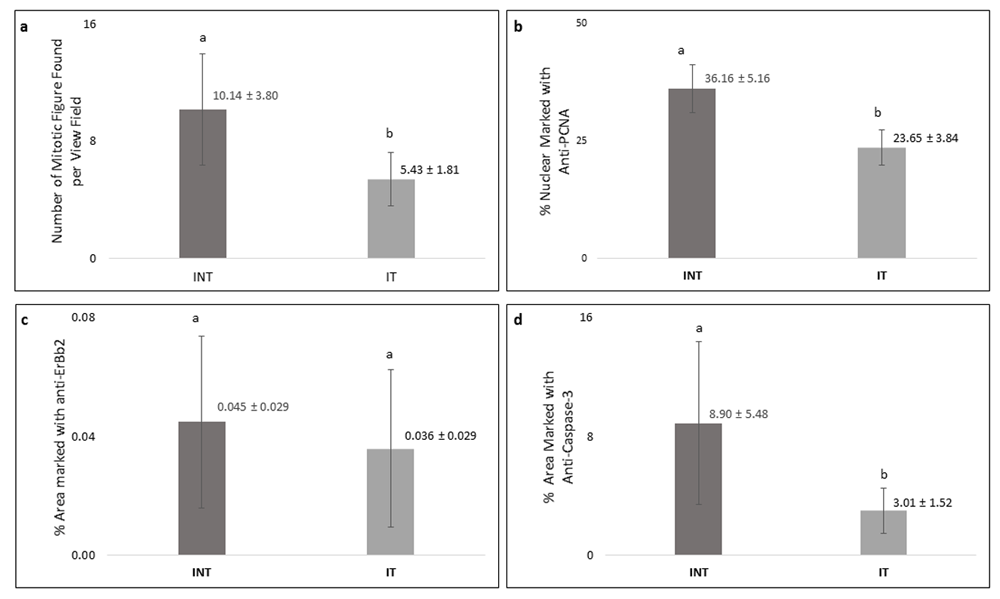
(a) Number of mitotic figures, (b) percentage of PCNA-positive cells, (c) percentage of ErbB2-positive cells, (d) percentage of caspase-3-positive cells. Error bars represent the standard error of the mean. Different letters (a, b) on top of the charts indicate significant differences (p<0.05).
Immunohistochemical staining with anti-CD68, anti-CD4 and anti-CD8 detected CD68+ macrophages, CD4+ and CD8+ T cells, respectively, in rat mammary tumors. The expression of CD68, CD4 and CD8 were detected on the cell membrane and in cytoplasm (Figure 7). In addition, CD68+ macrophage infiltration was found in mammary tumors of INT and IT groups, particularly in necrotic areas and hollow regions (Figure 7a and Figure 7b, respectively). The hollow regions in IT group were seen to be more numerous and larger in size than those found in INT group. In addition, CD68+ macrophage infiltration into the hollow regions of mammary tumors in IT group were also seen to be greater than that in INT group. Conversely, CD68+ macrophages infiltration in necrotic areas was seen to be higher in INT group (Figure 7a) than that in IT group (Figure 7b), since the distribution of necrotic areas in INT group was also wider than that in IT group. Similarly, CD4+ and CD8+ T cells were also found around the proliferating tumor cells that may respond to certain antigens in the mammary tumor cells, within blood vessels, and in the necrotic areas12,13 which were characterized by inflammation33,34 (Figure 7c and Figure 7d for CD8, Figure 7e and Figure 7f for CD4).
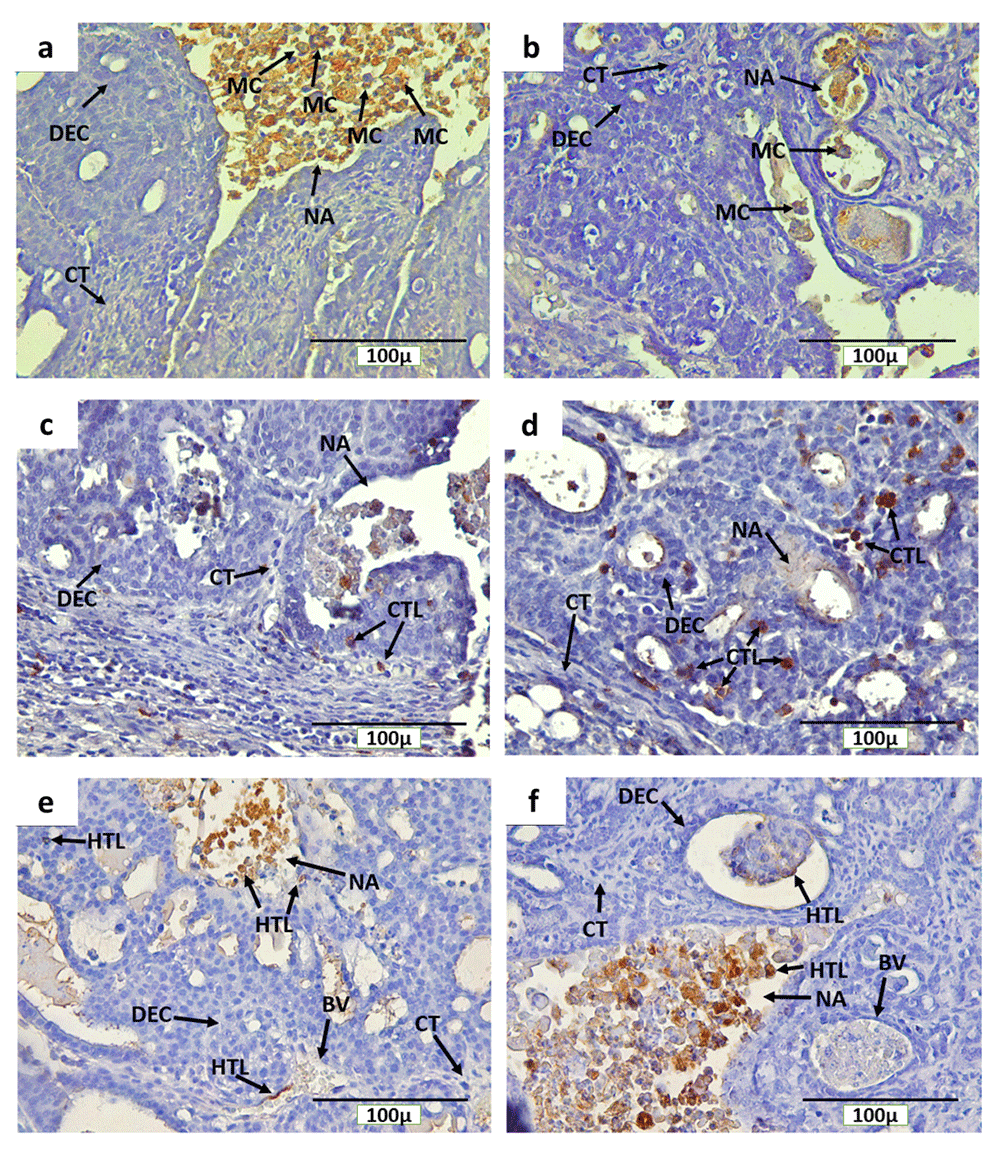
(a) CD68+ macrophages in non-therapy (INT) group, (b) CD68+ macrophages in therapy (IT) group, (c) CD8+ T cells in INT group, (d) CD8+ T cells in IT group, (e) CD4+ T cells in INT group, (f) CD4+ T cells in IT group, Brown color chromogen indicates positively stained cells. MC: Macrophage cells, CT: Connective Tissue, BV: Blood Vessel, DEC: ductal epithelial cells, NA: Necrotic area, CTL: Cytotoxic T Lymphocyte, and HTL: Helper T Lymphocyte.
Significantly higher number (p<0.05) of CD68+ macrophages was found in the INT group (8.20 ± 0.52) than that in IT group (4.87± 0.30, Figure 8a) with lower growth rate (Figure 2a). The percentage of positively stained areas of CD4 in INT group (0.42 ± 0.08%) was higher than that in IT group (0.32 ± 0.08%), but the difference was not statistically significant (p>0.05, Figure 8b). In contrast, the percentage of positively stained areas of CD8 in INT group (0.21 ± 0.04%) was significantly lower (p<0.05) than those in IT group (0.64 ± 0.11%, Figure 8c). Consequently, the ratio of CD4/CD8 in INT group (7.16 ± 2.60) was higher than that in IT group (2.44 ± 1.08, Figure 8d).
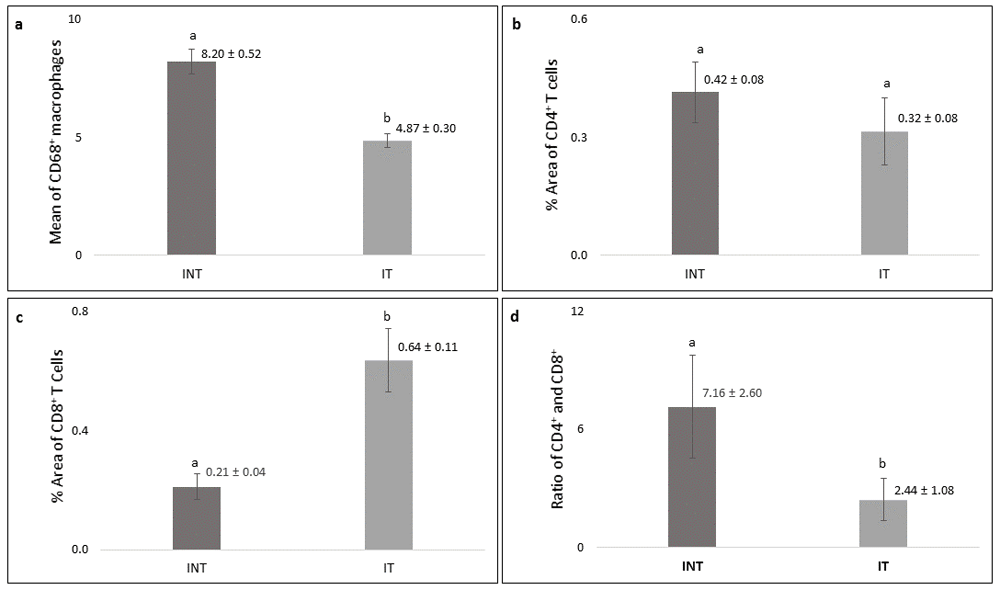
(a) Number of CD68-positive macrophages, (b) percentage of CD4-positive T cells, (c) percentage of CD8-positive T cells, (d) ratio of CD4/CD8. Error bars represent the standard error of the mean. Different letters (a, b) on top of the charts indicate significant differences (p<0.05).
In the present study, the significantly lower growth rate of rat mammary tumors in the IT group with a negative mean value of SGR compared to INT group with a positive mean value of SGR (Figure 2a) showed the inhibitory effect of non-contact electric fields of ECCT against mammary tumor cells that are actively dividing in the animals, as we have previously reported in our preliminary study in mice11. In addition, the development of mammary tumors exposed to 100 kHz non-contact electricfields of ECCT was decreased, whereas in the study of Pratiwi et al.22 which used a frequency of 150 kHz, it was increased. This may suggest that differences in the frequency of the electric fields can give different therapeutic results in cancer37,38 and the 100 kHz was the appropriate frequency of non-contact electric fields in inhibiting the proliferation of mammary tumor cells.
The absence of tumors in the placebo (NIT) group (Figure 4b) demonstrated the safety of non-contact electric fields of ECCT to healthy or normal animal models, as we have previously reported11. In addition to its safety, this electric fields exposure did not produce acute damage to rat skin tissue or chronic pain which usually occurs after radiation therapy, including breast cancer radiation therapy4,39,40. Instead, it helped wound healing after cancer treatment in rats of IT group, indicated by the drying wound and the reduction of wound size (Figure 3b and Figure 3c). Endogenous electric fields are known to play as a signal that direct cell migration in epithelial wound healing and external electric fields exposure may accelerate wound healing by directing the migration of T cells19 and fibroblast, as well as their proliferation and transdifferentiation41,42. In addition, Hoare et al.20 reported that external electric fields may contribute to the coordination and regulation of macrophage functions, including wound healing. The effect of non-contact electric fields of ECCT in directing immune cells was also seen in the fluid contained in the mammary tumors of the IT group as indicated by the presence of macrophages and lymphocytes infiltration into the tumor area (Figure 7) as we have previously reported11,22. This immune cell infiltration can promote the destruction of cancer cells12,21,43 as demonstrated in the IT group (Figure 2a and Figure 2c).
The cellular effects of electric fields exposure on PCNA expression during S-phase may indicate the incidence and the growth rate of mammary tumors44. Since PCNA is a S-phase marker in interphase that has a central role in DNA replication and repair45, the decrease of PCNA expression in mammary tumor cells in IT group (Figure 6b) may indicate a decrease in the incidence and the growth rate of mammary tumors44. In addition, alternating electric fields exposure with intermediate frequency and low intensity used in this study can cause tumor cells that are dividing to experience uneven segregation of chromosomes and mitotic catastrophe which followed by programmed cell death46. This is supported by the study of Mujib et al.47 which reported that p53 protein can serve as a marker for cancer cell death via apoptosis due to non-contact electric fields exposure. Moreover, the hollow cavities, also called dead cell islands, with cells debris, may be the spaces that used to be the place for mammary tumor cells that then died through apoptosis48,49, since caspase-3 was found in this place in the IT group (Figure 5f). In addition, within these dead cell islands, the expression level of caspase-3 was very low49, as we found in the IT group (Figure 6d).
The lower expression of caspase-3 in the IT group (Figure 6d) may suggest the mechanism of mammary tumor cell death under exposure to non-contact electric fields of ECCT through caspase-independent cell death. Meggyeshaz et al.50 reported tumor destruction by electric fields exposure and concomitant heat with elevated DNA fragmentation and low level of caspase-3 expression localized to inflammatory cells which dominantly follows a caspase-independent pathway. Meanwhile, higher caspase-3 expression in INT group (Figure 6d) suggested that higher apoptosis events may have occurred in this group, resulted from oxidative stress due to elevated reactive oxygen species in mammary tumors51,52. Although apoptosis occurred in the mammary tumors, the tumor cell death rate was relatively much lower than the proliferation rate, so that mammary tumor size increased53, as seen in the INT group (Figure 2a and Figure 2b).
The type of receptor possessed by breast cancer cells in this study was the ErbB2 receptor (Figure 5c and Figure 5d). The generation of mammary tumors with ErbB2 receptors were the results of carcinogenesis due to DMBA administration into the animal tumor model54. Despite such insignificant results in the expression level of ErbB2 between INT and IT groups (Figure 6c), tumor size in the INT group continued to increase from baseline, and its tumor growth rate was higher than that in the IT group (Figure 2a). In addition, the number of mitotic figures and the percentage of PCNA-positive tumor cells in the INT group were also higher than those in the IT group (Figure 6a and Figure 6b, respectively). This suggests that INT group experienced higher tumor cell proliferation than IT group. Moreover, the higher number of CD68+ macrophages as one of inflammatory cells seen in the tumor microenvironment of INT group (Figure 7a and Figure 8a) was capable to induce tumor growth by contributing to cellular migration of tumor cells and metastasis12,14,55. Ong et al.56 also reported that rat mammary carcinoma had significantly higher percentage of CD68+ macrophages compared to benign mammary tumors with a lower growth rate. CD68+ macrophages that infiltrate to the necrotic areas of INT group (Figure 7a) may be considered as M2 macrophages which play a role in tumor growth and provide a poor prognosis12,56,57. In addition, increased M2 macrophages or Tumor-associated macrophages (TAMs) infiltration in mammary tumors would be accompanied by an increase in tumor size14,15,58, as shown in the INT group (Figure 2a and Figure 8a). Conversely, the lower the number of infiltrating TAMs in rat mammary tumors, the smaller the size of the tumors12,15, as shown in the IT group (Figure 2a and Figure 8a). Moreover, the expressions of chemokine (C-C motif) ligand 2 (CCL2) and interleukin 18 (IL18) that have main roles in the development of metastatic breast cancer and excessive angiogenesis by amplifying M2 macrophage polarization59,60 were down-regulated under the exposure to non-contact electric fields of ECCT at 150 kHz frequency22. Similarly, non-contact electric field (100 kHz) exposure in our study may also inhibit the amplification of M2 macrophage polarization resulted in the negative mammary tumor growth rate in rats of IT group (Figure 2a). The infiltration of CD68+ macrophages in mammary tumors of IT group suggested that exposure to non-contact electric fields of ECCT may direct macrophages, likely of M1 phenotype, to the tumor areas11,21 and then engulf and digest dead cell, debris and tumor cells12,61 that eventually produced hollow cavities48,49 as seen in Figure 7b. This macrophage-mediated clearance of dead cells was occurred in caspase-3-independent death62, since the IT group had lower expression of caspase-3 than the INT group (Figure 6d).
Immunogenic tumor cell death induced by the electric fields exposure in the IT group can also be caused by the interaction of lymphocyte with receptors on membrane of mammary tumor cells with the production of cytokines21,46. The distribution of CD4+ and CD8+ T cells within blood vessels and mammary tumors of IT group revealed that non-contact electric fields of ECCT may direct the spreading of these lymphocytes from blood vessels to tumor areas11,19,21, including necrotic areas in it (Figure 7d and Figure 7f). The presence of CD4+ T cells in mammary tumors would induce the activation of CD8+ T cells to kill the tumors12,63,64 as an adaptive immune response12,13,65. However, since the percentage of CD4+ T cells in INT and IT groups was not significantly different (Figure 8b), the presence of CD4+ T cells in tumor areas may not always indicate tumor elimination, instead they may indicate tumor progression12,30,66. Moreover, electric field exposure can decrease CD4+ T cells polarisation19, resulted in the lower percentage of CD4+ T cells in IT group compared to INT group (Figure 8b). Conversely, the presence of CD8+ T cells in the necrotic areas which characterized by inflammation33,34, indicated their role in the inflammatory response to clear dead cells12,67 recognized as foreign agents13. In addition, a higher percentage of CD8+ T cells in IT group (Figure 8c) indicated increased anti-tumor immunity and immunological ability against tumor progression66,68,69. CD8+ T cells may release granzymes during granule exocytosis when tumor cells are marked for elimination12,13,70, and the granzymes may induce caspase-independent apoptotic pathways71,72 which support the presence of hollow cavities in the tumor areas with low caspase-3 expression49 as seen in the IT group (Figure 5f and Figure 6d).
The ratio of CD4/CD8 is correlated to clinical aspects and could be used as prognostic factor for breast cancer, where higher CD4/CD8 ratio in INT group (Figure 8d) was correlated to mammary tumor progression73. Conversely, the higher positive area of CD8+ T cells and the lower ratio of CD4/CD8 in IT group suggested a good prognosis in rat breast cancer12,66,73 with non-contact electric field exposure. Further study will be conducted to investigate the potency of non-contact electric fields exposure in wound healing upon breast cancer treatment by observing the presence of cytokines, as well as the migration of immune cells and fibroblast that regulate wound healing20,41,42 by using tissue samples from the IT group. Based on the evidence of its efficacy and safety on preclinical studies, the 100 kHz non-contact electric field has been used in phase I clinical trials of ECCT for healthy volunteers.
In summary, low intensity (18 Vpp) and intermediate frequency (100 kHz) non-contact electric fields exposure showed inhibition of mammary tumor growth in rats by inducing CD8+ T cells that lead to tumor cells death supported by decreased CD4/CD8 ratio. It also showed inhibition of M2 macrophage polarization resulted in the negative tumor growth rate. The proposed therapy gives good prognosis in rats with mammary tumors and potentially helps in wound healing of the tumor aftermath during the treatment.
Open Science Framework: Cytotoxic T cells response with decreased CD4/CD8 ratio during mammary tumors inhibition in rats induced by non-contact electric fields, https://doi.org/10.17605/OSF.IO/3P79474 (registered on 11th December 2020: https://osf.io/b6d4m).
This project contains the following underlying data:
- Ethical Clearance document
- Growth rate of mammary tumors and wound healing data
- IHC scoring data
- Raw images for IHC and hematoxylin figures
- Statistical analysis with GraphPad Prism ver.8.4.3
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
We would like to thank Mrs. Ardaning Nurliani from Faculty of Biology of Universitas Gadjah Mada for helping the antibodies procurement.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical imaging, cancer therapy, MRI, image guided therapies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 04 May 21 |
read | |
|
Version 1 19 Jan 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)