Keywords
Averrhoa bilimbi, obesity, brown adipocytes, white adipocytes, adipomyocytes
This article is included in the Plant Science gateway.
Averrhoa bilimbi, obesity, brown adipocytes, white adipocytes, adipomyocytes
We are pleased to submit this revised article with an updated title “Fractionation of Averrhoa bilimbi hexane extract corresponding to brown adipocytes stimulation”. The article has been revised according to the reviewers’ comments. No changes were made to figures and dataset. The word “isolation” has been removed and replaced by “extraction” and “fractionation” to better indicate the work that had been carried out in this study. Tables 1 and 2 have been updated. “Mass spectrum” has been replaced with “GC Chromatogram” in the figure 5-8’s legends. The content has also been rephrased to reflect the current findings and potential future work.
See the authors' detailed response to the review by Yusuf Andriana
See the authors' detailed response to the review by Nor Akmalazura Jani
Averrhoa bilimbi, also known as bilimbi by locals, is widely found in many countries of tropical Asia including Malaysia, the Philippines, Indonesia, and India. Bilimbi is a fast growing and long-lived plant species that is commonly 16-33 feet tall.1 It belongs to the same Oxadilaceae family as the carambola or star fruit. Scientific studies have been conducted on various parts of this plant, with medicinal benefits including antioxidant, hepatoprotective, anti-cancer, wound healing, anti-diabetic, anti-hyperlipidemic, anti-hypertensive, anti-hypercholesterolemic, anti-ulcerative colitis effects, as well as attenuated hyperglycermia-mediated oxidative stress and anti-thrombotic activities.2–5 Its beneficial effects in brown adipogenesis activation, a phenomenon that combats obesity, has also been recently reported.6
There are two main types of adipose tissue. White adipose tissue (WAT) is the primary site of fat storage and its amount increases in obesity. Brown adipose tissue (BAT) on the other hand plays specific roles in promoting energy expenditure and maintaining body temperature via heat generation. Although BAT and WAT are originated from distinct lineages, the plasticity of WAT allows brown adipocyte-like cells to emerge upon appropriate and adequate stimulation. This process is termed “browning” or “beiging” of WAT, which induces thermogenesis in cellular and animal models.7–9 Fundamental to the development of obesity is an imbalance between caloric intake and energy expenditure. The induced BAT development from WAT may help to increase energy consumption as well as reduce adverse effects of WAT to improve metabolic health. The stimulation of BAT development and activity can also be strategised to combat obesity.
Although the molecular effects of bilimbi in stimulating BAT activity to combat diet-induced obesity has been investigated recently, the corresponding phytocompounds to the reported pharmacological effects remain poorly studied. Here, we identify the phytocompounds of plant partition, and its active fractions that induce BAT associated adipomyocytes differentiation and enhance brown adipogenesis activities.
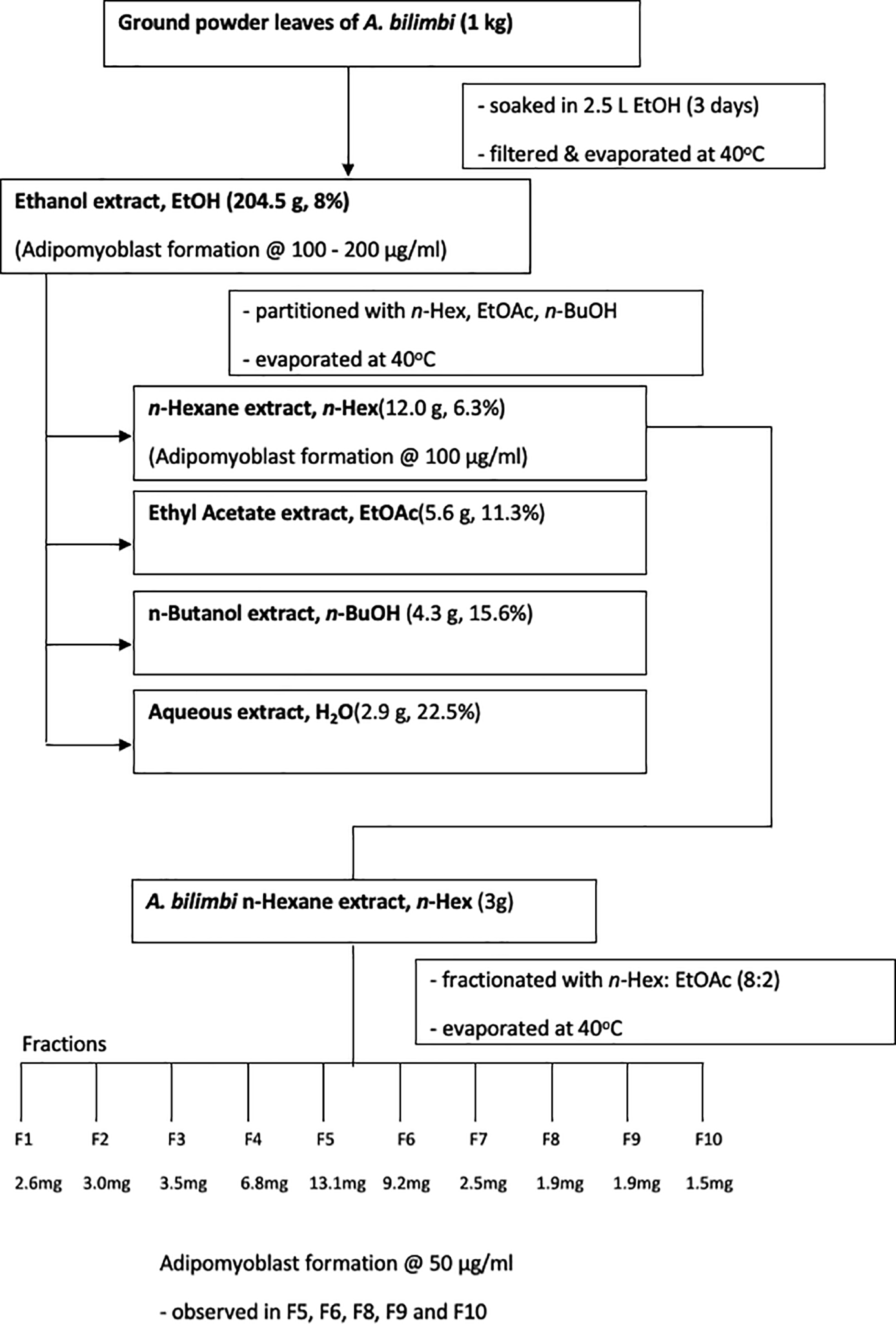
Bilimbi leaves were sampled from an orchard with the owner’s permission. The botanical authentication of the specimen was conducted by botanists of the School of Biological Sciences, Universiti Sains Malaysia (USM) and a voucher specimen 11738 was deposited in the herbarium of USM. Fresh leaves were dried in an oven at 45°C until the percentage yield of moisture content value was less than 10%. Dried leaves were then ground to a fine powder using a grinder then weighed, recorded and kept in a plastic bag. The powder was soaked in ethanol (EtOH) for 3 days at room temperature. After filtration with Whatman No. 1 filter paper, the solvent was removed under reduced pressure at 40°C using a rotary evaporator (Buchi, Switzerland) to obtain the crude ethanolic extract. The weight and percentage yield were recorded, stored in an amber bottle, labelled and kept refrigerated until further use. Next, the crude was sequentially extracted with organic solvents, namely n-hexane (n-Hex), ethyl acetate (EtOAc), and n-butanol (n-BuOH) with increasing polarity respectively. The filtrate was then concentrated and dried under reduced pressure at 40°C using a Buchi rotary evaporator. The remaining aqueous layer was evaporated and lyophilised in a freeze-dryer. The weight and percentage of each yield was recorded and kept refrigerated. A flow diagram showing the step-by-step extraction and partitioning processes is depicted in Figure 1.
After the bioactivity of these extracts was confirmed in cell-based assays, 200 g of dried leaves was soaked in 15 L of n-Hex to yield 12 g of extract. Fractionation was conducted with normal phase silica gel flash column chromatography. Mobile phases in the separation consisted of gradient of n-Hex, EtOAc, and methanol.
HPTLC has advanced separation efficiency and detection limits compared to conventional TLC Chromatography. The pre-coated silica gel Merck, TLC silica gel plates 60 F 254, 200 × 100 mm were used. 2 μl of the sample solution was applied on the plate using a CamagLinomat V automatic sampler applicator in the form of bands (length: 5 mm, width: 1 mm, distance between two bands: 10 mm) by using a 100 μl Camag Microlitre Syringe (Hamilton, Bonaduz, Switzerland). A constant application rate of 150 nl/s with mobile phase of n-hexane: ethyl acetate (80:20, v/v) was adopted. The plate was then placed in the mobile phase, and ascending development was performed to a distance of 8.5 cm, the plate was then air dried and performed densitometry scanning at 250 nm. Images of the TLC plates were captured using the Camag’s TLC Visualizer documentation system. The system provided illumination with white light (remission, transmission or a combination of both), where the exposure of RT white was optimised at 1.482 s, R 254 at 0.185 s, and R 366 at 0.877 s.
C2C12 myoblast was purchased from the American Type Culture Collection (Manassas, VA, USA) and was maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Adipocyte differentiation in the myoblast was initiated after the cells reached confluent by adding 0.5 mM isobutyl methylxanthine (IBMX), 1 μM dexamethasone (DEX), 1 μg/ml insulin, and 1 μM rosiglitazone (ROSI). Fresh differentiation medium containing DMEM, 10% FBS, 1 μg/ml insulin and 1 μM ROSI was replaced two days later. These cells were then stabilised in DMEM containing 10% FBS for another two days until the formation of oil droplets was observed.8
Cells were washed once with PBS and fixed with 4% paraformaldehyde for ten minutes. Lipid droplets produced by adipocytes were stained by Nile Red solution and were visualised using the IN Cell 2200 Analyzer High Content Screening System (GE Healthcare, PA, USA) at an excitation wavelength of 460 nm.10
The GC/MS analyses of non-polar hexane partition extract and active fractions were carried out using the Agilent 5977A Series GC/MSD system with an HP-5ms ultra inert column (30 m × 0.25 mm id, 0.25 μm film thicknesses). Spectroscopic detection by CG/MS involved an electron ionisation system which utilised high energy electrons (70 eV). Pure helium gas (99.995%) was used as the carrier gas with a flow rate of 1 ml/min. The initial GC oven temperature was set as 110°C for two minutes and programmed with increasing rate of 10°C/min to 200°C in five minutes. Finally, the temperature was increased to 250°C at 10°C/min in five minutes and held at that temperature for 13 minutes. The split ratio was 1:25 with a scan range of 40 to 550 amu.11 Hexane fractions (1 μl, 1 mg/ml) and partition extract (3 μl, 10 mg/ml) diluted in n-Hex were injected into the GC/MS via an auto-injector. Compounds present in the partition extract and active fractions were analysed by the mass fragmentation pattern. The National Institute of Standards and Technology (NIST) 14 and Wiley 10 library (W1ON14.L) were used for the identification of compounds with spectral similarity score higher than 60%.12
Four partitioned extracts were in the yield: n-Hex, EtOAc, n-BuOH and H2O. The yield of each component is indicated in Figure 1.
Flash column chromatography was conducted on the partition extract with an equilibration solvent ratio of n-Hex and EtOAc (95:5). A Hi Flash Column with a size of 3 L (46 × 130 mm silica gel) was used. With a flow rate of 60 ml/min, a total of 80 fractions were collected and spotted on a plate, employing the HPTLC. Plate visualisation was done under short (254 nm) and long wavelength (366 nm) ultraviolet lights, as well as white light illumination. Fractions with similar separation profiles were pooled. A total of ten fractions were collected with respective TLC profiles as shown in Figure 2.
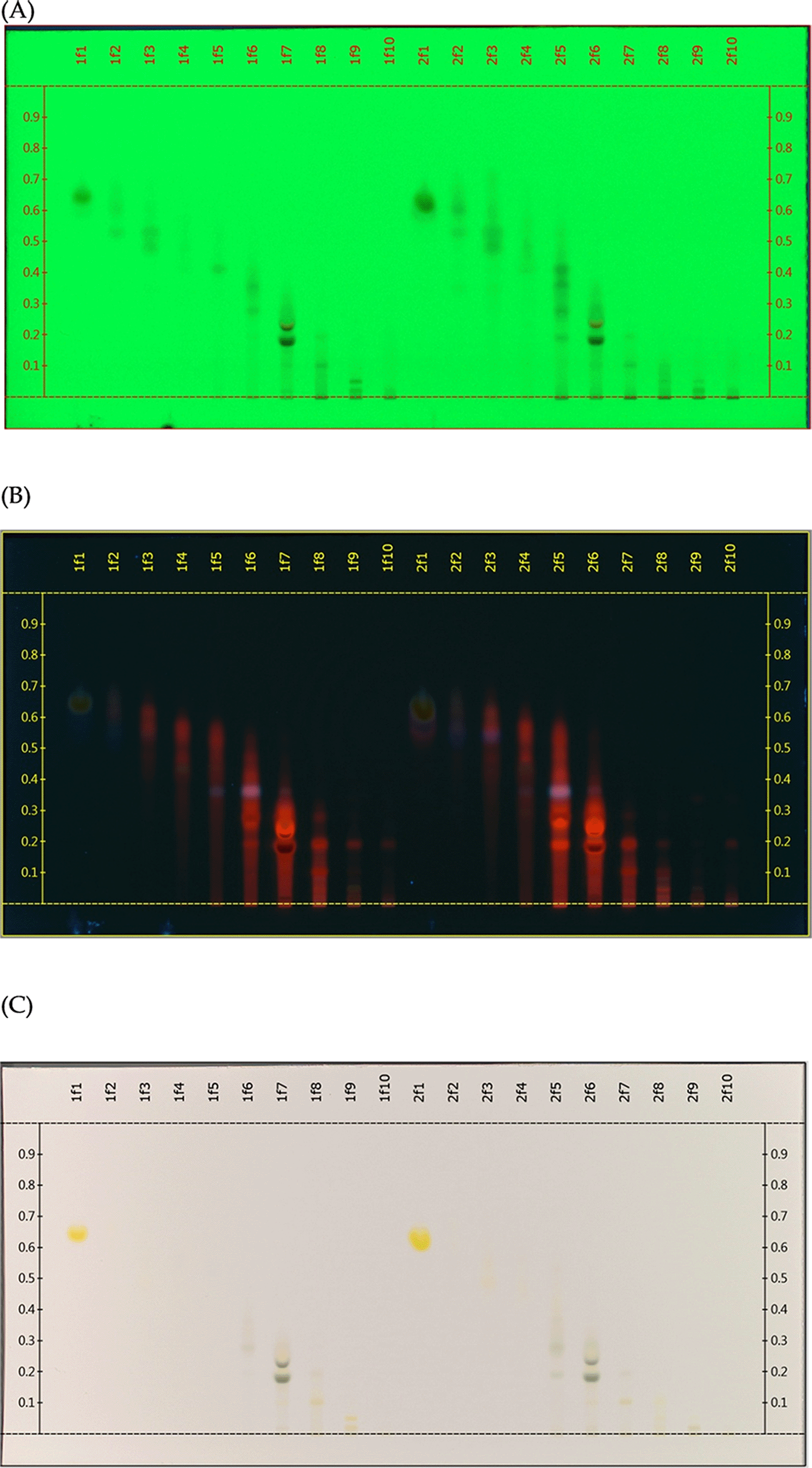
Two batches (1 & 2) of fractionation samples were collected and run on the same plates (A) TLC plate image viewed under UV 254 nm. (B) TLC plate image viewed under UV 366 nm. (C) TLC plate image viewed under white light illumination.
The partitioned extracts and fractions corresponded to the growth stimulation of brown adipocyte were investigated via cell-based study. The myf5-positive characteristics of C2C12 murine myoblast allow the co-development of mature myotubes and brown adipocytes upon appropriate stimulations. ROSI was served as the positive control. Adipogenesis was observed on day four after treatment with 1μM ROSI. A similar phenomenon was found in 200 μg/ml EtOH and 100 μg/ml n-Hex treated cells. Myotube development was observed in all treated cells. All positively treated cells showed intracellular lipid droplet production after 7 days of incubation. These lipid droplets were captured by Nile Red staining and imaged at 20× magnification (Figure 3). Among the four partitioned extracts tested, n-Hex exhibited the highest amount of lipid droplet production.
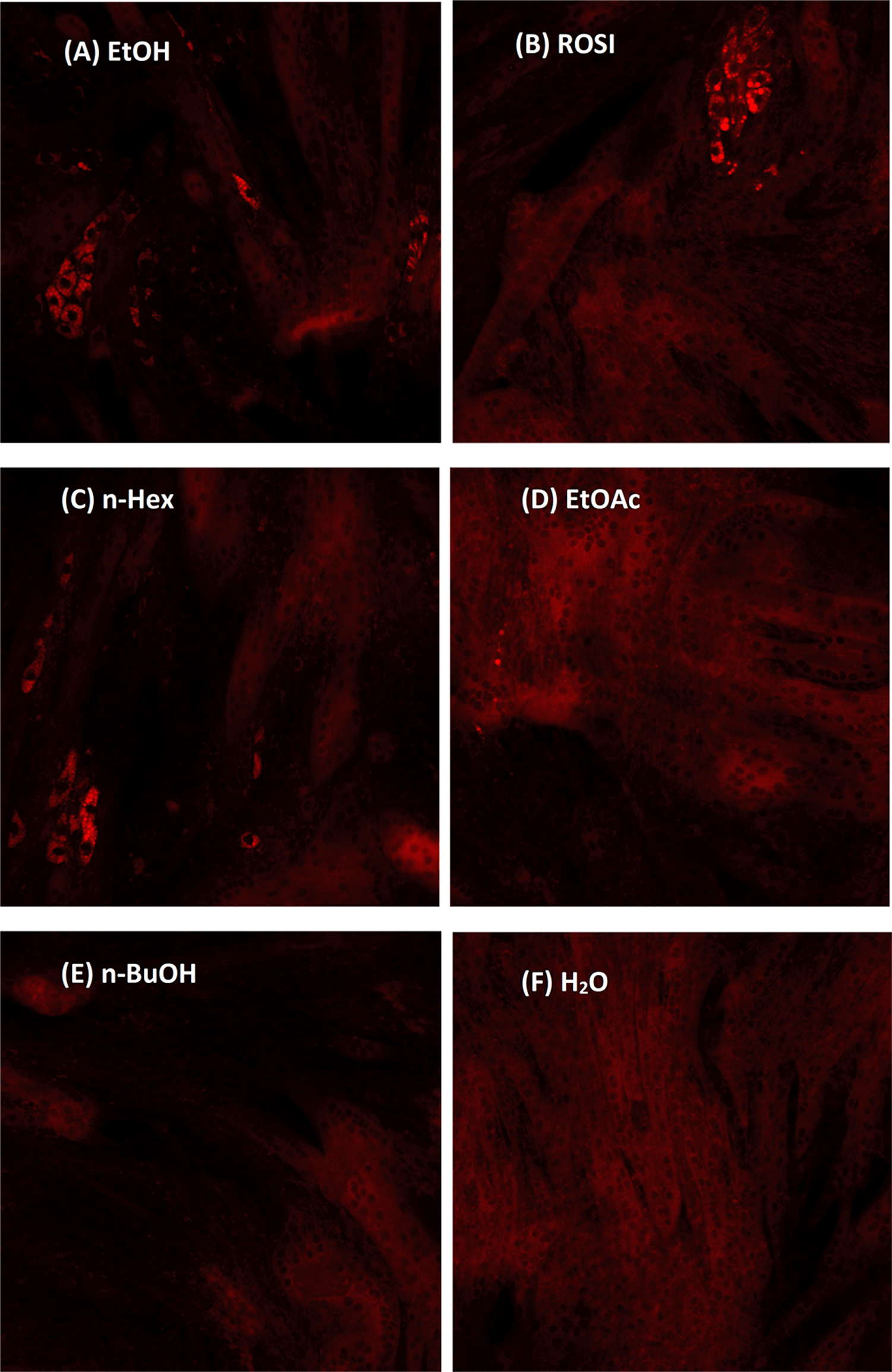
Lipid droplets formation upon adipogenesis upon treatment by (A) 200 μg/ml EtOH, (B) 1 μM ROSI, (C) 100 μg/ml n-Hex, (D) 100 μg/ml EtOAc, (E) 100 μg/ml n-BuOH and (F) H2O were stained by a Nile Red solution. Treatment of myoblasts with adipogenesis stimulants facilitated myocytes and adipocytes co-differentiation.
A bioassay guided fractionation technique was used to identify the active components present, as well as to evaluate the corresponding phytocompounds in brown adipocyte activation.
The cell-based study was then repeated on ten n-Hex fractions yielded from the HPTLC fractionation. Cell morphology and lipid droplet accumulation was observed for seven days with treatments by ten fractions. Myotube development was observed in all treated cells. Adipocyte differentiation was also observed in f5, f6, f8, f9 and f10 treated cells, with lipid droplet formation as depicted in Figure 4. Massive death was observed in f7 treated cells on day 3, and cell death was also found in f6 treatment from day 5 onwards. DMSO was used to replace bilimbi and ROSI in non-treated cells. These negative control cells were supplemented with adipogenesis culturing media and were fully developed into myotubes.
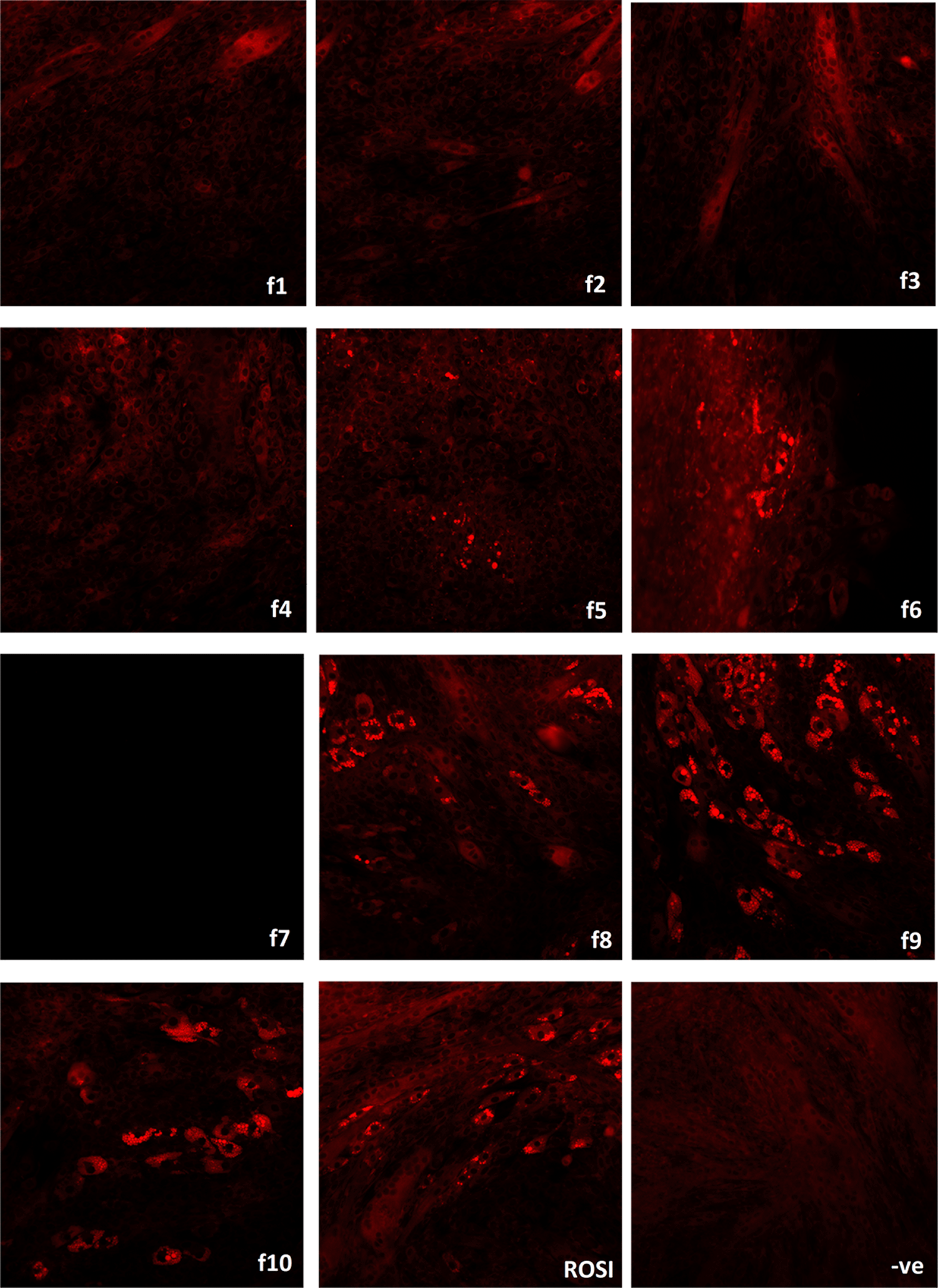
Lipid droplet formation upon treatment by n-Hex fractions (f1 – f10) and 1 μM ROSI were stained by a Nile Red solution. n-Hex fractions with adipogenesis stimulating components facilitated myocyte and adipocyte co-differentiation. Cells treated with 0.5% DMSO served as negative control (-ve) and demonstrated a complete process of myogenesis.
A GC/MS based qualitative analysis was performed on n-Hex and subsequently three active fractions, namely f8, f9, and f10, to determine the types of compounds present (Figure 5-8). Major constituents identified are presented in Tables 1 & 2. Studies on f6 & f7 were discontinued after the demonstration of cytotoxic effect in these treated cells, as shown in Figure 4.
The database information search via the MassHunter Library WION 14.L revealed the presence of nine predominant compounds in f8, ten in f9, and five in f10. (1.alpha., 4.alpha., 4a.alpha., 10a.alpha.) -1, 4, 4a, 5, 6, 7, 8, 9, 10, 10a -decahydro- 1, 4, 11, 11-tetramethyl-1, 4-methanocycloocta [d] pyridazine and i-propyl 3- (phenylamino) -2- (phenylseleno) -3- (phenyl) propanoate were detected in all the three fractions whereas nonanal and hexadecanoic acid were commonly present in f9 and f10. The high abundance compounds include 3-nonen-2-one; 2(4H)-Benzofuranone, 5, 6, 7, 7a-tetrahydro -6- hydroxy-4, 4, 7a-trimethyl-, (6S-trans)-; hexadecanoic acid; (12R)-(9Z)-12-hydroxy-9-octadecenoic acid; spiro [2.4] heptanes-5-carboxaldehyde; and i-propyl 3 -(phenylamino) -2- (phenylseleno) -3- (phenyl) propanoate.
Brown adipocytes and myocytes, precursor cells that give rise to skeletal muscle, are developed from a common adipomyocyte precursor. White adipocytes on the other hand are derived from pericytes that embedded within the vascular vessel walls. In this study, a straightforward cell-based assay was adopted based on the unique characteristics of precursor cells, where the adipomyocytes containing a mixture of brown adipocytes and myocytes, or solely the myocyte differentiation program, was activated via its initiation factors. Gene program commitments in myoblast-brown adipocyte transition were observed in bilimbi treated cells and the up-regulation of PGC-1α, UCP1 and PRDM16 proteins which influenced the cellular metabolism and mitochondrial function.6
Our observations in this study showed that bilimbi n-Hex partition was able to induce a higher cellular response in signalling the co-development of brown adipocytes and myocytes than its crude extract. The induction of brown adipocyte differentiation indicates the presence of active phytocomponents that are responsible for the program. Major phytocomponents in the n-Hex partition include hexadecenoic acid, phytol, 9-Octadecenoic acid (Z)- and squalene, which agree with an earlier report.13,14 Bioassay-guided fractionations yielded some promising bilimbi fractions from the n-Hex partition which further enhanced the brown adipogenesis program. Major chemical compounds of these fractions were then isolated and are summarised in Table 2. Some of these compounds are highly related to the obesity research in the past few years. For instance, Terbutaline is a β2-adrenergic receptor agonist that has been recognised to increase lipolysis and thermogenesis.15 2(4H)-Benzofuranone,5,6,7,7a-tetrahydro-6-hydroxy-4,4,7a-trimethyl-,(6S-trans)- is a compound that has been found to ameliorate hyperglycaemia, dyslipidaemia, and obesity in high-fat diet-fed mice.16 (12R)-(9Z)-12-hydroxy-9-octadecenoic acid, also known as ricinoleic acid, is an unsaturated omega-9 fatty acid and hydroxyl acid. Ricinoleic acid is linked to the activation of EP3 prostanoid receptor for prostaglandin E2 regulations in adipogenesis and lipolysis of WAT.17,18 i-Propyl3-(phenylamino)-2-(phenylseleno)-3-(phenyl) propanoate, detected in all the three bioactive fractions, is a short-chain fatty acid which regulates gut hormone release, suppresses food intake, and protects against diet-induced obesity.19 Hexadecanoic acid, also known as palmitic acid, is a controversial component in obesity research, where over-accumulation may result in dyslipidaemia and other obesity associated diseases.20
Evolutionary processes in natural products enhance their structural diversity and shape the pathways of secondary metabolite production. Many of these metabolites play important roles as the starting points for drug discovery in both traditional and modern medicines. Besides, plants are used in the form of spices and herbs based on their natural antioxidant values. Several plant-derived nutraceuticals are found to regulate BAT activity and induced WAT metabolis, including capsenoid, curcumin, quercetin, and resveratrol, without major adverse events.21 Food ingredients that could increase BAT activity include chili peppers, turmeric, thyme, cinnamon, garlic, onion, green tea, mulberry, and cocoa. Their white-to-brown adipose tissue conversion and calorie burning properties are identified through in vitro and in vivo models.22,23 Nevertheless, the involvement of bilimbi fractions and their bioactive compounds in BAT program enhancement are reported for the first time in this study.
It is estimated that 40-50g of active BAT can increase daily energy expenditure in humans by 20%.24 This could be implemented either by enhancing the activity of BAT or by increasing the number of WAT browning. Dietary components have been shown to increase thermogenic capacity by augmenting BAT activity via several mechanisms of action. Capsaicin that is commonly found in red peppers has been shown to activate ADRB3, a member of the G protein-coupled receptor family, that induces adenyl cyclase after activation, triggering a cascade of responses in the brown adipogenic program. Resveratrol extracted from grapes and berries on the other hand activates SIRT1 to recruit the BAT program via a series of PRDM16 pathway modulators including PPARγ, C/EBPβ, PGC-1α, and UCP1.25 Bilimbi, in our studies, acted similarly by up-regulating the expression of relevant proteins which influenced the cellular metabolism and mitochondrial function in adipocyte browning. Therefore, the molecular mechanism of action behind each active compound of bilimbi should be further studied to constitute a therapeutic option for obesity treatment in the future.
In summary, n-Hex extract of bilimbi activated the adipocyte program of adipomyocytes. Several bilimbi fractions showed strong activity in brown adipocyte differentiation from adipomyocytes. Further compound isolation and purification steps are necessary to confirm their biological effects in brown adipocyte stimulation.
All data underlying the results are available as part of the article and no additional source data are required.
F.H., A. A. and W.K.L. conceived and designed the experiments, collected and analysed the data; the manuscript was written by W.K.L. and reviewed by F.H. & A.A.
This work was supported by the Fundamental Research Grant Scheme of the Ministry of Higher Learning of Malaysia (FRGS/1/2019/STG05/MOSTI//4).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural product chemistry
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural product chemistry
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Andriana Y, Xuan TD, Quy TN, Minh TN, et al.: Antihyperuricemia, Antioxidant, and Antibacterial Activities of Tridax procumbens L.Foods. 2019; 8 (1). PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: natural product chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 18 Jan 22 |
read | |
|
Version 2 (revision) 08 Dec 21 |
read | |
|
Version 1 18 May 21 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)