Keywords
cancer, oligometastases, survival, review, meta-analysis, tumours
This article is included in the Oncology gateway.
cancer, oligometastases, survival, review, meta-analysis, tumours
We have updated the table data as requested. We have changed a sentence in the final abstract section. We modified introduction by shortening the length. We also improved discussion by discussing the emergent problem of oligometastatic disease in light of the new imanging modalities. We discussed also the potential curative role of targeted local therapies (e.g RT) in some disease (lung, renal carcinoma) supported by recent clinical trials. Some requests of the reviewer are not satisfied because data lack into included studies (exact burden of disease, treatments received in oligo vs polymetastatic subgroups). We extracted only data about timing of metastases (synchronous vs metachronous) that is reported in the table.
See the authors' detailed response to the review by Dario Baratti
See the authors' detailed response to the review by Luca G. Campana
The vast majority of metastatic solid tumors are incurable, and despite the evolution of treatments, patients ultimately die because of their disease. The modern concept of oligometastatic (OM) state was initially developed in 19951 to describe patients with a low burden of disease (e.g. 1 to 3-5 metastases) with a potential for cure with local ablative treatments. This assumption also relies on the hypothesis that metastatic spread follows a hierarchical pattern in time and number of localizations.2 Large consensus on the definition and management of OM patients is currently lacking. Clinically, those cancers with a lower burden of metastatic disease have a favorable prognosis and they may be amenable of local treatment for the primary and distant tumors. Recently, infact, advances in imaging and local ablative therapies have permitted the treatment of these patients with additional locoregional treatment in addition to systemic therapies, and some patients may be cured and attain long term survival.3 This scenario has been best elucidated in genitourinary, lung and melanomas.4,5 In these settings oligometastatic cancers may be treated in oligoprogressive sites continuing systemic therapy that control the remaining disease. One of the first published trials proving benefit of an aggressive local treatment of oligometastases was published in Lancet during 2019. In the SABR-COMET randomized study median overall survival (OS) was 28 months (95% CI 19-33) in the control group versus 41 months (26-not reached) in the stereotactic body radiotherapy to all metastases group (hazard ratio 0.57, 95% CI 0.30-1.10; P = .09).6
The aim of this systematic review and meta-analysis was to investigate and establish the prognostic survival of OM compared to non-OM solid tumors. In particular, we evaluated if patients with oligometastatic solid tumors do better than patients with non-oligometastatic tumors.
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines.
A comprehensive search was performed with the following terms: (advanced or metastatic or recurrent or relapsed or synchronous or metachronous) and (site or oligo* or “oligometastastic” or oligorecurrence or oligoprogression or single or multiple or 1-3 or >3 or >4 or >5 or 1-2 or 1-3 or 1-5 or number) and (synchronous or metachronous or metastases or relapse or recurrence or progression) and (tumor or tumour or cancer or carcinoma or melanoma or sarcoma) and (“hazard ratio”) and (cox or multivariate or multivariable) and survival. We searched PubMed, the Cochrane Library and EMBASE for studies eligible for this meta-analysis published in English language from inception up to October 30th, 2020. To be eligible, studies needed to have evaluated survival of patients with OM cancers (1 up to 3/5 metastases regardless of anatomic sites) regardless of line of therapy and to provide data of outcome according to the number of OM sites used by each author. Studies were excluded if they enrolled less than 10 patients, pediatric subjects, and hematological diseases. Commonly we define polymetastatic cancer as any disease with more than three or more than five metastases. Studies were searched and screened independently by three authors (FP, MG and GT).
The primary endpoint was overall survival (OS) and the secondary endpoint was progression-free survival (PFS). Quality assessment of the included studies was performed using the Newcastle-Ottawa Scale (NOS) for observational or retrospective studies (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). With NOS scale, studies were defined as poor, sufficient or good quality if scores (the sum of points attributed to each domain) were <6, 6 or 7-9 points, respectively.
The extracted data (from six reviewers) included the type of study, number of patients, cancer type, median age of included patients, performance status 0-1 (rate), treatment received, timing of oligometastases (synchronous or metachronous), number of OM sites used for comparison, and median follow up. Hazard ratios (HR) for OS and PFS with their 95% CIs, were extracted preferentially from multivariate analyses where available. The heterogeneity in the included studies was evaluated by the Chi-square-based Q-test and I2 (I2 = 0% to 25%, no heterogeneity; I2 = 25% to 50%, moderate heterogeneity; I2 = 50% to 75%, high heterogeneity; I2 = 75% to 100%, extreme heterogeneity). When I2 was larger than 50%, a random effects model was used; otherwise, the fixed effects model was used. Sensitivity analyses for OS were performed according to type of cancer, timing and number of oligometastases to find the potential heterogeneity among the included studies. If the number of studies was less than or equal to one, we did not carry out the subgroup analysis. The possibility of publication bias was explored by the Egger's and Begg's tests and Trim and Fill method.7,8 Begg's test explores bias with a funnel plot, conversely Egger's test is a linear regression of the effect estimates (OS) on their standard errors weighted by their inverse variance. The trim-and-fill method aims at estimating potentially missing studies due to publication bias in the funnel plot and adjusting the overall effect estimate. All analyses were performed using RevMan v.3 software.9
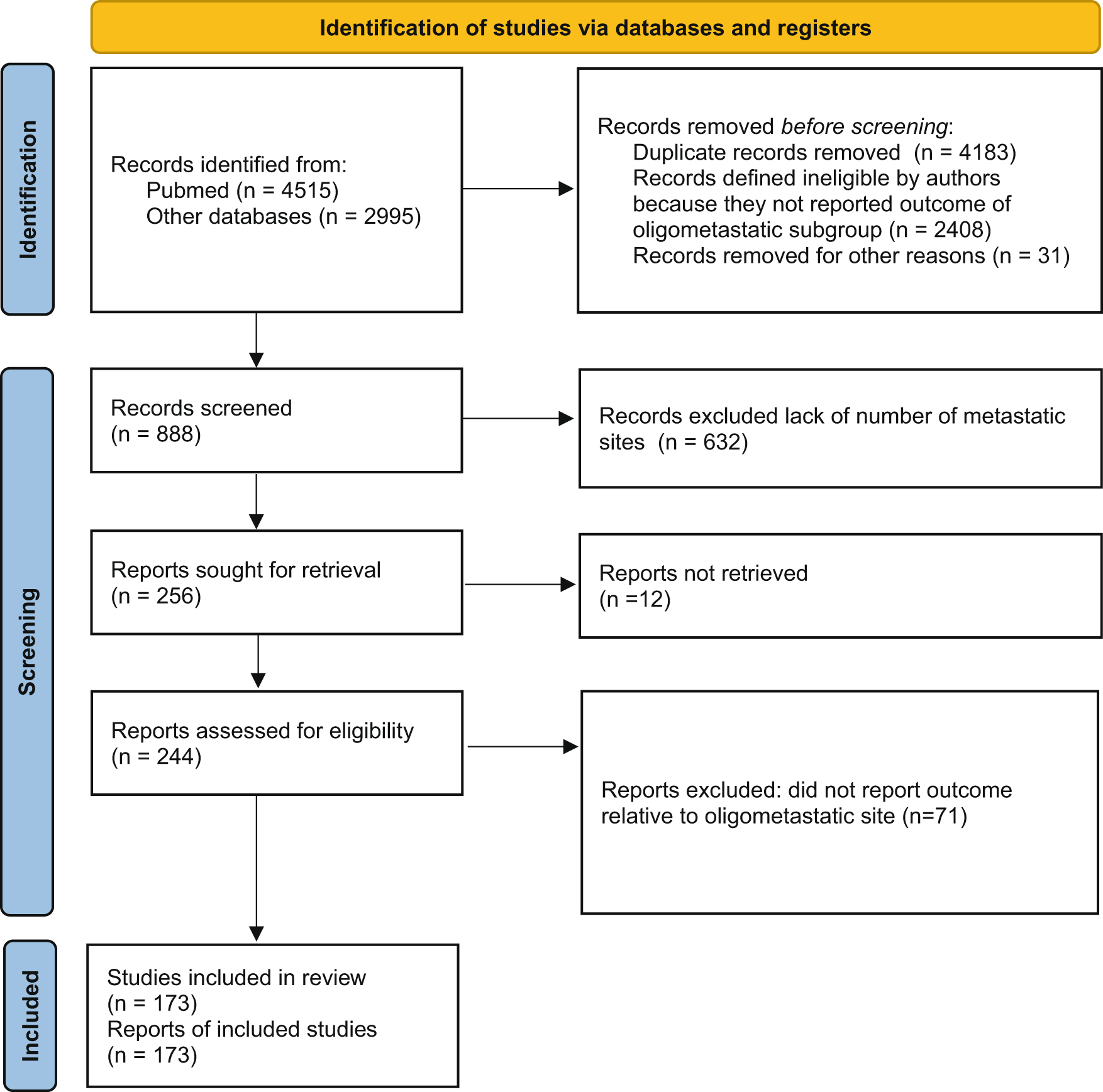
Among the publications retrieved using electronic search (n = 7510), 173 studies were eligible for meta-analysis, for a total of 104,234 patients10 (Figure 1). Baseline characteristics of the included studies and treatments received are presented in Table 1.
Progression-free survival was better in patients with OM disease (HR = 0.62, 95% CI 0.57–0.68; P < .01; n = 69 studies; Figure 2). Additionally, in the OS analysis, OM cancers were associated with a better OS (HR = 0.65, 95% CI 0.62–0.68; P < .01; n = 161 studies; Figure 3). Results were significant for all analyzed disease subgroups except biliary tract cancer and cervical cancer (only three studies included). In colorectal (CRC), breast, non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC), which constituted the more representative series, the reduction in the risk of death for OM patients were 35, 38, 30 and 42%, respectively (Figure 3). Timing of onset (synchronous vs metacronous disease) did not influence the risk of death. Most studies reported OS analysis for up to three metastases (152 out of 161 studies). After exclusion of eight studies that reported outcomes for up to five metastases the final results remained unchanged (HR = 0.64, 95%CI 0.61-0.67; P < .01). No cut-off was associated with a better outcome (1 vs 2 vs 1-2 vs 1-3 metastases).
Risk of bias through Begg's funnel plot was not significant for the OS analysis. Conversely, Egger's test showed evidence of bias (P < .01) (Figure 4). Trim and Fill analysis incorporated 29 missing studies. The overall effect measure (95% CI) based on this analysis was 0.7 (0.67-0.73), which became slightly weaker compared to the originally reported overall effect measure. Compared with cancers with more than three to five metastases, high-certainty evidence indicates OM tumors are associated with better prognosis in particular for CRC, breast, NSCLC and RCC. Despite the subgroup difference is not significant likely for less studies included in other groups, the results for these 4 cancers remain robust.
The definition of oligometastatic refers to malignancies with a limited metastatic spread which may be amenable of radical treatment for both primary and each distant site, and that generally have a better prognosis compared to polymetastatic cancers. A very recently published paper clearly explains the timely clonal evolution of somatic mutations and consequently the metastatic process of many cancer types.11 It may be hypothesized that OM cancer is associated with a more indolent spread and therefore may represent a less fatal disease. With the expansion of the oncological armamentarium, many efforts have been made over the years to improve outcomes of patients with minimal metastatic burden. Advance in imaging may also have improved in the last years the diagnosis of oligometastases with the possibility of a more targeted approach toward primary tumor and every single oligometastatic site. This may have created a bias compared to older series, where less accurate imaging modalities were available and more frequent cases of oligometastases could now be overdiagnosed.
We have performed the most exhaustive systematic review of the literature to quantify the prognostic value of OM stage in various cancers. Overall, OM cancer patients have a risk of death and progression that is a third less than the polymetastatic counterpart. The OM state is frequently calculated as an independent favorable prognostic variable, which means that these patients do well independent from other clinical-pathological characteristics. The effect size was calculated from 173 studies including more than 100,000 patients. The final results were similar in all the most frequent histologies including CRC, breast cancer, NSCLC, RCC and sarcoma with inferior survival in OM gastric, melanoma and head and neck cancers.
Prognosis of OM cancer may be also different according to site of oligometastases. For example in CRC, breast and RCC lung metastases have a generally more favourable outcome than liver (or peritoneal ones in CRC). In our series, sites of oligometasteses were mixed or not described at all so a subgroup analysis was not performed.12
There is also evidence from randomized clinical trials13-15 that ablative therapies improve survival in patients with OM cancer. For example, in some cancers small randomized studies13-21 already provide evidence of survival improvement in patients that received both systemic and local therapies compared to those that received systemic therapies alone. As a matter of fact, resection of colorectal cancer liver metastases nowadays represents an essential curative option and a primary endpoint in multiple clinical trails.13 Furthermore, Gomez et al.14 found that in OM NSCLCs, adding local consolidative therapy to active oligometastases and to primary disease may improve OS from 17 to 41 months. Also, in RCC the treatment of indolent lung metastases may permit delaying the start of systemic treatment and obtain an excellent control.15 A large burden of evidence now supports local therapy for minimal oligoprogressive cancers treated with targeted therapies or immunotherapy. Here, metastases-directed therapy could delay the switch of systematic therapy by radical local treatment of all progressive metastatic sites.16,17 With the advent of immunotherapy, the combination of immune check point inhibitors and radiotherapy to single OM lesions may facilitate a potentiation of the immune response, increasing the chances of achieving an abscopal effect. This term describes an event in which focalized radiotherapy discharge systemic anti-tumoral action that can result in distant responses.18 For example, in lung cancer the combination has a good safety profile and achieves high rates of local control and greater chances of obtaining abscopal responses than radiotherapy alone, with a relevant impact on outcome.19 Oligometastatic cancers can also regarded as extended locoregional disease if, after proper conversion therapy, all sites of metastases and primary tumor may be radically resected with curative purposes. Such a strategy has been employed in largely incurable cancers as gastric and pancreatic carcinomas where selected cases with small liver-limited recurrences were managed with surgery.20,21 Melanoma and head and neck OM cancers are also associated with better prognosis. In these settings isolated recurrences (lymph nodes, lung nodules or brain metastases) may be radically treated with surgery or radiotherapy.
This meta-analysis has several limitations. First, our review does not evaluate the absolute benefit of any local treatment and the prognosis and management of oligoprogressive disease or down staged polymetastases to an OM state. Second, the literature search covered a large lifetime span and may include older series where radiological evaluation did not include more advanced modalities that can now increase the accuracy of oligometastases detection. Third, most of studies have an observational design and outcome was retrospectively analysed. Likely publication bias may influenced the prognosis of this population. Finally, the optimal number of lesions defining the OM state cannot be defined in this paper.
A consensus paper of EORTC and ESTRO societies attempted to provide definitions of various OM conditions either naïve or attained after therapy and either synchronous or metachronous.22
Some large, randomized studies have included local therapies for OM cancers. An NRG Oncology randomized phase II/III trial study compares therapy with stereotactic radiosurgery and/or surgery with standard of care therapy alone in treating patients with breast cancer that has one or two locations in the body (limited metastatic) that are previously untreated. The PREST study will assess the efficacy of ablative radiotherapy (stereotactic body radiotherapy applied to all oligometastases) administered to all tumor sites (metastases and prostate if applicable), in oligometastatic hormone-sensitive prostate cancer patients. Finally, an ECOG-ACRIn phase III study compared standard chemotherapy to consolidative radiotherapy in patients with oligometastatic HER2 negative esophageal and gastric adenocarcinoma (https://clinicaltrials.gov/ct2/show/NCT02364557; https://clinicaltrials.gov/ct2/show/NCT04115007; https://clinicaltrials.gov/ct2/show/NCT04248452). In all ongoing studies the aim is the optimal timing (after a good shrinkage during systemic therapy) and integration of systemic medical therapy and local ablation/resection with the scope of improving long-term survivals.
In conclusion, this meta-analysis tried to quantify the prognosis associated with OM compared to cancers with more extensive diffusion. Based on our findings, we suggest that every metastatic patient should be accurately evaluated for the number of distant sites of disease, and a treatment strategy that involves both the primary and the metastases should be carefully considered. Patients could be reassured about their life expectancy and about the possibility of integrate both systemic and local therapy with the hope, in certain cases, for definitive cure. In others, focal treatment on the metastases may delay the immediate use of more toxic drugs (for example in elderly or indolent diseases). Also, we propose that these patients should be stratified when included in clinical trials and dedicated studies should be designed.
Mendeley Data: Extended data for ‘Better survival of patients with oligo- compared with polymetastatic cancers: a systematic review and meta-analysis of 173 studies’.
http://dx.doi.org/10.17632/8kycvdnp6v.1.10
This project contains the following extended data:
Mendeley Data: PRISMA checklist for ‘Better survival of patients with oligo- compared with polymetastatic cancers: a systematic review and meta-analysis of 173 studies’.
http://dx.doi.org/10.17632/8kycvdnp6v.1.10
Data are available under the terms of the Creative Commons Attribution 4.0 license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Peritoneal surface malignancies, advanced colorectal cancer
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: General Surgery; Surgical Oncology; Clinical Research; Melanoma; Colorectal Cancer; Soft Tissue Sarcomas; Skin Cancer
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical oncology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Peritoneal surface malignancies, advanced colorectal cancer
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Peritoneal surface malignancies, advanced colorectal cancer
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical oncology, locoregional therapies (limb perfusion/infusion, intraperitoneal chemotherapy, electrochemotherapy), melanoma, sarcoma, breast cancer, peritoneal malignancies, colorectal cancer.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 4 (revision) 12 May 22 |
read | read |
|
Version 3 (revision) 15 Nov 21 |
read | read |
|
Version 2 (revision) 25 Aug 21 |
read | |
|
Version 1 27 May 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)