Keywords
Oral ulcer, Areca nut, Chrysanthemum, TNF-α, Collagen
Oral ulcer, Areca nut, Chrysanthemum, TNF-α, Collagen
Abstract: We made a revision regarding the number of experimental groups
Table 4.: The revision of the number of rats (n= 72). We added the data of the changes in the normal group's ulcer size and body weight. This was also added to the Discussion.
Figure 1: Replacement with a new figure.
See the authors' detailed response to the review by Ali Akbar Nekooeian
See the authors' detailed response to the review by Nurrul Shaqinah Nasaruddin
See the authors' detailed response to the review by Gunawan Indrayanto
See the authors' detailed response to the review by Mingsan Miao
Oral ulceration is the most common presentation in the oral cavity caused by many etiologic factors.1 Most of the etiologies of ulcerative lesions on the oral mucosa are divided into four categories; namely, infectious (bacterial and virus infection), immune-related (autoimmune and allergic), traumatic (mechanical trauma), and neoplastic (oral squamous cell carcinoma).2 Traumatic ulcers are injuries to the oral mucosa caused by mechanical or physical trauma such as sharp food, accidental biting during mastication, biting while speaking, punctured by sharp objects, fractured, malformed, carious, or malposed teeth on the superficial epithelial layer or underlying connective tissue or may involve both.3
Currently, increasing the resolution of wound healing is one of the main priorities in the medical field to accelerate the healing of chronic wounds and traumatic injuries.4 The process of tissue regeneration and repair occurs immediately after lesion onset. The linear tissue repair and regeneration involves growth factors that induce cell proliferation, especially parenchymal cells, followed by dynamic changes in soluble mediators, blood cells, and the extracellular matrix.5 The unique oral cavity environment shows advantages in accelerating wound healing compared with skin repair. This is due to differences in the response to inflammation, differentiation and proliferative programs, modulation of stem cells, collagen synthesis, the role of macrophages, and epithelial remodeling.6,7 Collagen synthesis and tumor necrosis factor-α (TNF-α) are important components during acute inflammation and responsible for a diverse range of signaling events within cells in the wound-healing process. TNF-α is one of inflammatory cytokine and regulates the immune system.8
Areca nut is the seed of the areca palm (Areca catechu Linn.; areca, Palmaceae), which grows thrive in tropical Pacific region, South and South East Asia, including Indonesia where it is known as “Pinang”. It has been consumed by people worldwide as part of ancient tradition, custom, or ritual for a long time. Several studies have been conducted to prove the healing ability of areca nut, including that the alkaloid and polyphenols content in areca nut could enhance the healing of burn wounds, leg ulcers, and skin graft surgery.9,10 Areca nut contains alkaloid, phenolic, tannin, and flavonoid compounds. The presence of catechin, quercetin, and jacareubin contributed antioxidant and cytotoxic activities of areca nut extract.1 Our previous study showed that areca nut extract had cytotoxic activity againts human oral cancer cell lines such as HSC-2 and HSC-3 with IC50 values of 629.50 and 164.06 μg/mL, respectively.2 The selectivity of the areca nut extract was verified by using normal cell line. Areca nut had no cytotoxic effect on human keratinocyte (HaCaT) cell line. Instead, it induced high rate of proliferation in HaCaT cells. Chrysanthemum or chrysanth (Dendrathema grandiflora; Asteracea) is a flowering plant originating from East Asia and dominantly grows in China. The flowers are generally consumed in herbal teas and as a supplement. This genus comprises 40 species and contains flavonoids (flavanone), terpenoids, anthocyanins, and steroids.3,4 The selectivity index of Chrysanthemum indicum and morifilium oils were calculated 1.8 and 1.5, respectively, indicating low selectivity against trypanosomes compared to their toxicity versus HL-60 cells.5 Previous studies reported the effect of increasing keratinocyte proliferation and skin regeneration derived from Chrysanthemum boreale.11 Chrysanthemums are usually used for treating allergies, anxiety, hypertension, inflammation, headache, cold, sore throat, and tinnitus by certain communities.12
However, scientific research has not been conducted related to the healing process on the oral mucosa by oral application of these herbal plants. This study aimed to evaluate the potential of the combination of areca nut and chrysanthemum oral gel on oral mucosa using the Sprague–Dawley rat model.
This study was approved by the Ethics Committee for Animal Research of Tropical Biopharmaceutical Research Center (Trop BRC), Bogor Agricultural University, West Java, Indonesia, with number 042-2020-KEH TROP BRC. All the procedures in the study were reported following the ethical roles, principles, and guidelines of Animal Research Reporting in Vivo Experiment (ARRIVE).
Areca nuts were obtained from the Pinang plant from Aceh Besar, Indonesia, which was documented and determined by the Botanical Division of Biological Research Center LIPI Cibinong, complete with its roots, stems, leaves, flowers, and seeds in 2017. 2 kg of areca nuts (gross weight) were cleansed of dirt and dried in the open air and sunlight. Further drying was done using an oven set at a temperature of 50°C. The nuts were crushed using a blender and then strained with a 20-mesh sieve. The maceration process was conducted using 96% ethanol diluent solvent for 7 days before being subsequently filtered and evaporated using a vacuum rotary evaporator at 30–40°C and then reconcentrated using a water bath until a solid dry powder extract was obtained. Chrysanthemum polyethylene (P.E.) (Product No. 1237X17911) was provided by Javaplant Company, Indonesia.
The composition of the optimum formula was determined from 3 concentrations of carboxymethyl cellulose-Natrium (CMC-Na), followed by an evaluation of the physical stability study. The formula included in the criteria parameters is selected as the optimum formula. The compositions of the formula are shown in Table 1. The gels were made by dissolving areca nut and chrysanthemum extract with distilled water and then heated at a temperature of 50°C. The CMC-Na was heated with the remaining distilled water on a magnetic stirrer with a stirring speed of 400 rpm at a temperature of 70°C. Methylparaben was added until dissolved. Propylene glycol and glycerin were mixed and then added to the mixture of CMC-Na and methylparaben. The liquefied extract was added to the mixture and stirred continuously until a gel was formed.
The prepared formulations were placed in an incubator at a temperature of 40°C for 4 weeks. After that period time, all formulations were placed in an incubator at 5°C for optimum formulation selection. The final results are shown in Table 2. Formulation F III was found to be the most stable after the preliminary study. This optimum formula was used for the next assays.
| Evaluation | Criteria parameter | Characteristics | ||
|---|---|---|---|---|
| F I | F II | F III | ||
| Homogeneity | Homogeneous | Inhomogeneous | Homogeneous* | Homogeneous* |
| Consistency | Soft viscous | Liquid | Liquid | Soft viscous* |
| pH | 4.5-6.5 | 5.72 ± 0.02* | 5.88 ± 0.15* | 6.02 ± 0.07* |
| Spreadability (cm) | 5-7 | 7.05 ± 0.32 | 7.02 ± 0.22 | 5.5 ± 0.34* |
The optimum formula of the areca nut and chrysanthemum P. E oral gel (F III) was evaluated for thermal stability by observing color, shape, odor, taste, homogeneity, pH, spreadability, and viscosity during storage with a constant relative humidity (RH) of 75 ± 5% and maintained at 40 ± 2°C, without direct light. The samples were analyzed at the initial time (t0) and 2, 4, 6, and 8 weeks after exposure to the atmospheric conditions. The homogeneity test was carried out by visual inspection after the gels have been set in the container.6 They were tested for their appearance and presence of any aggregates. The pH test is carried out to observe the acidity level of the gel preparation to ensure that the oral gel does not irritate the skin. The pH criteria of oral gel are in the interval 4.5-6.5.7 The spreadability test was carried out to ensure the even distribution of the gel when applied to the skin.8 The gel was weighed as much as 0.5 grams and then placed in the middle of a round glass scale. On top of the gel is placed another round glass and ballast so that the weight of the round glass and ballast is 150 grams, allowed to stand for 1 minute, then the diameter of the distribution is analyzed. The good spreadability is between 5-7 cm. The viscosity test was analyzed by putting the gels in the Brookfield Viscometer and was rotated at 50 rpm using spindle no. 64. The corresponding dial reading was noted.6
The extract was formulated into four gel dosage forms with various concentrations of the extract combination, namely F1 (20% of areca nut:80% of chrysanthemum), F2 (50% of areca nut:50% of chrysanthemum), F3 (80% of areca nut:20% of chrysanthemum), and base gel (gel without areca nut and chrysanthemum). Preparation of the extract oral gel began with making two mixtures. The first mixture consisted of a carbamoyl and extract combination of areca nut and chrysanthemum and mixed with 10 mL of water at 70°C. The second mixture consisted of methylparaben dissolved in a little water and then mixed with a mixture of glycerin and propylene glycol. This mixture was then combined and stirred with water so that it is mixed homogeneously.
A total of 72 adult male Sprague–Dawley rats weighing 200–240 g were provided by the animal laboratory at Tropical Biopharmaceutical Research Center (Trop BRC), Bogor Agricultural University. The rats were kept for adaptation at 25°C in the well-ventilated laboratory for 14 days under a 12/12 h light/dark cycle and fed with a standard pellet diet and tap water ad libitum before being entered into the experiment. All the animals were given an initial examination for systemic health conditions and stored in boxes with sawdust.
Ulcer induction was performed after the animals were anesthetized by intraperitoneal injection of 33 mg/kg of ketamine and 13 mg/kg of xylazine (2%). The left buccal mucosa was smeared with 10% povidone–iodine on cotton pellets. An abrasion was made with 5 mm diameter, 1 mm depth, and was limited to the mucosa without muscular involvement. The ulceration was performed by using a number 15 scalpel blade. The standardization area was marked with an 8-mm diameter demarcator. The surgical and technique was standardized for animals and performed by the same operator.13 The ulceration was not performed in the normal group. The animals were placed in a thermic bag to avoid hypothermia and were observed during the return of the anesthetic plane. The formation of the ulcers could be observed after 24 hours.
The animals were randomly divided into six groups with 12 animals each: negative control group (rats with base gel treatment), positive control group (rats treated with triamcinolone acetonide), F1 (treatment with 20% areca nut:80% chrysanthemum), F2 (treatment with 50% areca nut:50% chrysanthemum), and F3 (treatment with 80% areca nut:20% chrysanthemum). A normal group (without ulcer and treatment) was added for the microscopic evaluation. All the groups were treated every 12 hours for seven days with topical application. The application of the oral gel was performed by using the individual sterile and disposable dental micro brush (SDent, USA) and hold for 1 minute above the wound. The application was carried out continuosly twice a day; in the morning and evening. Each group was sacrificed gradually through the end of the seventh-day study period. At the end of this experimental study, all rats were anesthetized through the intramuscular injection of 50 mg/kg BW ketamine 100 mg/mL and 5 mg/kg BW of xylazine 100 mg/mL. After the animals were sacrificed, a section of buccal mucosa containing the ulcer of each rat was collected. Histopathology, immunohistochemistry, and collagenases evaluation were analyzed in Primate Research Center, Bogor Agricultural University, Indonesia.
The animal body weights and diameter of the ulcers were measured before and after treatment on the second and seventh days. The diameter of the ulcers were measured with the naked eyes using a digital stainless vernier caliper which is capable of measuring diameter in 0.05 mm increments.14,15 All measurements were performed by the same operator.
The collected fragments of ulcers were identified and immersed in 10% formol for 24 hours. After fixation in formol, the specimens were macroscopically analyzed, subjected to dehydration in crescent alcoholic series, diaphanized in xylol, impregnated in paraffin, and melted at 60°C. The fragments were packed in paraffin-forming blocks at room temperature. The fragments were sectioned to 5 μm in thickness through the use of microtome and histology using routine coloration by hematoxylin-eosin (HE) was performed. The histopathological parameters were determined and scored from 0 to 4 according to previously published criteria (Table 3).16
The selected tissue sections of the ulcers (2.5 μm) were deparaffinized and rehydrated with distilled water. Blocking endogen peroxidase activity was done using 3% hydrogen peroxide solution for 15 minutes, followed by washing with phosphate-buffered saline (PBS) three times every 5 minutes. After protein blocking, the dripping of Biocare’s Background Sniper was performed and the specimens were incubated for 15 minutes at 37°C. The dripping of normal serum was performed and incubated for 60 minutes at 37°C. The specimens were washed with PBS and anti-TNF-α antibody (RRID: AB_2892586; TNFA/1172) (ab220210Abcam), was diluted at 1:100, and then dripped and incubated at 4°C for two days. After washing with PBS, the secondary antibody goat anti-rabbit IgG H&L horseradish peroxidase (HRP) (RRID: AB_955447; ab6721-Abcam) was given for 30 minutes at 25°C and followed by Betazoid DAB chromogen solution (BDB2004H-Biocare Medicare LLC). It was used for increasing stability and staining intensity for HRP detection in the specimens. The specimens were washed with distilled water and checked with the microscope. After counterstaining with hematoxylin for 30 seconds, the specimens were rinsed in tap water, dehydrated, purified, and mounted. The percentage of nuclear and cytoplasmic expression in the connective tissue was divided into four scores, namely: 0: no positive cells; 1 (mild): 1–33% of positive cells; 2 (moderate): 34–66% of positive cells; 3 (intense): 67–100% of positive cells. Two observers analyzed the same scores until they were considered as the final scores.17
The same fragments were sectioned to a thickness of 3 μm, de-wax, hydrate paraffin section, and stained using picrosirius red (solution A) for one hour. After washing in acidified water (solution B), the specimens were dehydrated and mounted for observation under polarized light microscopy. The collagen bundles shown in red in the image were then calculated using Adobe Photoshop CC 2017 (RRID:SCR_014199; GNU Image Manipulation Program (RRID:SCR_003182) is an open-access alternative) and ImageJ (v1.50i) (RRID:SCR_003070) software. The percentage of collagen was derived from collagen area pixel divided by tissue pixel and multiply by 100%. The mean of three percentages was used as a sample unit.18
All experiments were carried out in triplicate and the data were expressed as mean ± standard deviation (SD). Statistical analysis was performed by using the SPSS 20 software (SPSS Inc., Chicago, USA) (RRID:SCR_019096); JASP (RRID:SCR_015823) is an open-access alternative. The spreadability and viscosity test analyzed by one-way analysis of variance (ANOVA/Tukey test). Clinical evaluation in changes of the area of ulcer and body weight of rats analyzed by the dependent t-test. Differences among samples in histopathological and immunohistochemistry analysis were evaluated by using the Kruskal–Wallis test. Collagenesis analysis was evaluated by using ANOVA/Tukey test. A significant difference was assumed at p < 0.05.
The selected formulation F III presented appreciable organoleptic properties during stability studies. The color, smell, taste, and homogeneity didn’t show significant changes, but a slight changes in shape and pH were seen in the 8th week (Table 5). Data of spreadability test in optimum formula gel showed that the longer the storage time, the gel will spread wider. One-Way ANOVA analysis showed that there was a significant difference in the average dispersion of the gel every 2 weeks, between week 0 with week 4, 6, and 8 (mean = 5.53 ± 0.09, 95% CI: [−1.37, −7.36]), so it can be concluded that this gel formula was unstable in spreading ability during 8 weeks storage in 40°C. Different results are found in the viscosity test. The viscosity of the gel showed the reduction of viscosity value every 2 weeks of storage time, but the difference was not significant (p > 0.05) These results can be seen in Table 5.
The change comparison of ulcer size analysis in the buccal mucosa of rats showed a significant decrease in ulcer size in F1, F2, F3, and positive control groups (Figure 1A–J, Table 4). This observation was carried out on the second day after the ulcer was formed and at the end of the experiment. The most significant decrease in ulcer area was seen in positive control (triamcinolone acetonide orabase), F2, and F3 treatment groups. Notably, the average ulcer size in positive control, F2, and F3 treatment on the second day was 10.10 ± 0.17, 10.08 ± 0.16, and 10.08 ± 0.18 mm2, respectively. On the seventh day, it was seen that the size of the ulcer had almost closed, namely 0.03 ± 0.01, 0.11 ± 0.07, and 3.49 ± 0.42 mm2, respectively. In the end of experimental period, the clinical evaluation showed that although ulcers in the F2 group has smaller size, but the ulcers in the F3 group have tighter healing wound closure and more cicatricial tissue formation than other treatment groups. These data analysis showed that treatment with areca nut and chrysanthemum oral gel caused significantly (p < 0.05) ulcer healing in a dose-dependent manner in clinical analysis. Analysis of changes in bodyweight showed a significant increase in the normal, positive control, and F2 groups, while a significant decrease in bodyweight occurred in the F1 and F3 treatment groups (Table 4). Meanwhile, in the negative control group, there was a decrease in bodyweight, but it was not significant.
| Evaluation | Weeks of storage | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 95% CI | p | |
| Organoleptic characteristics | |||||||
| Color | Dark chocolate | Dark chocolate | Dark chocolate | Dark chocolate | Dark chocolate | ||
| Aroma | Typical aromatic | Typical aromatic | Typical aromatic | Typical aromatic | Typical aromatic | ||
| Taste | Bitter | Bitter | Bitter | Bitter | Bitter | ||
| Shape | Condensed | Condensed | Liquid | Liquid | Liquid | ||
| Homogeneity | Homogeneous | Homogeneous | Homogeneous | Homogeneous | Homogeneous | ||
| pH | 5.50 ± 0.03 | 5.52 ± 0.02 | 5.50 ± 0.03 | 5.45 ± 0.02 | 4.49 ± 0.01 | ||
| Spreadability (cm) | 5.53a ± 0.09 | 5.72a ± 0.08 | 6.21b ± 0.18 | 6.73c ± 0.15 | 6.79c ± 0.09 | −1.37, −7.36d | 0.000 |
| Viscosity (m.Pas) | 202.24e ± 1.12 | 184.52e ± 4.67 | 181.03e ± 0.98 | 161.96e ± 2.73 | 155.82e ± 0.45 | −4.54, 35.42d | 0.129 |
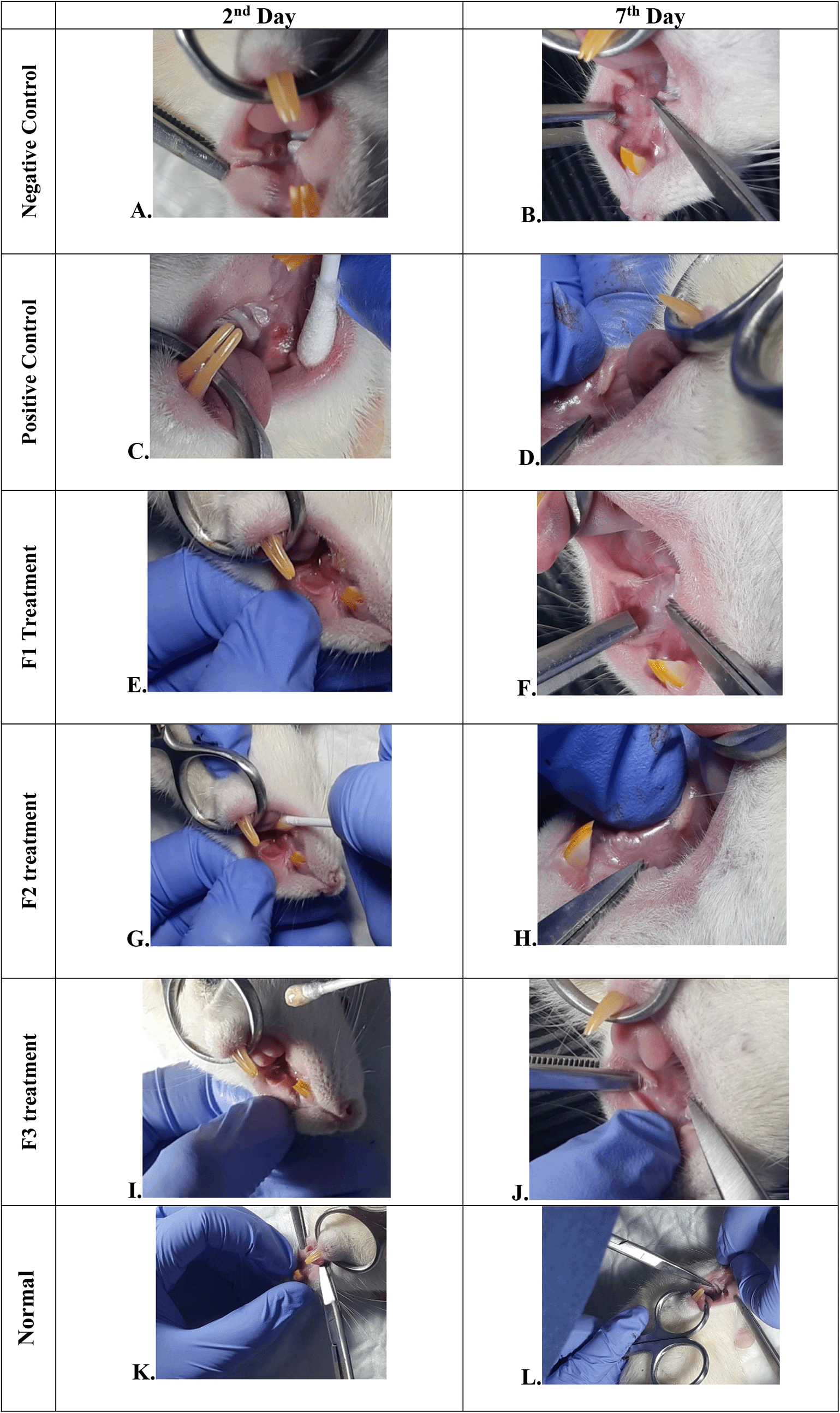
Negative control: base gel treatment group (A and B); Positive control: triamcinolone acetonide treatment (C and D); F1 treatment: 20% areca nut:80% chrysanthemum (E and F); F2 treatment: 50% areca nut:50% chrysanthemum (G and H); F3 treatment: 80% areca nut:20% chrysanthemum (I and J); Normal: without ulcer and treatment (K and L).
Effect of areca nut and chrysanthemum oral gel on histological scores, number of connective tissue cells that expressed TNF-α, and percentage of collagen deposition area on the 7 th experimental day (n = 18).
| Parameter | Microscopic evaluation | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | ||||||||
| Negative control | Positive control | F1 | F2 | F3 | Normal | 95% CI | p | |
| Histological scores | 0 | 1.34 ± 1.53 | 1.67 ± 1.53 | 0 | 1.34 ± 0.58 | 0 | 0.107 | |
| TNF-α immunostaining scores | 1.33 ± 0.58 | 2.67 ± 0.58 | 1.00 ± 1.53 | 2.33 ± 0.58 | 1.33 ± 0.58 | 1.00 ± 0 | 0.025 | |
| Collagen deposition area (%) | 5.63a ± 2.28 | 14.49 ± 0.95 | 17.73b ± 9.32 | 12.63c ± 0.92 | 25.4d ± 2.12 | 9.40 ± 1.05 | 1.61, 23.93e | 0.001 |
To verify the mechanism of ulcer healing by areca nut and chrysanthemum oral gel, the ulcers were subjected to histological examination. As seen in Table 6, the mean of histological scores on the seventh day showed that positive control, F1, and F3 groups indicated that there were no ulcers in the epithelial layer, presence of fibrosis, and slight chronic inflammation in the conjunctive tissue. The score 0 indicating no ulcers and remodeling in the conjunctive tissue was seen in the negative control, F2, and normal groups, but significant differences between groups were not detected in histology examination. Analysis of photomicrograph of ulceration showed loss of ulcer with remodeled connective tissue and accompanied by a slight inflammatory infiltrate in Figure 2B, 2C, and 2E (score 1). There were no visible inflammations in Figure 2A, 2D, and 2F (score 0).
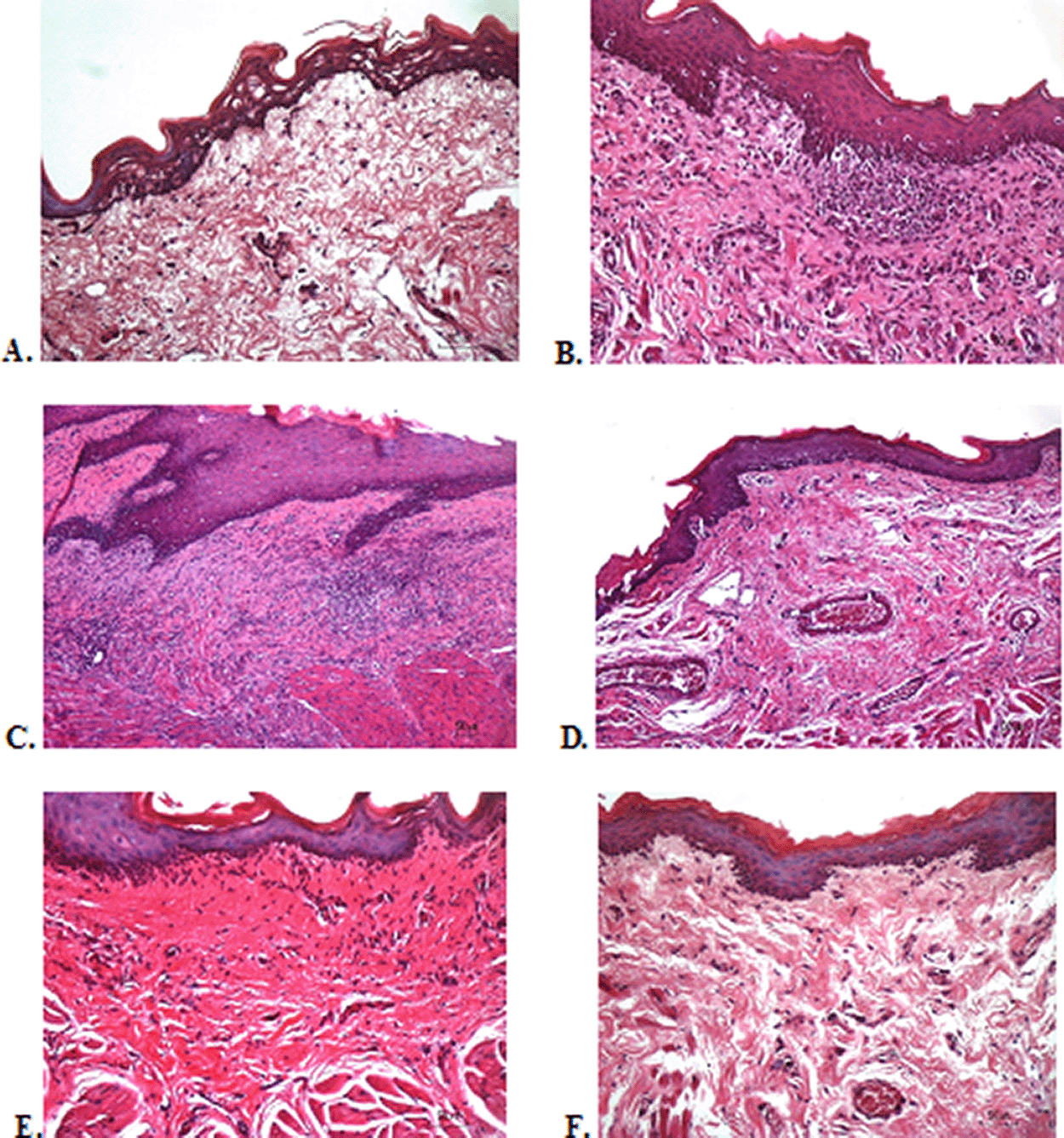
(A) Negative control: base gel; (B) Positive control: triamcinolone acetonide in orabase; (C) F1: 20% areca nut:80% chrysanthemum; (D) F2: 50% areca nut:50% chrysanthemum; (E) F3: 80% areca nut:20% chrysanthemum; (F) Normal: without ulcer and treatment (Hematoxylin and eosin, 40×).
The immunostaining for TNF-α aims to show changes in TNF-α expression released by connective tissue cells in the cytoplasm during the healing process ( Figure 3). The increase in the expressions with a mean score of 2 (moderate) was showed by positive control (2.67 ± 0.58) and F2 groups (2.33 ± 0.58) (Table 6). The lower scores (1-mild) were seen in the negative control, F1, F3, and normal groups. This study showed significant differences between the six groups with changes in TNF-α expression. These results were in line with the pictures that revealed brown diffuse granular-like staining pattern of the connective tissue and epidermis in the Figure 3B and 3D, while the other groups did not show dominant TNF-α expressions except in the F3 group, the immunostaining of TNF-α was prominently found in the basal cell layer (Figure 3E).
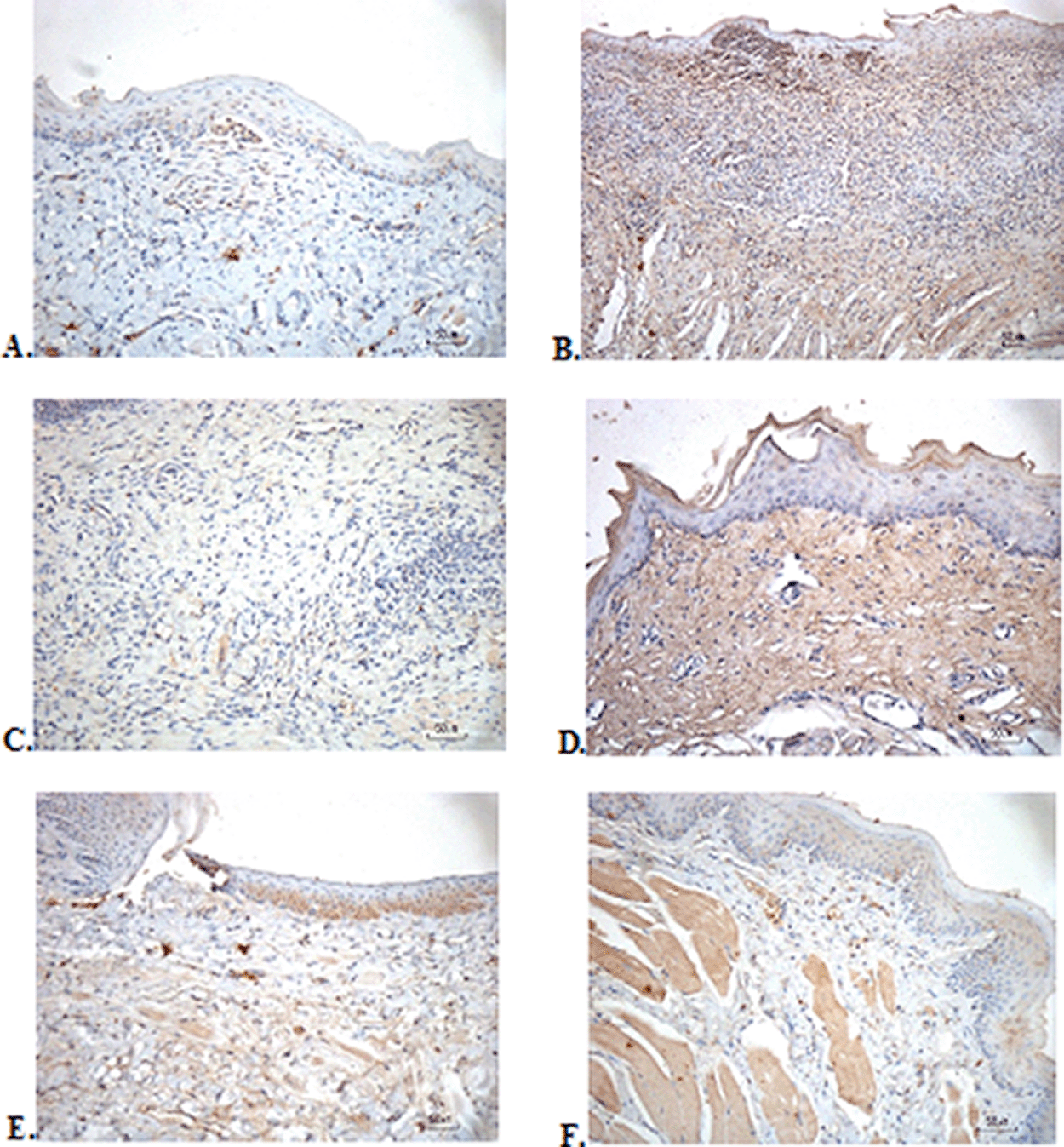
(A) Negative control: base gel; (B) Positive control: triamcinolone acetonide in orabase; (C) F1: 20% areca nut:80% and chrysanthemum; (D) F2: 50% areca nut:50% chrysanthemum; (E) F3: 80% areca nut:20% chrysanthemum; (F) Normal: without ulcer and treatment (TNF-α immunostaining, 40×).
The results of red picrosirius staining on the last experimental day showed the highest collagen deposition in the F3 group with a mean of 25.4 ± 2.12, while collagen deposition in low level was seen in the F2, normal, and negative control group (12.63 ± 2.28, 9.04 ± 1.05, 5.63 ± 2.28, respectively) ( Table 6). Figure 4B (Positive control) and 4D (F2 group) showed regular, dense, and thick collagen, whereas, in the negative control (Figure 4F), collagen deposition showed thin, irregular, and many empty gaps. The ANOVA analysis showed significant differences (p < 0.05) in collagen expression between groups (Table 6). The Tukey test demonstrated significant differences between F2 and F3 groups. Other significant differences were evaluated between F1, F2, F3, and negative control groups.
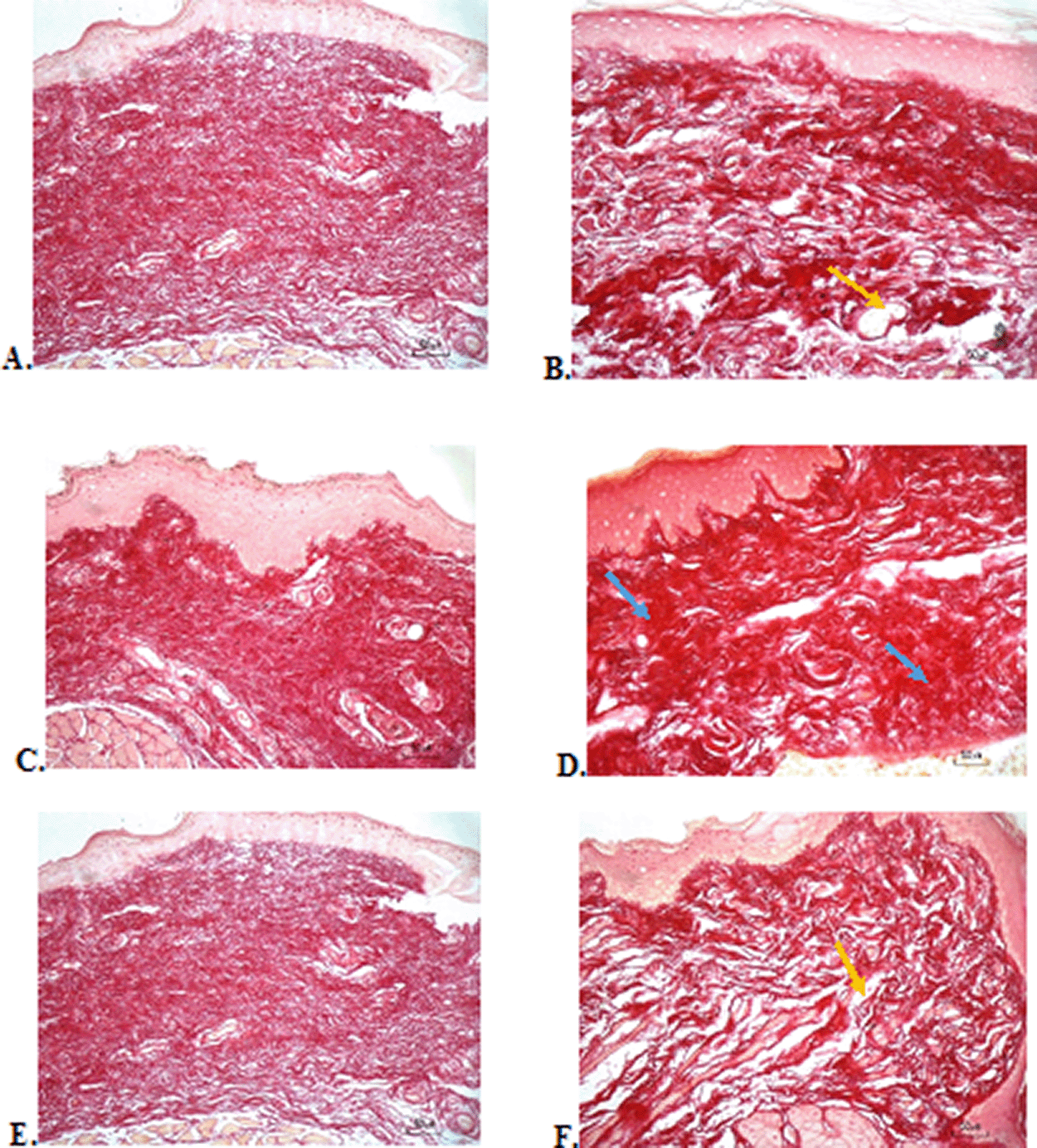
(A) Negative control: base gel; (B) Positive control: triamcinolone acetonide in orabase; (C) F1: 20% areca nut:80% chrysanthemum; (D) F2: 50% areca nut:50% chrysanthemum; (E) F3: 80% areca nut:20% chrysanthemum; (F) Normal: without ulcer and treatment (Picosirius red staining, 40×). Yellow arrow: Disintact collagen bundles. Blue arrow: Intact collagen bundles.
The preparation of the oral topical gel in this study using a CMC-Na-based gel. The gel form was chosen because it is easy to dry and provides a cool feeling on the mucosal surface. The gel formulation was carried out by trial-error by modifying the gelling agent. The aim of designing a stable oral topical gel is to deliver antioxidant properties effectively over its shelf life. Gel stability can be affected by environmental stress such as temperature and humidity. The decrease in the effect of the active gel ingredients can be observed from physical and chemical changes such as color, shape, texture, odor, and pH. The stability test in our research is a step in optimizing the preparation based on the selection of the appropriate gelling agent level so that the oral gel can be stable in various storage settings. The organoleptic characteristics of the optimum gel (F III) showed promising stability because there were no significant changes in color, odor, texture, and shape. The gel is said to be homogeneous if there is an even color equation and no different particles are found. The consistency, acidity, and spreadability of the gel are related to the comfort of use on the surface of the oral mucosa. The disadvantages of the gel are that it cannot adhere tightly to the mucosa surface and is easily soluble in saliva. This research showed that viscosity stability is an important consideration in the flow and texture properties of the gel. The stability of the constituents extract in the gel is also important because the pharmacological properties depend on the chemical viability of the extract. As pointed out above, the catechins are pharmacologically active constituents of the areca nut; however they are more stable at high concentrations around pH 4 and low temperature.9 Their stability is affected by several factors such as pH, temperature, and concentration in solution state.10
In the present study, we demonstrated that application of the oral gel to ulcerated lesions for 6 days showed significant lesion healing in the composition of the oral gel containing 50% areca nut:50% chrysanthemum and 80% areca nut:20% chrysanthemum. This significant wound healing was based on the clinical appearance such as ulcer size reduction, reepithelization over the ulcer base, and formulation of granulation tissue around the ulceration. These results have the same clinical appearance as the positive control group that using triamcinolone acetonide. Areca nut, as one of the main ingredients, contains phenolic and flavonoids which function as antioxidants.19 Catechin is a component of flavonoids present in areca nut that acts as an antioxidant by chelating the ions and scavenging the free radicals particularly, superoxide (O 2–), peroxyl, hydroxyl radicals (·OH), and hence inhibit both DNA damage and lipid peroxidation, which can cause membrane damage.20 Catechin can reduce the expression of interleukin-6 (IL-6) and IL-8 which function to overcome inflammation and increase the wound healing process by chemotactic for fibroblasts, accelerates their migration and stimulates deposition of tenascin, fibronectin, and collagen I during wound healing in vivo.21–24 The success of using areca nut in oral gel to heal the ulcers is in line with a previous study using ointment with 2% ethanolic extract on burns on the skin.10 This study also used a combination with chrysanthemum. Previous studies have shown that chrysanthemum has antioxidant, antiproliferative, antimicrobial, acetylcholine esterase inhibition, antitimelanogenic, and antiviral activities, especially Chrysanthemum morifolium, Chrysanthemum indicum, and Chrysanthemum coronarium. The active phytochemistry content of chrysanthemum is apigenin-7-O-glucoside.25
The wound in untreated rats would recover without treatment for a certain period. The wound healing process normally begins immediately after the damage occurs, but the mechanism and speed of repair of damaged tissue depend on the type of wound and the body’s defense system. A normal wound healing progresses through inflammatory, proliferative, and remodeling phases in response to tissue injury. Impairments of any of these phases can cause the wound to be in a chronic, non-healing state. Although the body has its wound healing mechanism without the help of external medicinal materials, the acceleration of wound healing can be assisted by intervention in the form of a supply of materials and nutrients that can be obtained from outside to guide the process back to completion. In this study, medicinal plants have roles in accelerating the process of wound healing. Collagen is an important extracellular matrix component in the regulation of the phases of wound healing in its native. The administration of a combination of areca nut and chrysanthemum extract gel on wounds of the oral mucosa can produce better-wound healing with the ability to stimulate collagen and TNF-α activity so that it can increase the average wound contraction and increase re-epithelialization. This condition is expected to significantly reduce wound healing time.
The wounds on the buccal mucosa of rats can heal on their own, but the results of our study prove that there is a difference in wound healing in the group that received treatment and the group without treatment. In addition to the results from clinical examinations, differences in healing speed were also supported by differences in test results for TNF-α levels and collagenase activity. The technique of applying the oral gel to the oral mucosa of rats is important in this research process because at the time of application the rats are not anesthetized, so there is a possibility that the gel is not maximally absorbed in the oral mucosa and most of it dissolves in saliva and is swallowed by rats. In this study, the oral gel was applied over the wound and held for 1 minute. The drug partitions dissolved into the mucous membrane and within a few minutes will reach equilibrium with the drug in solution in the oral cavity. One of the previous studies revealed the existence of the term pseudo-equilibrium which refers to the balance of a drug in solution and a drug in tissue in a relatively short contact time in the oral cavity before a dose of a drug is swallowed. This pseudo equilibrium has an absorption half-life of about 0.87 minutes (52 seconds).26 It means that the drug partition in the oral gel was absorbed by the oral mucosa.
Several factors that influence the fast or slow absorption of drugs on the mucosal surface are permeability and blood supply.27 The buccal mucosa is the part that has a high permeability making it easier for drugs to enter. The buccal mucosa has a thin layer of nonkeratinized epithelium consisting of the stratum basale, filamentous, and distendum which facilitates absorption of the drug substance into the epithelium. In addition, the buccal mucosa lacks the intercellular lamellar bilayer structure found in the stratum corneum and hence is more permeable. Another factor that increases tissue permeability is the rich blood supply in the oral mucosa, which causes the absorption of drugs from the oral cavity into the mucosal tissue to be faster.
This present study observed the body weight of rats before and after application. There are similarities between ulcer repair and weight gain. A significant increase in body weight in the F2 and positive control showed faster healing of ulcers, making it easier for rats to consume more food. The use of healthy rats aims to evaluate the effect of ulcers in the oral cavity purely based on the ability of rats to gain healing process without being affected by systemic diseases that can affect rat’s body weight. Areca nut is capable of repressing prostaglandin E 2 (PGE2) and arachidonic acid-induced inflammation, thereby helping to accelerate ulcer healing.28 The analgesic or antinociceptive activity of the areca nut is originated by inhibition of prostaglandin synthesis. The oral gel form was chosen to facilitate absorption in the oral mucosa and it has a rapid cool soothing effect, although the drawback is that the gel is easily soluble in saliva. Application twice a day in the morning and evening regularly showed a fairly good cicatrization process in both groups (F2 and positive control). The increase in body weight in the rat showed that in the last few days the diet was not disturbed by the size of the ulcer. In normal group, we also reported that there was a significant increase of body weight after feeding with areca nut. This is probably due to the areca nut extract’s ability to increase body weight. Its mechanism of action is that the main component of areca alkaloids is an inhibitor of the gamma-aminobutyric acid (GABA) receptor.29 The inhibitory effects of GABA receptor may increase appetite with eventual fat accumulation in general and central area of the body. Another mechanism that might play role in gain weight is that the areca nitrosamines derived from alkaloids may induce insulin resistance which can cause hyperglycemic conditions. Another study has reported that areca nut chewing has closely associated with metabolic syndrome, general obesity, and type-2 diabetes mellitus.30
Histological examination with HE staining on day seven showed no significant difference between all treatment groups, normal, positive, and negative control groups. The oral gel containing a balanced composition of areca nut and chrysanthemum, negative control, and normal groups did not show any ulcers and the epithelial tissue had been completely remodeled. Meanwhile, the other group still showed fibrosis and inflammation without ulcers. Fibrosis, persistent neutrophil infiltration, and poor angiogenesis in the ulcer base are factors involved in the underlying mechanism of delayed healing ulcers.31 The ulcer healing mechanism begins with ulcer base contraction which includes contraction of the ulcerated area, re-epithelization over the ulcer base, and formation of granulation tissue. The next step is epithelial regeneration and mucus secretion. This epidermal repair is regulated by a key growth factor (KGF) that is expressed at very high levels within 24 h of injury on dermal skin.32 The buccal mucosa is a non-keratinocyte layer, so it is probably the most involved in the re-epithelization process in the oral mucosa is salivary mucin. Mucin is the primary gel-forming component of mucus that provide a critical layer of protection on the wet epithelial surface including gastrointestinal, female genital, and respiratory tracts.33 Angiogenesis is the next step of ulcer healing. It allows the supply of nutrients and oxygen to the ulcerated area thereby giving the collagen in connective tissue and epithelium an opportunity to recover. Matrix formation is the last step of ulcer repair. This remodeling formation is regulated by the temporospatial expression of the fibrillar and basement membrane collagen (types I, III, IV), and matrix metalloproteinase-2 (MMP-2). Fibronectin is important for cell proliferation, adhesion, differentiation, and matrix formation.
In the present study, the analysis of TNF-α showed that the increase in the moderate expressions was showed by positive control and F2 groups. This is in line with research by Kobayashi et al. which showed that the water-soluble component in dried chrysanthemum has antioxidant ability to increase TNF-α. This activity was observed within 3 hours after ulcer formation.8 TNF-α is a cytokine produced primarily by immune cells such as monocytes/macrophages, but several cell types, such as T and B lymphocytes, natural killer cells, neutrophils, fibroblasts, and osteoclasts can also secrete TNF-α in smaller quantities.34 TNF-α levels which had the same score between F2 and positive control on the seventh day showed that the amount of this cytokine was still high detected in the local connective tissue. Prolonged or remaining elevated expression of TNF-α may occur for up to the third to the fifth day following the traumatic injury.35 Prolonged increase in TNF-α especially in delayed healing reflects their augmented blood TNF-α, suggesting an association between impaired healing and hard work TNF-α in the inflammatory response so that wound healing is achieved.36 In this study, we did not examine the level of TNF-α in the blood so that the role of areca nut-chrysanthemum oral gel to accelerate wound healing systemically cannot be explored.
TNF-α is a cytokine that has pleiotropic effects on various cell types. The pleiotropic effects mean that TNF-α can affect multiple systems or exert multiple pharmacological activities. It has been identified as a major regulator of inflammatory responses and is known to be involved in the pathogenesis of some inflammatory and autoimmune diseases. Although the precise role of TNF-α is not fully understood, among the pro-inflammatory cytokines, TNF-α is an important cytokine because it is quickly released and initiates inflammation in wound tissues. Ritsu et al. reported that TNF-α is involved in the early process of wound healing.37 TNF-α is released rapidly by vascular endothelial cells, keratinocytes, and fibroblasts in the lesion area and initiates the inflammatory phase by promoting inflammatory leukocyte recruitment into the wounded tissues. The major cellular sources of TNF-α are changed into recruited neutrophils and macrophages, and this process yields a positive amplification circuit for extending the inflammatory responses. An addition of inflammatory phase, TNF-α also plays a role during the proliferation phase in the wound healing process. TNF-α secreted from macrophages promotes the formation of the extracellular matrix in the wounded tissues by inducing the generation of proteoglycan and fibronectin by fibroblasts. This process is further enhanced by collagen synthesis from the proliferating fibroblasts, which are promoted by tumor growth factor (TGF)-β.
Triamcinolone acetonide was chosen as a positive control because this preparation has efficacy as a synthetic corticosteroid that has anti-inflammatory, antipruritic, and anti-allergic effects. This preparation has the advantage of being an emollient dental paste that can attach the drug to the oral mucosal tissue so that it is not easily soluble in saliva. An important role of steroids in triamcinolone acetonide is to alter the localization of high junction proteins thereby increasing the adhesion density between epithelial cells.38 A tight epithelial protective barrier can be achieved through tight cell adhesion. Contacts between adjacent cells are made up of tight junctions, adherens junctions, and desmosomes which help in preserving tissue homeostasis. This study showed that with the use of triamcinolone acetonide, F2, and F3 group on the seventh day, the ulcers had closed tightly without any inflammation. These results prove that there might be a possibility of areca nut–chrysanthemum oral gel also can close the adhesion bonds between epithelial cells so that the level of wound closure density which is similar to that of the group positive control (triamcinolone acetonide).
Collagenase analysis showed a lot of collagen deposition in the F3 group. The results of this study are in line with the fact that the alkaloids in areca nut are potent inducers to increase the amount of collagen. Previous research has shown that a series of synthetic arecaidine esters by oral mucosa fibroblast was closely correlated with the extent of collagen synthesis.39 Alkaloids in the areca nut in a long term play a major role in the accumulation of collagen in oral submucous fibrosis (OSF). Collagen is a key component of the extracellular matrix that plays a critical role in the regulation of the phases of wound healing. Collagen exposure due to injury in the inflammatory phase will activate the clotting cascade, resulting in a fibrin clot that stops the initial bleeding. It also acts as potent chemoattractants for neutrophils, enhancing phagocytosis and immune response and modulating gene expressions. Collagen promotes anti-inflammatory, proangiogenic wound macrophage phenotype via microRNA signaling. In the angiogenesis phase, collagen stimulates angiogenesis in vitro and in vivo through the engagement of specific integrin receptors. The C-propeptide fragment of collagen I recruit endothelial cells, potentially triggering angiogenesis in the healing wound.40
The results of the evaluation of collagen deposition after treatment, with picrosirius red staining, showed a picture of collagen with thicker density, large fiber size, and lots of collagen fibrils in the oral gel with higher composition of areca nut which consisted 80% of areca nut. This shows the good work of collagen to repair ulcers. Although the result of the collagenase assay has a significant p value, the range in the confidence interval (CI) is very wide. It indicates that these results have great uncertainty about the effect of the extract content in the gel for ulcer healing.11 Further research is needed by using a larger sample size so that it can produce narrower estimated CIs and the estimated effect could become more reliable.
The present study provides the activity of the healing effect of combination areca nut and chrysanthemum. This oral gel can improve ulcer wound healing through the role of TNF-α and collagen with the same results as topical steroid therapy.
Dryad: Underlying data for ‘Evaluation of clinical, histology, TNF-α, and collagen expressions on oral ulcer in rats after treatment with areca nut and chrysanthemum oral gel’. https://doi.org/10.5061/dryad.8w9ghx3mw.
The project contains the following underlying data: body weight, oral ulcer’s size, histology and tnf-α score, collagen percentage, and Author checklist-E10 ARRIVE.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology of Traditional Chinese Medicine
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cardiovascular Pharmacology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pathology, histology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: pathology, histology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Gandevia S, Cumming G, Amrhein V, Butler A: Replication: Do not trust your p‐value, be it small or large. The Journal of Physiology. 2021; 599 (11): 2989-2990 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical Method Development and its Validation; Natural Product Chemistry, Herbal Drugs
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 7 (revision) 12 Sep 25 |
read | |||
|
Version 6 (revision) 06 Sep 23 |
||||
|
Version 5 (revision) 23 Nov 22 |
read | |||
|
Version 4 (revision) 17 Oct 22 |
read | |||
|
Version 3 (revision) 19 May 22 |
||||
|
Version 2 (revision) 19 Oct 21 |
read | read | ||
|
Version 1 21 Jul 21 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
1. For example, it is better to have a simple model group.
Answer: The research was conducted with 6 groups for the histopathology, collagenase, ... Continue reading The comments for the Version 5:
1. For example, it is better to have a simple model group.
Answer: The research was conducted with 6 groups for the histopathology, collagenase, and immunohistochemistry analysis ( All groups showed differences in response to the ulcer healing formation. :
- Treatment group (3 groups)
- Positive control group
- Negative control group
- Normal/Standard group (without ulcer and treatment)
2. It is better to provide the appraiser and Latin of betel nut and chrysanthemum for research; There should also be the extraction method of chrysanthemum.Answer:
- We chose to put names that are commonly heard in the article because we want to make it easier for readers to recognize or remember the types of plants we used in the research. We do not show the pictures of betel nut and chrysanthemum flowers in the article, so we want to make sure that the reader can recognize the plants we use in the study. The use of the word betel nut also refers to a term that is popular and commonly used in society, as well as chrysanthemum flower petals. We've written the Latin name in the background and methods section.
- The extraction method of chrysanthemum: We have added in the manuscript (Extract preparation method section)
3. The weight range of rats is a little large, generally the same experiment does not exceed 20 grams.Answer:
Thank you for your detailed correction. We have submitted the body weight data in Dryad. The weight of the rat is between 115-128 g. The range is 13 g. In the previous data (200-240), we got this information first from the laboratory assistant in the animal center before he bought the rats. Underlying data for ‘Evaluation of clinical, histology, TNF-α, and collagen expressions on an oral ulcer in rats after treatment with areca nut and chrysanthemum oral gel’. https://doi.org/10.5061/dryad.8w9ghx3mw
4. The level and certificate number of rats shall be provided; Provide the license number of rat animal center; Provide the source and batch number of main reagents and drugs.
Answer:
1. We have asked the Tropical Lab about the license number of the rats, but they did not give me the license number.
2. We added the reagent specification for immunohistochemistry analysis. We added the batch number of PBS, H&E staining solution, and Picro-Sirius red solution.5.
5. There are 12 in each group in the method, why is the result 18?
Answer:
In our research, we divided the rats into 6 treatment groups consisting of 12 rats per group. The number of 18 does not indicate the number of rats in each group but indicates the number of biopsy specimens examined. So we took 3 biopsy specimens from the buccal mucosa of rats in each group. We have added the word “specimens” to avoid this confusion. Thank you for the correction.
6. Pathology is best to have semi-quantitative results; The basis for sample selection, extraction process and sample quantity in the process of immunohistochemistry should be supplemented.
Answer:
Determination of the minimum number of samples is done by using the Federer formula and followed by the Resource Equation Method formula. Thank you for the correction, we added in the manuscript
7. It is reported in the literature that long-term use of betel nut may cause oral cancer. Why should we choose betel nut to treat oral ulcer?
Answer:
Among the many benefits of consuming areca nuts, other studies have shown that these nuts are carcinogenic because they can cause oral malignancies.1 Areca nuts actually do not contain carcinogens, however, this carcinogenic effect is caused by nitrosamine products produced by the nitrosation process by alkaloids (arecoline) from dry areca nut when chewed and digested in the stomach under acidic conditions in the long term and uncontrolled.2 The combination with nitric oxide produced by bacteria causes the formation of a substance methylnitrosaminoproprionitrile which is proven to cause carcinogenesis in animal tests. 2 This endogenous nitrosation is significantly higher especially significant in patients with poor oral hygiene. 3 If areca nut is mixed with tobacco and then chewed by people with poor oral hygiene, a large amount of nitrosamine product will occur.4 This process occurs continuously over a long period of time because these nuts have addictive properties. Several studies have also reported an increase in reactive oxygen species such as hydroxyl oxide in the oral cavity due to the combination of auto-oxidation of polyphenols from areca seeds with a high alkaline lime pH.5 If areca nuts are chewed together with betel leaf and lime, these two ingredients will cause mucosa membrane erosion, so that the carcinogens can penetrate more easily into cells through the mucosa. 6 Some people in India and Pakistan use factory-packaged areca seeds called gutka and pan masala. Gutka consists of areca nut, betel leaves, tobacco, and lime, while pan masala is made without tobacco. About 40% of these gutka and pan masala packages have been contaminated with carcinogenic aflatoxins from Aspergillus flavus, Aspergillus niger, and Rhizopus spp.7
Although several studies have shown a carcinogenic effect of areca nut, there is other literature proves the presence of high antioxidant activity in areca nut.8
References:
- Zain R. Cultural and dietary risk factors of oral cancer and pre-cancer: A brief overview. Oral Oncol 2001;37:205-10.
- Bhisey RA, Boucher BJ, Hsi-Chen TH, Gajalakshmi V, Gupta PC, et al. Monographs on the Evaluation of Carcinogenic Risks to Humans.Betel-Quid and Areca-Nut Chewing and some Areca-Nut Related Nitrosamines. IARCS Monographs 2004;85:1-293.
- Nagao T, Ikeda N, Warnakulasuriya S, Fukano H, Yuasa H, et al. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol 2000;36:466-70.
- Carossa S, Pera P, Doglio P, Lombardo S, Colagrande P, et al. Oral nitric oxide during plaque deposition. Eur J Clin Invest 2001;31:876-9.
- Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis 2004;19:251-62.
- Johnson NW, Jayasekara P, dan Amarasinghe HK. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 2000. Singapura: John Wiley and Sons; 2011.
- Anttila A, Bhat RV, Bhond JA, Bhorgoff SJ, Bosch FX, et al. Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARCS Monographs 2002;82:171-4.
- Xing Z, Jiao W, Zhuang H, Li MW, Fu DH. Antioxidant and cytotoxic phenolic compounds of areca nut (Areca catechu L.). Chem Res Chinese Universities 2010;26(1):161-4.
8. The discussion of the experimental results can be divided into categories and appropriately compressed.Answer:
We've removed some of the sentences we felt were less effective as reviewers instructed. We have divided the Result arrangement into:
1. For example, it is better to have a simple model group.
Answer: The research was conducted with 6 groups for the histopathology, collagenase, and immunohistochemistry analysis ( All groups showed differences in response to the ulcer healing formation. :
- Treatment group (3 groups)
- Positive control group
- Negative control group
- Normal/Standard group (without ulcer and treatment)
2. It is better to provide the appraiser and Latin of betel nut and chrysanthemum for research; There should also be the extraction method of chrysanthemum.Answer:
- We chose to put names that are commonly heard in the article because we want to make it easier for readers to recognize or remember the types of plants we used in the research. We do not show the pictures of betel nut and chrysanthemum flowers in the article, so we want to make sure that the reader can recognize the plants we use in the study. The use of the word betel nut also refers to a term that is popular and commonly used in society, as well as chrysanthemum flower petals. We've written the Latin name in the background and methods section.
- The extraction method of chrysanthemum: We have added in the manuscript (Extract preparation method section)
3. The weight range of rats is a little large, generally the same experiment does not exceed 20 grams.Answer:
Thank you for your detailed correction. We have submitted the body weight data in Dryad. The weight of the rat is between 115-128 g. The range is 13 g. In the previous data (200-240), we got this information first from the laboratory assistant in the animal center before he bought the rats. Underlying data for ‘Evaluation of clinical, histology, TNF-α, and collagen expressions on an oral ulcer in rats after treatment with areca nut and chrysanthemum oral gel’. https://doi.org/10.5061/dryad.8w9ghx3mw
4. The level and certificate number of rats shall be provided; Provide the license number of rat animal center; Provide the source and batch number of main reagents and drugs.
Answer:
1. We have asked the Tropical Lab about the license number of the rats, but they did not give me the license number.
2. We added the reagent specification for immunohistochemistry analysis. We added the batch number of PBS, H&E staining solution, and Picro-Sirius red solution.5.
5. There are 12 in each group in the method, why is the result 18?
Answer:
In our research, we divided the rats into 6 treatment groups consisting of 12 rats per group. The number of 18 does not indicate the number of rats in each group but indicates the number of biopsy specimens examined. So we took 3 biopsy specimens from the buccal mucosa of rats in each group. We have added the word “specimens” to avoid this confusion. Thank you for the correction.
6. Pathology is best to have semi-quantitative results; The basis for sample selection, extraction process and sample quantity in the process of immunohistochemistry should be supplemented.
Answer:
Determination of the minimum number of samples is done by using the Federer formula and followed by the Resource Equation Method formula. Thank you for the correction, we added in the manuscript
7. It is reported in the literature that long-term use of betel nut may cause oral cancer. Why should we choose betel nut to treat oral ulcer?
Answer:
Among the many benefits of consuming areca nuts, other studies have shown that these nuts are carcinogenic because they can cause oral malignancies.1 Areca nuts actually do not contain carcinogens, however, this carcinogenic effect is caused by nitrosamine products produced by the nitrosation process by alkaloids (arecoline) from dry areca nut when chewed and digested in the stomach under acidic conditions in the long term and uncontrolled.2 The combination with nitric oxide produced by bacteria causes the formation of a substance methylnitrosaminoproprionitrile which is proven to cause carcinogenesis in animal tests. 2 This endogenous nitrosation is significantly higher especially significant in patients with poor oral hygiene. 3 If areca nut is mixed with tobacco and then chewed by people with poor oral hygiene, a large amount of nitrosamine product will occur.4 This process occurs continuously over a long period of time because these nuts have addictive properties. Several studies have also reported an increase in reactive oxygen species such as hydroxyl oxide in the oral cavity due to the combination of auto-oxidation of polyphenols from areca seeds with a high alkaline lime pH.5 If areca nuts are chewed together with betel leaf and lime, these two ingredients will cause mucosa membrane erosion, so that the carcinogens can penetrate more easily into cells through the mucosa. 6 Some people in India and Pakistan use factory-packaged areca seeds called gutka and pan masala. Gutka consists of areca nut, betel leaves, tobacco, and lime, while pan masala is made without tobacco. About 40% of these gutka and pan masala packages have been contaminated with carcinogenic aflatoxins from Aspergillus flavus, Aspergillus niger, and Rhizopus spp.7
Although several studies have shown a carcinogenic effect of areca nut, there is other literature proves the presence of high antioxidant activity in areca nut.8
References:
- Zain R. Cultural and dietary risk factors of oral cancer and pre-cancer: A brief overview. Oral Oncol 2001;37:205-10.
- Bhisey RA, Boucher BJ, Hsi-Chen TH, Gajalakshmi V, Gupta PC, et al. Monographs on the Evaluation of Carcinogenic Risks to Humans.Betel-Quid and Areca-Nut Chewing and some Areca-Nut Related Nitrosamines. IARCS Monographs 2004;85:1-293.
- Nagao T, Ikeda N, Warnakulasuriya S, Fukano H, Yuasa H, et al. Serum antioxidant micronutrients and the risk of oral leukoplakia among Japanese. Oral Oncol 2000;36:466-70.
- Carossa S, Pera P, Doglio P, Lombardo S, Colagrande P, et al. Oral nitric oxide during plaque deposition. Eur J Clin Invest 2001;31:876-9.
- Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis 2004;19:251-62.
- Johnson NW, Jayasekara P, dan Amarasinghe HK. Squamous cell carcinoma and precursor lesions of the oral cavity: epidemiology and aetiology. Periodontology 2000. Singapura: John Wiley and Sons; 2011.
- Anttila A, Bhat RV, Bhond JA, Bhorgoff SJ, Bosch FX, et al. Monographs on the Evaluation of Carcinogenic Risks to Humans. Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene. IARCS Monographs 2002;82:171-4.
- Xing Z, Jiao W, Zhuang H, Li MW, Fu DH. Antioxidant and cytotoxic phenolic compounds of areca nut (Areca catechu L.). Chem Res Chinese Universities 2010;26(1):161-4.
8. The discussion of the experimental results can be divided into categories and appropriately compressed.Answer:
We've removed some of the sentences we felt were less effective as reviewers instructed. We have divided the Result arrangement into: