Keywords
cadmium tolerance, flow cytometry, ICP-OES analysis, DUF2256 domain, heavy metal, microalgae
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Plant Science gateway.
cadmium tolerance, flow cytometry, ICP-OES analysis, DUF2256 domain, heavy metal, microalgae
This revised version had changes incorporating the reviewers’ recommendations. In this version the clarifications on the amount and kinds of replication were added in the figure legends and in the methods. In this version there were changes done in order to improve figure quality. More specifically in terms of figure quality: Figures 1 and 2 are no longer line graphs, but changed to bar plots. The description was changed to arbitrary units, the y-axis was labeled Chlorophyll fluorescence (a.u.) and Cell size (a.u.) in Figures 1 and 2, respectively. In Figure 3 just Cd2+ (ppb) was used and the word “measured” was removed. The x-axis labels in Figures 1 and 2 are changed to CdCl2 (µM) instead of concentration (uM). In Figure 3 the x-axis is changed to CdCl2 (µM). The labels of the strains, instead of just 1 and 2, were changed to wild-type (CC-4425) and cia7 (CC-5013) in both Figures 1 and 2. With regards, to the comment on the effect of other metals, here in this revised version the results of a preliminary study done in our lab have been included in the discussion section. More specifically, we reported that the metal accumulation in strains cc4425 and cc5013 conducted by Vazquez et al. (2018) using Pb 2+ in the form of Pb (NO 3) 2 at concentrations 0 μM and 150 μM showed no significant differences in the bioaccumulation of Pb 2+ as measured analytically by ICP-OES. This revised version also has additional recommendations for future studies. The affiliations for the two co-authors have been revised in order to add a new affiliation.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Public health and environmental concerns from heavy metal contamination have increased in recent times. In fact, among all the pollutants, heavy metals have received particular attention due to their toxic nature, persistence in the environment, and bioaccumulative nature (Ali et al., 2019; Al Osman et al., 2019). Heavy metals are naturally occurring elements that have high atomic weights and densities at least five times greater than that of water. Arsenic, cadmium (Cd), chromium, lead, and mercury are among the priority metals of public health significance; this, in part, is due to their high degree of toxicity. These metals are systemic toxicants known to induce multiple organ damage, even at lower levels of exposure (Sevin et al., 2020; Tchounwou et al., 2012).
Heavy metals are known to be naturally occurring elements; however, anthropogenic activities introduce them in large quantities in the environment (Briffa et al., 2020). The presence of heavy metals in nature can arise from environmental processes like volcanic emissions and rock weathering (Jan et al., 2015; Mason et al., 2021). On the other hand, anthropogenic activities include mercury (Hg) contamination from gold mining, lead (Pb) from its use as a gasoline additive, and Cd from fertilizers, batteries, and sewage water (Sevim et al., 2020; Mashhadikhan et al., 2022). Human activities are contaminating the environment and its resources; these activities are discharging heavy metals at concentrations greater than what the environment can handle (Masindi and Muedi, 2018).
The final deposition points of heavy metal leachate are often aquatic environments, where microalgae are widely distributed and therefore exposed to these metals. Literature evidence suggests that toxic heavy metals may induce deleterious damage to the metabolism and physiology of microalgae (Xie et al., 2019; Abu Bakar et al., 2015). The damage that heavy metals exert at the cellular level is composite and targets different mechanisms. For example, intracellular heavy metal ions induce a variety of biochemical changes that affect the normal growth and metabolic activity of the cell system. These changes include blocking the functional groups of biomolecules, displacing essential metal ions, and modifying the native conformations of biomolecules (Priyadarshini et al., 2019). Heavy metals are also known to interfere and inhibit protein activity. Proteins whose active domains possess sulfhydryl groups are especially susceptible to deleterious effects of heavy metals due to their affinity with them, resulting in irreversible binding (Fein et al., 2019; Hua and Li, 2014). In addition, heavy metals can interfere with metalloproteins or metal-protein complexes by competing with an essential metal cofactor for an enzyme’s binding site (Pinter and Stillman, 2015; Viehweger, 2014). A reported symptom of heavy metal toxicity in a cell is an increase in its oxidative stress, marked by an increase in reactive oxygen species (ROS) (Elbaz et al., 2010; Jiang et al., 2016). ROS, due to their oxidative character, damage cellular structures and macromolecules by interfering with non-covalent interactions.
To study the deleterious effects of heavy metals and their interactions within biological systems, eukaryotic microalgae have been deemed appropriate subjects (Leong and Chang, 2020; Miazek et al., 2015). Microalgae have evolved resistance to heavy metal stress through their own metabolic pathways, which include metal immobilization by cell walls and extracellular polymeric substances (EPS), and transport through the cell membrane via transporters (Lu et al., 2019; Liu et al., 2016). It is this acclimatization that makes microalgae suitable candidates for heavy metal studies; they can survive relatively high metal concentrations, enabling the observations and analysis of heavy metal-induced effects.
Chlamydomonas reinhardtii is a unicellular green alga from the Chlorophyta lineage, and is the best-developed reference organism for studying algal biology, especially with respect to metal metabolism (Blaby-Haas and Merchant, 2012). C. reinhardtii has garnered scientific interest in the context of heavy metal toxicity due to its broad range of heavy metal resistance and tolerance (Zheng et al., 2020; Ibuot et al., 2017; Nowicka et al., 2016). C. reinhardtii is also a potential candidate for phytoremediation, the process of utilizing plants and algae to bioremediate contaminated areas (Leong and Chang, 2020).
In C. reinhardtii, a primary line of defense against heavy metals is the cell wall. The cell wall displays a high affinity towards metallic cations, and cell wall-less mutants commonly exhibit greater sensitivity towards metals (McFie et al., 1994; Collard and Matagne, 1990). Cell walls contain multiple negatively charged groups that form interactions with metal cations, thus sequestering metals and preventing metal uptake into the cytoplasm (Priyadarshini et al., 2019; Samadani et al., 2018; Hanikenne, 2003). Whereby, intracellularly, heavy metals are compartmentalized in vacuoles, allowing the cell to control the cytoplasmic metallic concentration, and neutralizing the heavy metal’s toxic effect (Perales-Vela et al., 2006). Mechanisms of heavy metal tolerance in C. reinhardtii also include transporters. For example, the mitochondrial half-size ABC (ATP-binding cassette) transporter is implicated in Cd-tolerance (Hanikenne et al., 2005). Heavy metal-binding peptide synthesis has also been shown to be preferentially developed in microalgae and some fungi, thus granting these organisms a higher metal tolerance (Perales-Vela et al., 2006).
C. reinhardtii showed various strategies to cope with Cd stress. Penen et al. (2017) previously reported that most Cd could diffuse in the whole cell, and complexed by thiol ligands. Thiol ligands were also shown to increase with cells’ exposure time to Cd, confirming thiol ligands’ important role in Cd stress regulation. There is also pyrenoidal sequestration of Cd, leading to limited CO2 fixation during Cd exposure (Penen et al., 2019).
Metal tolerance studies including Cd in C. reinhardtii have been extensively reported in the literature. In reported studies of exogenous metallothionein gene expression in C. reinhardtii, Cd was reported as an inducer. The generation and characterization of Cd-resistant and hypersensitive C. reinhardtii mutants have also been reported in studies of heavy metal tolerance (Samadani et al., 2018; Hanikenne, 2003; Hu et al., 2001). In particular, the overexpression of metal transporters in C. reinhardtii for bioremediation is an attractive approach; it may allow both increased metal accumulation into the cell and increased metal tolerance, such as by transfer of a toxic metal out of the cytosol and into an internal compartment (Ibuot et al., 2020; Ibuot et al., 2017). The genetic manipulation of C. reinhardtii has been shown to be successful in creating stable, inherited phenotypes (Pollock et al., 2017). Changes in the heavy metal biosorption capacity of C. reinhardtii have been reported in genetically modified strains (Ibuot et al., 2020; Cheng et al., 2019; Aksmann et al., 2014; Lin et al., 2013; Mayfield et al., 2007; Shimogawara et al., 1998).
A novel gene, Cia7, was isolated from an inorganic carbon-acquiring, and high carbon dioxide-requiring C. reinhardtii mutant, cc5013 (Ynalvez and Moroney, 2008). The gene designated as Cia7 was hypothesized to play a role in heavy metal tolerance or metal detoxification due to its translational product’s amino acid sequence. Sequence alignment utilizing the U.S. National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST, RRID:SCR_004870) revealed homology with many uncharacterized proteins conserved in bacteria; proteins classified as belonging to a family of DUF2256 domain-containing proteins. Members of this family of hypothetical bacterial proteins have domains of unknown functions (DUF). Homologous proteins also included computer modeling predicted proteins, TRAP transporter small permease (92% identity) and an uncharacterized metal binding protein (72% identity). The protein sequence of CIA7 contained a conserved, repeated motif of four cysteine residues whose thiol functional group is hypothesized to chelate heavy metals (Ynalvez and Moroney, 2008). Based on this observation, the aim of this study was to investigate whether Cia7 plays a potential role in metal tolerance in C. reinhardtii.
This study compared two strains of C. reinhardtii: cc4425, the wild-type with the functional CIA7 protein and cc5013, the strain that has a disruption in the Cia7 gene due to a mutant cia7-. It is hypothesized that CIA7, due to its conserved cysteine residue motif, could play a role in the molecular defense mechanism to attenuate heavy metal toxicity. More specifically, when subjected to metal (Cd2+) stress, C. reinhardtii cc4425 cells that express a functional CIA7 would exhibit higher growth rate and chlorophyll fluorescence, but a decreased metal bioaccumulation when compared to the mutant cc5013. The objectives of this study were to compare cell size, chlorophyll fluorescence, and metal (Cd2+) bioaccumulation between the two strains cc4425 (wild-type) and cc5013 (mutant). Flow cytometry was used in this study. This technique rapidly analyzes single cells or particles as they flow past single or multiple lasers while suspended in a buffered salt-based solution (McKinnon, 2019). Flow cytometers are suitable for microalgae applications as the latter are often a collection of single cells, and microalgae possess endogenous pigments which autofluoresce, making immediate measurements possible, without the need for pre-treatment of cells (Jamers et al., 2009). Flow cytometry analyses in metal studies using C. reinhardtii are relatively scarce. Therefore, this study will also provide a framework for future, related studies. In addition, the results of this study could provide a basis for the further investigation on the role of CIA7 in C. reinhardtii’s heavy metal tolerance or the further investigation on the potential use of Cia7 expression as a biomarker for heavy metal contamination.
C. reinhardtii strains cc4425 (wild-type) and cc5013 (mutant cia7-) were obtained from the Chlamydomonas Resource Center in the University of Minnesota. Cells were maintained as stocks in 1.5% Tris-acetate-phosphate (TAP) agarose solid media (Harris, 1989), stored at room temperature and subcultured every seven days. Liquid cultures were initiated in 50 mL of TAP medium by using two loopfuls (4 mm spherical scraping) of cells for inoculation. Culture flasks were placed in an orbital shaker (VWR DS2-500-1) at 130 rpm under normal fluorescent room lighting until cultures reached optical density (OD650) of 0.800 +/- 0.050, measured using a Spectronic Genesys 8 spectrophotometer. This OD650 of 0.800 has been calibrated to be equal to 1.0-3.0 × 106 cells/mL, which is log phase in C. reinhardtii.
Cells from both strains were cultured in liquid TAP to an OD650 = 0.400 and subjected to their respective metal treatments (CdCl2 at concentrations of 0 μM, 5 μM and 10 μM). Cd treatments in ¼X TAP amounted to a 50 mL final volume. Treatment flasks were prepared and cultured in replicates, one of them serving as the sampling culture flask, and the other as the supplemental culture flask. From the sampling culture flask, 900 μL of cells were taken for analysis, and the sampling flask was replenished with 900 μL of cells from the supplemental culture flask. The replenishment step was done in order to maintain the same volume of cells in the sampling culture flask throughout the 96-hour period. Samples for flow cytometry analysis were diluted 5:1 in saline buffer. Chlorophyll intensity level and cell size were measured using a Cytek DxP8 FACSCalibur Flow Cytometer acquiring 10,000 events. Chlorophyll intensity level was determined by utilizing laser BluFL3 (Cytek) with a wavelength filter 695/40 nm. Cell size was determined as a function of the forward scatter signal (FSC). Cell cultures cc4425 and cc5013 were treated with 0, 5, and 10 μM CdCl2 at midlog phase (OD650 = 0.400). Samples were analyzed at 24-hour time points for a duration of 96 hours via flow cytometry.
Each of the 24 treatment combinations in this 2 × 3 × 4 factorial experiment has two replications. This factorial arrangement of treatment combinations was the result of two levels of strain (cc5013, cc4425), three levels of Cd concentration (0 μM, 5 μM, and 10 μM), and four levels of time (1, 2, 3, and 4 days). In this experiment a block comprised only 1 replicate: each treatment combination appears only once in a block; thus this experiment has two blocks or two replicates. An analysis of variance (ANOVA) associated with a 2 × 3 × 4 factorial experiment in randomized complete block design was performed for data analysis.
To compare significant main and interaction effects, post-hoc tests in the form of least squares means comparison were performed using the PROC GLM tool of the SAS 9.4 (Statistical Analysis System, RRID:SCR_008567) statistical software. The usual levels of type-I error rates were used (i.e., * if p < 0.05, ** if p < 0.01 and *** if p < 0.001).
C. reinhardtii strains were cultured in liquid media. Cells were kept under constant movement (130 rpm) in an orbital shaker until the cultures reached an OD650 of 0.800 +/- 0.050 as determined by a Spectronic Genesys 8 spectrophotometer. An absorbance of OD650 = 0.800 was calibrated to be equal to 1.0-3.0 × 106 cells/mL (log phase in C. reinhardtii) (Lin et al., 2013; Kirst et al., 2012). Results from preliminary experiments established the sub-lethal Cd concentration of cc4425 and cc5013 at 10 μM. In this regard, cells were harvested and cultured in media treated with CdCl2 at concentrations of 0 μM and 10 μM (0 ppb and 1830 ppb) to a final volume of 150 mL. Two different growth media were used: a minimal (Min) medium (Harris, 1989) and a diluted, by a factor of ¼, TAP medium (Harris, 1989). Phosphorus has been reported as reducing the bioavailability of free metal ions due to its chelating character; therefore, the TAP medium was diluted (Nowicka et al., 2016). Similarly, the Min medium was used to determine whether a difference existed in C. reinhardtii’s metal bioaccumulation between TAP and the acetate-deprived Min media.
Metal treatment flasks were washed with 1% HNO3 to prevent metal cross-contamination among replicates. The treatment period was 4 days, after which cells reached the logarithmic phase of growth. On the 4th day, cells were equilibrated at OD650 = 0.800 (an absorbance at log phase) and harvested by centrifugation at 3,000 rpm for 10 minutes. The pellets were washed twice with millipore H2O, then resuspended, followed by centrifugation at 3,000 rpm for 5 minutes to rinse pellets of extracellular metals. Washed pellets were desiccated at 100°C, and either frozen at −80°C or used for downstream analysis. Dry pellets were acidified with 5 mL of HNO3, incubated for 20 minutes, and digested in a MARS 6 microwave following the machine’s internal protocol for plant material. Following digestion, samples were diluted 1:10, and analyzed for Cd, by ICP-OES (Agilent Varian 720-ES).
Each of the eight treatment combinations in this 2 × 2 × 2 factorial experiment has three replications. This factorial arrangement of treatments was the result of two levels of strain (cc5013, cc4425), two levels of media (¼X TAP and Min), and two levels of Cd concentration (0 ppb and 1830 ppb). To analyze the data, an analysis of variance (ANOVA) associated with a 2 × 2 × 2 factorial experiment in randomized complete block design was performed. To compare significant main and interaction effects, a post-hoc test in the form of least squares means comparison was performed using the PROC GLM tool of the SAS 9.4 (Statistical Analysis System, RRID:SCR_008567) statistical software. To further explore the nature of a significant interaction between medium and concentration, a t-test comparing concentration levels was performed for each medium level. The usual levels of type-I error rates were used (i.e., * if p < 0.05, ** if p < 0.01 and *** if p < 0.001).
A statistically significant difference in chlorophyll fluorescence was observed between cc4425 and cc5013 (6,127 and 3,858 counts respectively) at 10 μM Cd (Table 1, Figure 1). However, at the control treatment and at 5 μM treatment, cc4425 and cc5013 chlorophyll signals at BluFL3 were not statistically significantly different from each other (Figure 1). It is interesting to note that as the Cd concentration increased, chlorophyll intensity increased for wild-type strain cc4425, while chlorophyll intensity decreased for cia7- mutant cc5013 (Figure 1). This negative trend in chlorophyll intensity level might be indicative of heavy metal toxicity, to which cc5013, whose Cia7 gene has been disrupted, might be more susceptible, including Cd susceptibility, compared to the wild-type cc4425.
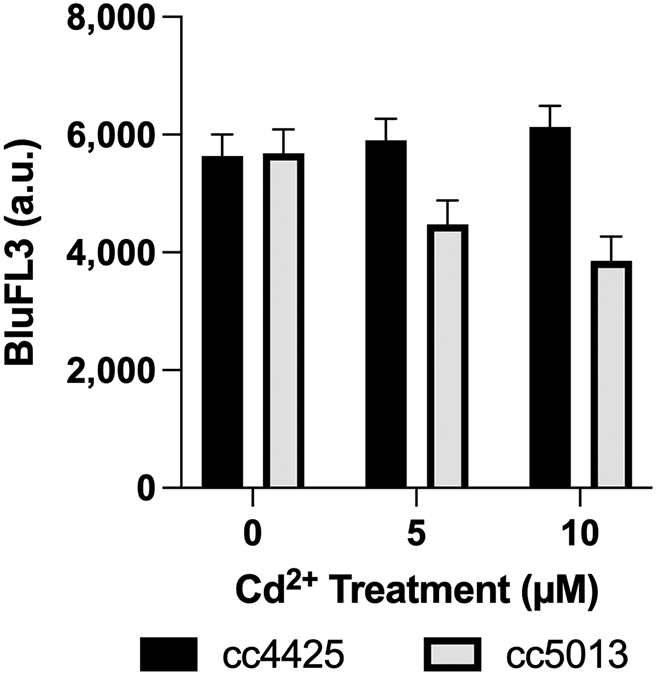
The data were generated from a 2 × 3 × 4 factorial experiment in randomized complete block design. The factorial arrangement of treatments was the result of two levels of strain (cc5013, cc4425), three levels of Cd concentration (0 μM, 5 μM, and 10 μM), and four levels of time (1, 2, 3, and 4 days). The bar graph depicts the strain × concentration (S×C) means. Error bars represent 95% confidence intervals. Statistical analyses of measurements are displayed in Table 1.
Cell size was determined as a function of the forward scatter signal (FSC) (Figure 2). A similar empirical trend in the strains was observed in cell size as measured by the FSC. The effect of Cd on cell size was determined to be statistically significant between strains (p < 0.0001) at all concentrations (Table 2, Figure 2). The mutant, cc5013 was observed to have greater cell size than wild-type cc4425 especially under 0 μM Cd2+. Although not statistically significant (p > 0.05), cc4425 showed an increase in cell size with increase in cadmium concentration, while cc5013 showed a decrease in cell size (Figure 2).
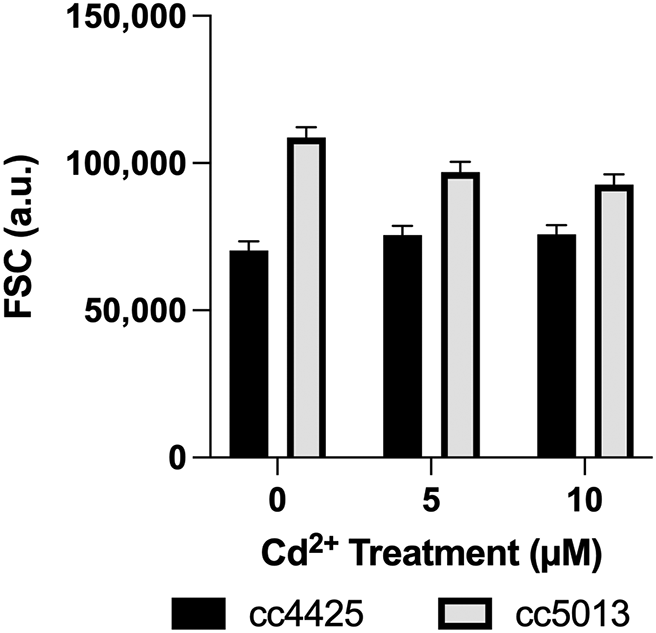
The data were generated from a 2 × 3 × 4 factorial experiment in randomized complete block design. The factorial arrangement of treatments was the result of two levels of strain (cc5013, cc4425), three levels of Cd concentration (0 μM, 5 μM, and 10 μM), and four levels of time (1, 2, 3, and 4 days). The bar graph depicts the strain × concentration (S×C) means. Error bars represent 95% confidence intervals. Statistical analyses of measurements are displayed in Table 2.
This bioaccumulation experiment aimed to investigate the interactions between the in vivo activity of CIA7 in differential Cd2+ concentrations (0 and 1830 ppb [10 μM])), in either a mixotrophic (¼X TAP) or photoautotrophic (Min) growth medium. Although the results are not indicative of a statistically significant correlation, the observations are nevertheless interesting. Due to the lack of cc5013 characterization studies and CIA7’s as-of-yet unknown function, it is difficult to predict what metabolic and physiological changes Cd2+ exposure could cause, and in which range. The factorial arrangement of treatments was the result of two levels of strain (cc5013, cc4425), two levels of medium type (¼X TAP and Min), and two levels of Cd concentration (0 and 1830 ppb). No significant statistical difference (p < 0.5905) was established between Cd2+ bioaccumulation in cc4425 and cc5013, regardless of growth medium (Table 3). In ¼ X TAP, cc4425’s Cd2+ content was measured as 128.63 ppb, and in cc5013 as 155.83 ppb. In Min, cc4425’s Cd2+ content was measured as 28.63 ppb, and cc5013’s as 29.14 ppb.
To further explore the nature of a significant interaction between medium and concentration, a t-test comparing concentration levels was performed for each medium level. A statistically significant difference was determined in the media by concentration effect (p < 0.0001) (Table 4). There was a significant difference in cells’ bioaccumulation of Cd2+ in 10 μM Cd (1830 ppb) treated ¼ X TAP (142.22 ppb) when compared to Cd2+ bioaccumulation in Min (28.88 ppb). The data represents pooled data from both strains, wild-type and mutant. On the other hand, as expected, there was no significant difference between ¼ X TAP and Min (14.53 ppb and 14.44 ppb respectively) at 0 ppb Cd (Table 4 and Figure 3). The main difference between the two media is the presence of acetate and phosphate in ¼ X TAP and their absence in Min. The pH of the two media were adjusted to pH 7.0. As previously mentioned, the TAP medium was diluted by a factor of ¼, as phosphorus has been reported as reducing the bioavailability of free metal ions due to its chelating character (Olaniran et al., 2013).
| Concentration | DF | SS | MS | Fc | p-value |
|---|---|---|---|---|---|
| ¼X TAP versus Min at 0 ppb | 1 | 0.04 | 0.04 | 0.00 | 0.9963 |
| ¼X TAP versus Min at 1830 ppb | 1 | 77077.0 | 77077.0 | 39.19 | <0.0001 |
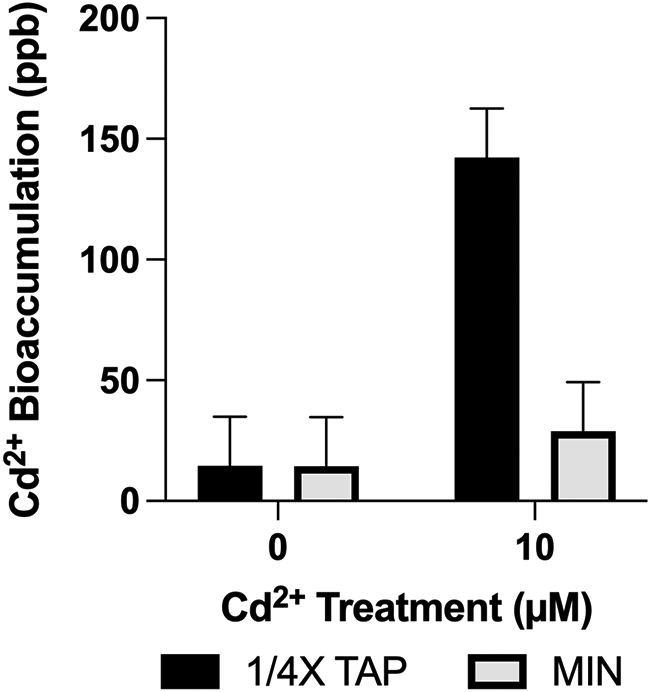
Statistical analyses of measurements are displayed in Table 4. The data were generated from a 2 × 2 × 2 factorial experiment in randomized complete block design. The factorial arrangement of treatments was the result of two levels of strain (cc5013, cc4425), two levels of media (¼X TAP and Min), and two levels of Cd concentration 0 ppb [0 μM] and 1830 ppb [10 μM]). In this experiment there were 3 blocks. ANOVA results showed no significant statistical difference (p < 0.5905) between Cd2+ bioaccumulation in cc4425 and cc5013, regardless of growth medium. In this regard, data represents pooled data from both strains. Error bars represent 95% confidence intervals.
A statistically significant difference in chlorophyll fluorescence was observed between cc4425 and cc5013 (6,127 and 3,858 counts respectively) at 10 μM Cd (Figure 2). A possible mechanism behind the phenomenon reported in cc5013 is a decrease in chlorophyll intensity level because of Cd substituting magnesium in chlorophyll, leading to the decrease in chlorophyll intensity (Grajek et al., 2020; Rydzyński et al., 2019; Kupper et al., 1996). The apparent absence of this phenomenon in cc4425 at the Cd concentrations used in this study might be, at least in part, contributed to by the biological activity of CIA7. This observed difference in chlorophyll fluorescence between the strains suggests that CIA7’s biological activity could play a direct or indirect role in increasing Cd tolerance in C. reinhardtii.
Although not statistically significant, cc4425 showed an increase in cell size with an increase in Cd concentration, while cc5013 showed a decrease in cell size. The trend observed in cell size in cc4425 is partly supported by similar findings in Jamers et al. (2013), in which at 5 μM Cd2+ after 72 hours treatment, cells were bigger than the control group (p < 0.05). Furthermore, the trend reported in this study is likewise partly supported by Franklin et al.’s (2002) findings on the effect of Cu2+ in microalgae: Selenastrum capricornutum and Chlorella sp. In the same study, it was argued that increases in cell size in Cu2+ treatments were due to an increase in Na+ uptake, or the uncoupling of cell growth and cell division. However, whether Cd2+ causes the same effect is not yet known.
Cd bioaccumulation differences were analyzed in C. reinhardtii strains cc4425 (wild-type) and cc5013 (mutant). Intracellular metal accumulation has been reported as an indicator of metal tolerance (Collard and Matagne, 1994; Fernandez and Novillo, 1996; Perez-Rama et al., 2002; Jamers et al., 2009). The mechanisms of metal tolerance in C. reinhardtii are dependent on metal concentration, availability of nutrients, as well as the general health of the cell (Igiri et al., 2018; Roach et al., 2013). Cd2+ bioaccumulation, for example, occurs once the concentration of the metal reaches critical levels sufficient to both saturate the binding capabilities of the cell wall, and hijack the transport proteins of essential metals with similar redox characteristics (i.e., Zn2+) (Brautigam et al., 2011).
In this study, cc4425 (wild-type) and cc5013 (mutant strain cia7-) were treated with Cd2+, 10 μM (1830 ppb) final concentration. Following a 96-hour exposure, the intracellular Cd2+ content was less than 10% of the total Cd2+ bioavailable. Due to similarities between this study and that by Fernandez and Novillo (1996), both including similar Cd2+ concentrations, prolonged exposure time and organism, it could be argued that the apparent lack of difference in terms of Cd2+ tolerance amongst cc4425 and cc5013 could be a result of the very well documented phytotoxic effects of Cd2+, reducing the uptake capabilities of both strains at 96 hours. A similar study on the metal accumulation in strains cc4425 and cc5013 was conducted by Vazquez et al. (2018) using Pb2+ in the form of Pb (NO3)2 at concentrations 0 μM and 150 μM, in which they reported no significant differences in the bioaccumulation of Pb2+ as measured analytically by ICP-OES. With both Cd2+ and Pb2+ being well-reported phytotoxic agents, it is possible that both of these metals exert irreversible photosynthetic damage after long-term exposures, and reduce the metal uptake capabilities. These could likely account for the non-significant difference in Cd2+ and Pb2+ bioaccumulation between the wild-type and mutant strains.
However, it was initially not expected that there would be a significant difference in cells’ bioaccumulation of Cd2+ caused by medium type. The presence of acetate and phosphate in the diluted TAP medium likely still contributed to a considerably higher buffering capacity compared to Min. Additionally, the pH affects the bioavailability of metals (Glaesener et al., 2013; Olaniran et al., 2013); for example, C. reinhardtii strains showed a higher uptake of Cd at pH 7 and higher Cd tolerance at pH 4 than pH 7 due to the exclusion of Cd at the cell wall surface, which was higher at pH 4 than pH 7 (Samadani et al., 2018). Thus, the choice of medium in this kind of studies is critical and should be given a much careful consideration.
As a nutritional-rich growth media, the composition of TAP could potentially influence the cell response towards a toxicant (i.e., metals). The acetate present in TAP growth media could potentially shield C. reinhardtii from the phytotoxic effects of Cd, particularly photosynthesis impairment, as it could be integrated into the energy-yielding metabolic pathways of the cells. The findings of this study suggest that acetate supplementation triggers a variation in the response of cc4425 and cc5013 towards Cd stress. These findings are supported by Nagel and Voigt (1995) who suggested that the biochemical or metabolic adaptations leading to an increase in Cd2+ tolerance resulted in heavy photosynthetic impairments, obligating the cell to upregulate carbohydrate metabolism as a means of energy, resulting in acetate overconsumption.
In evaluating biological parameters in C. reinhardtii treated with heavy metals, the growth medium’s composition may display an effect in the cells’ responses to heavy metals. Acetate has been described as capable of alleviating photosynthetic stress, with the cell incorporating it into its glyoxylate or citric acid cycle, while under photosynthesis impairment (Roach et al., 2013). The previously reported recovery in growth and increase in reparation in the presence of acetate (Heifetz et al., 2000; Jiang et al., 2016) could have also contributed to the higher Cd bioaccumulation observed in ¼ X TAP.
Heavy metal contamination poses an environmental hazard, and the elucidation of mechanism of tolerance will be relevant in developing techniques and strategies for attenuating this threat. This protein, although of an unknown function, is likely to be significant because it is highly conserved in many organisms. Here we conclude that the results from the comparative analysis in this study indicate that cc5013 (cia7- mutant strain) is more sensitive to Cd2+ than the wild-type strain cc4425, which possesses a functional Cia7 gene. Neither cc4425 nor cc5013 displayed a statistically significant difference in Cd2+ bioaccumulation at 10 μM (1830 ppb) CdCl2.
However, a statistically significant interaction between Min media (absence of acetate and phosphate) and Cd2+ was observed, implying that a CIA7-mediated role in metal tolerance in cc4425 could require a supplemental catabolite because of a diminished photosynthetic efficiency, as reported in the literature. Furthermore, the presence of a catabolite could potentially shield the detrimental effects of the metals, as observed in this study. It was established that cc5013 was subjected to an increased photochemical quenching in the presence of Cd2+ as compared to cc4425. This could be attributed, at least partly, to Cd2+ substituting Mg2+ in the porphyrin group of chlorophylls. Cell size differences in the presence and absence of Cd were observed in both cc5013 and cc4425. The cell size differences could be attributed, at least partly, to an uncoupling of cell growth and cell division due to heavy metal stress.
For future studies, the determination and comparison of intracellular Cd2+ bioaccumulation between cc4425 and cc5013 assessed over a shorter period of time (6-12 h) is recommended. This assessment will likely provide a more dynamic analysis of the extent of metal accumulation with fewer generational gaps. The results of this study also provide a basis for recommendations for future studies: (1) to address why and how this mutant is more sensitive against cadmium (2) to investigate the effect on C. reinhardtii wild type strain (cc4425) and the mutant (cc5013) of other metals including lead in comparison to cadmium and (3) to address why the mutant (cc5013) has a statistically significant greater cell size compared to wild type strain (cc4425).
Dryad: cell size, chlorophyll fluorescence and cadmium bioaccumulation between wild-type and mutant strains of Chlamydomonas reinhardtii upon exposure to cadmium, https://doi.org/10.5061/dryad.rn8pk0pb4 (Ynalvez et al., 2021).
This project contains the following underlying data:
- ICP_OES_data_ynalvez_etal.csv (raw ICP-OES data)
- ODValues_ynalvez_etal.csv (raw OD values for each strain, Cd treatment and growth medium)
- raw_flowcytodata_chlorophyll_cellsize_ynalvez_etal.csv (raw forward scatter, flow cytometry and chlorophyll measurements)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Morphology, migration and transformation of metal elements at the environment-biological interface and remediation of heavy metal pollution in soil/water by microorganisms (mainly microalgae).
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: In recent years I have been carrying out research concerning the response of Chlamydomonas reinhardtii to heavy metal-induced stress. I am particularly interested in examining the role of cellular antioxidants in the acclimatization and adaptation to heavy metals.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I work on trace metal metabolism in Chlamydomonas reinhardtii, regularly conducting elemental analysis of the alga on an Agilent 8900 ICP MS/MS system.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Algae OMICS toxicology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 25 Apr 23 |
read | read | ||
|
Version 1 16 Aug 21 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)