Keywords
cognition, vestibular dysfunction, vertigo, P300, dizziness, event related potentials, cortical auditory evoked potentials, VEMP
This article is included in the Manipal Academy of Higher Education gateway.
cognition, vestibular dysfunction, vertigo, P300, dizziness, event related potentials, cortical auditory evoked potentials, VEMP
Vestibular dysfunction is caused by pathologies in the peripheral and central vestibular system. The peripheral pathologies constitute 90% of cases with vertigo.1 It involves lesion in the end organs of the inner ear and/or the eighth cranial nerve. The central vestibular pathologies involve lesion in the cortical and sub-cortical pathways of the vestibular system. Vestibular dysfunction results in several adverse physical outcomes such as postural instability, abnormal gait and falls. Further, the majority of individuals with vestibular dysfunction are also found to have anxiety and depression. In addition, the loss of vestibular sensory information is shown to alter cognitive abilities related to the processing of spatial and non-spatial information.2
Several studies have investigated the cognitive abilities of individuals with vestibular dysfunction. According to literature, parabrachial nucleus and the hippocampus are the anatomically two regions that account for the relation between the vestibular system and neural networks involved in cognitive and emotional processing.3 The different cognitive skills associated with vestibular function include attention, visuospatial orientation, executive function, memory, metacognition and self-control.4 Research on cognition assessment pertaining to vestibular function has mainly been based on spatial orientation, attention, memory and executive function.5 Smith (2017) reported that cognitive impairment is usually seen in any vestibular dysfunction such as either peripheral or central vestibular dysfunction.
The P300 is an event-related potential, elicited when the target stimuli in the odd-ball paradigm is identified by the participant. It serves as an index for the assessment of cognitive ability to assess cerebral information processing in the context of various neurological diseases.6 Several studies have documented abnormal P300 in individuals with cognitive dysfunctions such as autism spectrum disorder,7 attention deficit hyperactivity disorder,8 schizophrenia,9 migraine,10 etc. It is usually performed with minimum attention to the stimuli without secondary tasks. The P300 is used to evaluate age-related cognitive dysfunction, reflecting attention and memory processes and overlapping function in cognitive deficit.11 Different areas of the brain that provide the generation of P300 response include subcortical structures, auditory regions in the cortex and frontal lobe and various association areas neo cortex.12 The subcomponents for P300, P3a is generated from the frontal working memory which helps in early attention whereas P3b is attention-driven stimulus generated from the temporal and parietal structures.6 The P300 amplitude response mainly depends on stimulus probability, stimulus significance, task effort, motivation and attentiveness.13 P300 amplitude is directly related to the amount of attention paid to perform a particular task associated with superior memory performance.14 P300 latency reflects stimulus processing time in contrast to response processing time, which corresponds to stimulus evaluation time and is independent of the response section.15
In the case of individuals with vertigo, the literature has reported reduced behavioural cognitive abilities.16 A national health and nutrition examination survey done in the US revealed an association between vestibular and cognitive function in the adult population.5 There is a steady accumulation of evidence that the vestibular lesion leads to cognitive deficit. It is not essentially directly related to reflexive signs and perceptual disturbances associated with vestibular dysfunction.17 And there is minimal scientific evidence on cortical potentials in individuals with vestibular dysfunction. Hence, the current study aims to compare the findings of cortical potentials (P1, N1, P2, and N2) and cortical potentials (P300 and N4) between individuals with and without vestibular dysfunction. The objective was to investigate the relationship of cortical and cognitive potentials peak latency and peak amplitude in individuals with and without vestibular dysfunction and the correlation between DHI score with P300 findings among individuals with vestibular dysfunction.
A total of 40 adults aged between 20 and 60 years (mean = 40.5, SD = 13.1) participated in this study. Group I included 20 adults with vestibular dysfunction. All participants in group I had abnormal findings on oculomotor examination or vestibular evoked myogenic potentials (VEMP) assessment. The oculomotor examination was performed using videonystagmography (VNG), the subtests included were saccade test, tracking test, and optokinetic test. Group II included 20 age-matched adults with no vestibular dysfunction. Twenty individuals with vestibular dysfunction in group I included 12 individuals diagnosed with peripheral vestibular lesion and eight individuals with central vestibular lesion. All participants in the study had hearing sensitivity within normal limits. Individuals under medication for vertigo and individuals diagnosed as having an autoimmune disease, systemic illness or neurodegenerative disorders were excluded from the study. The study was approved by the institutional ethics committee of Kasturba Medical College, Mangalore (IECKMCMLR11-18/456) and written informed consent was obtained from all participants before they participated in the study.
All participants in group I completed a Dizziness Handicap Inventory (DHI) questionnaire.18 It assesses quality of life of participants on three domains: functional (nine questions), emotional (nine questions) and physical (seven questions). The participants were instructed to provide responses such as “Yes” when the symptom is present always, “Sometimes” when the symptoms is present sometimes, and “No” when it is absent. Item scores were summed, and the maximum score was 100 and the minimum score was 0. Answers were graded according to 0 for a “No” response, 2 for a “Sometimes” response and 4 for a “Yes” response.
The P300 was recorded using the IHS Smart EP version 3.92 evoked potential system (Intelligent Hearing Systems, USA). During the recording of the P300, participants were made to sit comfortably on a reclining chair in a sound-treated room. The electrode sites were cleaned using Nu-prep Skin Prep Gel (Weaver and Company, USA). Gold plated disc electrodes were placed on the electrode sites using conduction paste and it was secured using adhesive tape. Two non-inverting electrodes were placed on the scalp, one on the vertex (Cz) and the other on the parietal lobe (Pz). Inverting electrodes were placed on both ear mastoid (linked mastoid), and the ground electrode was placed on low forehead (Fpz). The electrode impedance was maintained below 5 kΩ for each electrode and the inter-electrode impedance was less than 2 kΩ. The P300 was elicited using pure-tones of 1000 Hz and 2000 Hz in an odd-ball paradigm. The 1000 Hz pure-tone served as standard stimuli and the 2000 Hz pure-tone served as deviant stimuli. The standard and target stimuli were presented at a ratio of 4:1. The pure-tones were presented to both ears of participants at 80 dB SPL using ER-3A insert earphones (Intelligent Hearing Systems, USA). A total of 300 stimuli were presented at a repetition rate of 1.1 stimuli/sec and the ongoing EEG was differentially recorded from the scalp. The EEG was amplified 50,000 times and filtered using a bandpass filter of 1 to 30 Hz. The duration of the analysis window was 600 msec with a pre-stimulus duration of 100 msec. The participants were instructed to count the target stimuli and report at the end of the recording.
The waveforms obtained from all participants for standard and target stimuli were grand averaged separately to identify various components or peaks of event related potentials. The averaged waveforms included peaks P1, N1, P2, and N2 between 50 msec and 250 msec. The broad positive peak after 250 msec from the stimulus onset in the waveform of target stimuli was referred to as P300. And the negativity peak following the P300 was considered as N4 (late negativity). The latency (in msec) and peak amplitude (in μV) of peaks P1, N1, P2, N2, P300 and N4 was measured at the electrode sites Cz and Pz. The peak amplitude was measured relative to the pre-stimulus baseline and the latency was measured from the stimulus onset. The dataset is published as underlying data in Mendeley Data.19
Statistical analysis was conducted using IBM SPSS software version 26 (RRID:SCR_002865). Initially, descriptive analysis and the Shapiro-Wilk test were carried out to check the normality of the data. The latency and peak amplitude of peaks P1, N1, P2, N2, P300, and N4 were normally distributed. Thus, the independent t-test was administered to investigate if the mean latency and amplitude of peaks were significantly different between groups. Correlation analysis was carried out to investigate the relationship between the DHI score and latency and amplitude of P300 in group I.
The grand averaged waveforms for standard and deviant stimuli for both groups are shown in Figure 1. In the figure, it is evident that both pure-tones elicited obligatory P1-N1-P2 response for the stimulus onset among participants in both groups. Further, the waveform for target stimuli showed a large positive peak at a latency of 280 ms following the P1-N1-P2 response in both groups of participants, referred to as P300. The identification rate of peak responses varied across groups at Cz and Pz positions, which is depicted in Figures 2 and 3 respectively.
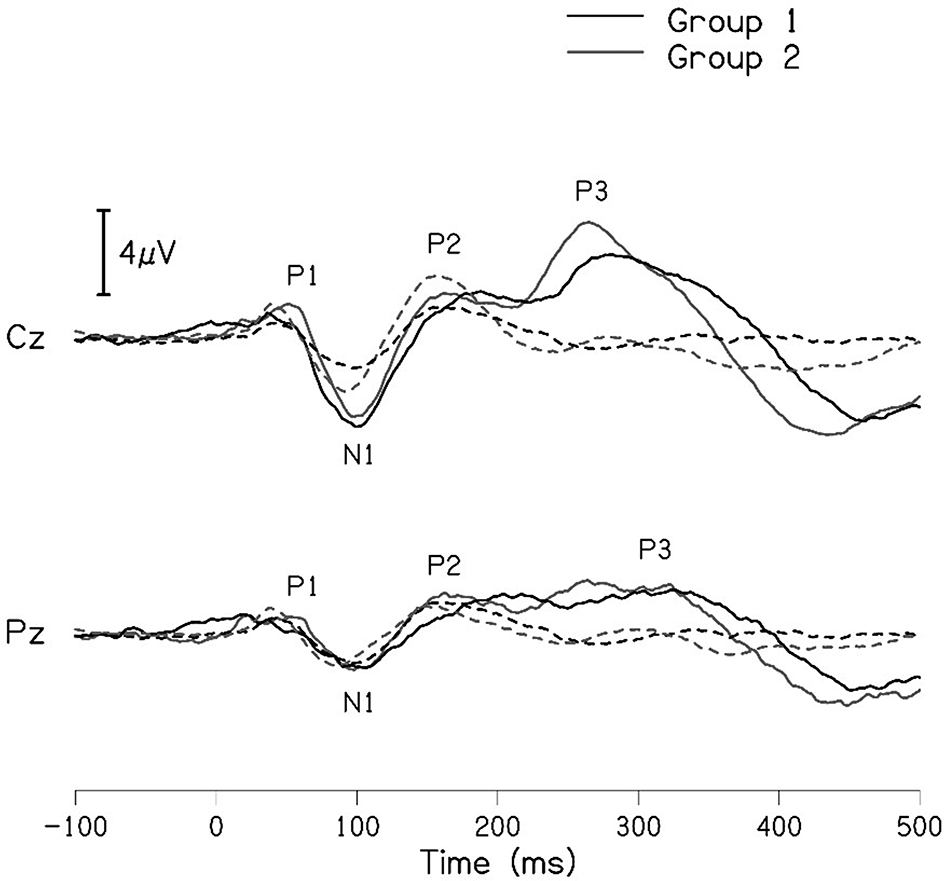
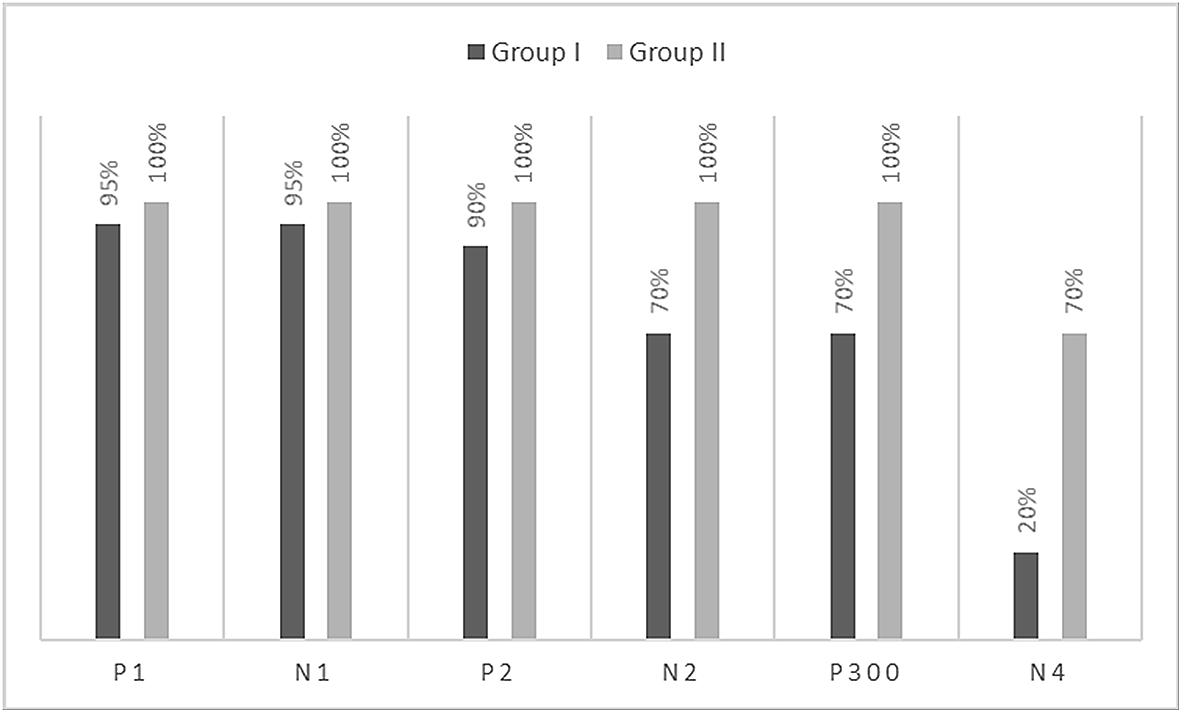
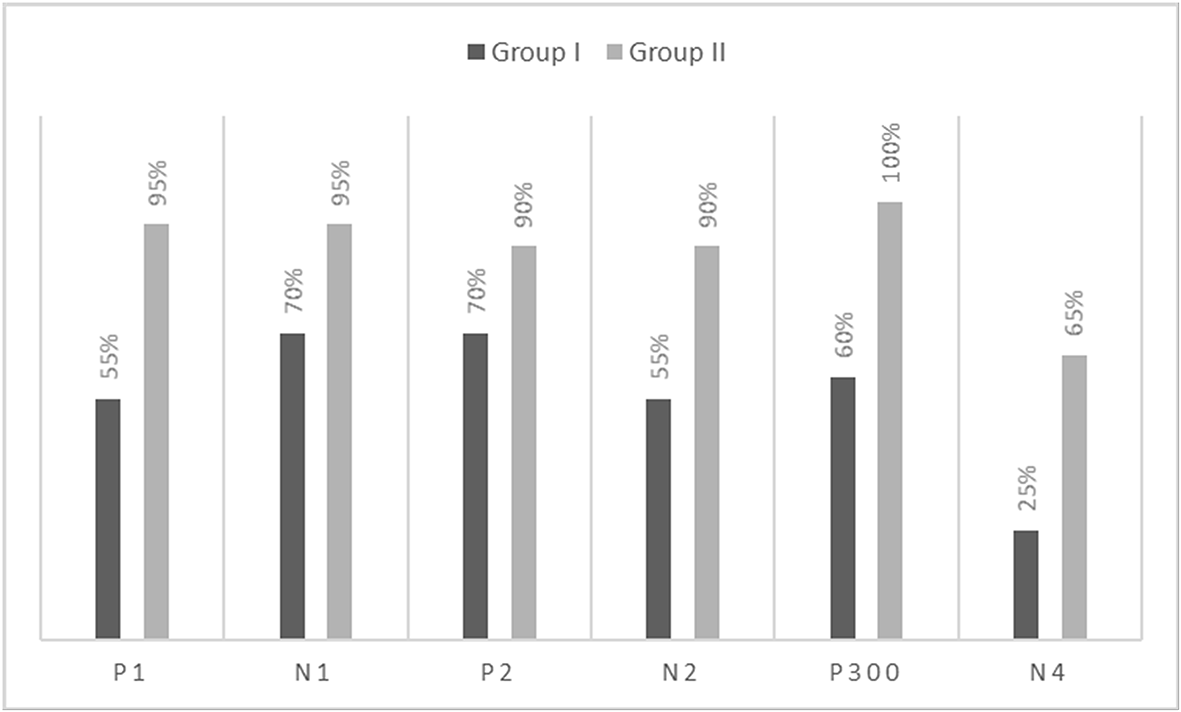
Table 1 shows the mean latency of peaks P1, N1, P2, and N2 at electrode sites Cz and Pz for both groups. The mean latency of peaks P1, N1, P2, and N2 were similar between groups at the electrode sites Cz and Pz. To investigate if the mean latency of peaks were significantly different between groups, the independent t-test was carried out. It showed no significant difference for the latency of peaks P1 [t(37) = -1.083, p = 0.286)], N1 [t(37) = -1.008, p = 0.320)], P2 [t(36) = 1.007, p = 0.321)], and N2 [t(32) = 1.822, p = 0.078)] at the electrode site Cz. Similarly, at Pz the latency of peaks P1 [t(28) = -0.029, p = 0.977)], N1 [t(31) = -0.029, p = 0.977)], P2 [t(30) = -1.059, p = 0.298)], and N2 [t(27) = 0.56, p = 0.956)] were not significantly different between groups.
Table 2 shows the mean amplitude of peaks P1, N1, P2, and N2 at electrode sites Cz and Pz for both groups. The mean amplitude of peaks was larger in individuals with no vestibular dysfunction (group II) at both Cz and Pz. To investigate if the mean amplitudes of peaks are significantly different between groups, independent t-test was carried out separately for Cz and Pz. It revealed no significant difference for the amplitude of peaks P1 [t(30) = -2.733, p = 0.10)], N1 [t(37) = -0.614, p = 0.543)], P2 [t(36) = -1.061, p = 0.296)] and N2 [t(32) = -0.268, p = 0.790)] at the electrode site Cz between groups. Similarly, the amplitude of peaks P1 [t(28) = -1.742, p = 0.093)], N1 [t(31) = -1.326, p = 0.194)], P2 [t(28) = -1.742, p = 0.093)] and N2 [t(27) = -0.318, p = 0.756)] at the electrode site Pz showed no significant difference between the groups. On peak-to-peak analysis between groups showed no significant difference in P1-N1 and N1-P2 peak to peak amplitude at Cz and Pz.
Table 3 shows the mean latency and amplitude of peaks P300 and N4 at Cz and Pz for both groups. A noticeable difference was observed for the mean amplitude of N4 between groups at both the electrode sites. The mean amplitude of P300 was larger in group I compared to group II, this finding is controversial when compared to the grand average waveform of both groups shown in Figure 1. The mean amplitude of P300 was found to be largest in group 1 compared to group II. To investigate if the mean difference for latency and amplitude were significantly different between groups, an independent samples t-test was carried out. The results showed no significant difference in the latency of P300 [Cz: t(32) = -0.173, p = 0.866); Pz: t(30) = -0.357, p = 0.724)] and amplitude of P300 [Cz: t(32) = 0.218, p = 0.829); Pz: t(29) = 0.797, p = 0.432)] at both Cz and Pz. Whereas no statistical significant difference was found for the N4 latency [Cz: t(16) = 0.415, p = 0.684); Pz: t(16) = 0.786, p = 0.443)] and significant difference was observed in N4 amplitude [Cz: t(14) = -2.178, p = 0.047); Pz: t(15) = -2.107, p = 0.052)] at both positions.
To investigate the relationship between the latency of P300 and DHI score, Pearson’s correlation analysis was carried out. The results showed a weak negative correlation between the latency of P300 and the DHI score; however, the correlation was not significant at both electrode sites [Cz: r = -0.201, p = 0.490; Pz: r = -0.401, p = 0.196]. Further, no correlation was found between the amplitude of the P300 and DHI score at both electrode sites [Cz: r = -0.167, p = 0.569; Pz: r = 0.087, p = 0.787].
The mean latency of late latency response and P300 of individuals diagnosed with peripheral vestibular lesion and central vestibular lesion are depicted in Tables 4 and 5 respectively.
| Latency | Cz | Pz | ||||
|---|---|---|---|---|---|---|
| msec | n | Mean | SD | n | Mean | SD |
| P1 | 11 | 56.09 | 5.12 | 6 | 65.33 | 12.50 |
| N1 | 11 | 104.54 | 9.63 | 7 | 107.71 | 9.12 |
| P2 | 10 | 166.00 | 14.03 | 7 | 167.42 | 16.71 |
| N2 | 8 | 230.37 | 15.74 | 5 | 235.20 | 13.19 |
| P300 | 9 | 345.22 | 25.49 | 7 | 349.57 | 25.99 |
The present study compared the latency and amplitude of cortical (P1, N1, P2 and N2) and cognitive potentials (P300) among individuals with and without vestibular dysfunction. The cortical potential includes auditory late latency responses P1, N1, P2, and N2. The cortical and cognitive potentials mainly involve the primary and non-primary auditory cortex of the temporal lobe, especially the primary pathway being more auditory sensitive. As per the literature, the generation of middle latency response is an interplay of primary and non-primary areas in the auditory thalamo-cortical pathway.20 Whereas late latency response is from non-primary cortical areas which measure the integrity of the auditory system beyond the brainstem.21 The P300 is a positive peak that comes after approximately 300 ms has extended importance in the study of cognition and executive functions. The waveform is obtained with parieto-central distribution and shows typical P300 topography for processing oddball stimuli via any sensory modality.22 As per a review, it is clear that cognitive impairment is observed in individuals with vestibular dysfunction, with respect to attention, spatial orientation, executive function and memory.5
The results of the present study showed no significant difference in the latency and amplitude of peaks P1, N1, P2, and N2 of the cortical auditory potentials. These findings are not in agreement with results of earlier investigation.23 However, findings of the present study could be explained based on the hearing sensitivity of participants in both groups and the characteristics of the P1-N1-P2 response. The P1-N1-P2 response is elicited for the onset of stimuli, therefore, the characteristics of the response are dependent on the onset of the stimuli. Further, the participants in both groups had hearing sensitivity within normal limits. Thus, the latency and amplitude of the peaks are expected to be similar in both groups.
The P300 was found to be absent in a greater number of individuals with vestibular dysfunction compared to the control group. It was absent in 30–40% of the individuals with vestibular dysfunction; this finding is consistent with results of the previous study.24 Further, when the P300 was present, the mean latency and amplitude of the P300 in both groups were similar. In contrast to the findings of the present study, earlier investigations have reported prolonged latency for P300 in individuals with vestibular dysfunction compared to the control group.19,20 The contrasting findings observed in the present study and earlier investigations could be differences in the site of vestibular lesion across studies. Studies in the literature have included individuals with peripheral vestibular lesions where the site of the lesion was localized to the lateral semicircular canal.23,24 The majority of the participants were reported to have unilateral caloric hypofunction. The findings of the above investigations showed prolongation of P300 latency in individuals with unilateral peripheral vestibular lesions (lateral semicircular canal) compared to the control group. In contrast, participants in the present study had peripheral vestibular lesion with abnormal findings on cVEMP and oVEMP indicating a lesion in the saccule and utricle. Therefore, the contrasting findings observed in the present study could be a consequence of differences in the site of the lesion. In addition, contrasting findings observed in the present study could be due to the degree of severity of dizziness. The majority of the participants with peripheral vestibular lesion in the present study were found to have mild severity/handicap based on DHI scores. The severity of dizziness might have an influence on the latency and amplitude of P300.
Studies on cognitive function assessment using a cognitive failure questionnaire in individuals with vestibular dysfunction revealed that cognitive dysfunction is prevalent in individuals with central and peripheral vestibular pathologies.25 The literature also showed a positive correlation between cognitive dysfunction and dizziness severity in terms of a self-rated questionnaire. DHI helps in evaluating the severity of dizziness based on its impact physically, functionally and emotionally with limited profile on cognition.26 In the current study, the correlation between DHI scores and P300 showed no significant correlation. The lack of correlation between the two measures could be due to the different areas of assessment. DHI is a measure of self-help obtained primarily based on daily activities, whereas the P300 is an electrophysiological measure that assesses cognitive functioning.12 Because of this direct correlation between DHI and P300 was found to be inconclusive in the present study. Similarly, no significant correlation was observed between P300 and vertigo symptoms, whereas another study stated that severity of vestibular symptoms seems to correlate with P300 responses.27 In support of the current study, a randomized controlled trial showed cognitive behaviour therapy influenced patients with chronic subjective dizziness with a significant reduction in DHI and no changes in psychological outcome measures.28 Similarly, other literature has reported that the functional and physical parameter of DHI showed a negative correlation, and the emotional parameter showed a weak significant positive correlation in 369 participants evaluated for functional tests such as electronystagmography, rotational testing, and platform posturography.29 The findings of various studies by several investigators have emphasized the role of the vestibular system’s role on cognition, such as perceptual/visuospatial ability, memory, attention, and executive function. Knowing the cognitive function of individuals with vestibular dysfunction facilitates the setting of vestibular rehabilitation therapy goals. Evidence reveals that in patients with intractable dizziness following vestibular rehabilitation there is a significant improvement in vestibular function and cognitive function including attention, visuospatial ability and executive function with coincidental improvement in DHI.30 The findings of this study are circumscribed to oddball auditory tasks only, which might be a limitation.
The present study, which includes a review of the literature, divulges that cortical and cognitive potentials might help us in assessing and understanding the cognitive function in individuals with vestibular dysfunction; however, a lot of research is required in this field. For individuals with vestibular dysfunction, cognitive assessment is necessary to understand the dizziness impact on daily activities. Knowing the cognitive function of individuals with vestibular dysfunction enables the planning of vestibular rehabilitation therapy, which enhances the quality of life in these individuals by improving the vestibular and cognitive function.
Mendeley Data: Underlying data for ‘Cortical auditory potentials and cognitive potentials in individuals with and without vestibular dysfunction’ https://www.doi.org/10.17632/hn6z8x5vkk.119
This project contains the following underlying data:
• Data file 1. Description.txt
• Data file 2. Event related potentials in individuals with vestibular dysfunction.xlsx
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Written informed consent for publication of the participants’ details was obtained from the participants.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Diagnostic Audiology including Electrophysiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Auditory Evoked Potentials, Auditory Processing Disorders, Psychoacoustics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 18 Oct 23 |
read | ||
|
Version 2 (revision) 16 Nov 22 |
read | read | |
|
Version 1 07 Sep 22 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)