Keywords
in vitro model, miniaturisation, animal reduction, high throughput, anticoccidial compounds, chicken coccidiosis, Eimeria species
This article is included in the NC3Rs gateway.
in vitro model, miniaturisation, animal reduction, high throughput, anticoccidial compounds, chicken coccidiosis, Eimeria species
Scientific benefit:
• Increased throughput for evaluation of new compounds aimed to control chicken coccidiosis by miniaturising the previously developed in vitro compound-screening model
3Rs benefit:
• Evaluation of novel anticoccidial compounds will require fewer chickens for the generation of parasite stocks
Practical benefits:
• Reduced variability: simultaneous evaluation of a larger number of anticoccidial compounds
• Cost effectiveness: less materials and reagents are necessary per evaluated compounds
• Streamlined protocols: 96-well plate format is used through the whole process (cell culture, DNA isolation and qPCR analysis)
Current application:
• High-throughput screening of potential anticoccidial compounds under investigation by commercial companies and/or public research organisations
Potential applications:
The use of in vitro models in biosciences and biomedical research has supported many scientific advances and has emerged as a suitable alternative to reduce time and cost associated with in vivo models by replacing and/or reducing the use of experimental animals in many areas. The implementation of in vitro models for anticoccidial drugs pre-screening as an alternative to in vivo tests is leading to the replacement and reduction of an important number of chickens used in research in coccidiosis control (Thabet et al., 2017). Chicken coccidiosis is an enteric disease caused by different species of the genus Eimeria (Apicomplexa) (Burrell et al., 2020), characterized by malabsorption, diarrhoea and haemorrhage with an important impact on chicken meat and egg production worldwide that cause >£10 billion per annum losses (Blake et al., 2020). Coccidiosis has also impacted on the ‘Five Freedoms’ of animal welfare, more in particular affecting the ‘freedom from discomfort’ and ‘freedom from pain, injury’ (Webster, 2001). Resistance against current anticoccidial drugs, as well as near-prohibition of ionophores antibiotics in some parts of the world and public concerns about the use of drugs in animals for human consumption, has led to an increased research in new substitute compounds, evidenced by the high number of new publications in the area in recent years (>25 in vivo studies within the past two years involving >7,000 chickens).
We previously developed an in vitro model to evaluate anticoccidial compounds effects in Eimeria tenella (Marugan-Hernandez et al., 2020) and a first direct application for the evaluation of two essential oils (Sidiropoulou et al., 2020) led to a reduction of the number of chickens used for in vivo testing. Results obtained in vitro showed that each of the essential oils exhibited anti-parasitic effects at different concentrations. This supported the selection of a single experimental group (90 animals + control group) of a combined treatment at a single dose, excluding the evaluation of the compounds separately since the in vitro model had already proved their dose-independent individual effects; therefore, it was not considered necessary to re-evaluated this in vivo. The in vivo experiment was reduced from six to two experimental groups (test and control), resulting on a reduction of 90×4=360 chickens in a single study (67%). This in vitro model has been standardised, applied and published using a 24-well plate format (Marugan-Hernandez et al., 2020); in this study, we aimed to adapt the model to a 96-well plate format. This miniaturisation will reduce the numbers of parasites needed for compound testing, and therefore the number of chickens used to generate parasites stocks, additionally allowing the evaluation of a higher number of compounds simultaneously. Eimeria spp. cannot complete their life cycle in vitro; therefore, the natural host (chicken) is necessary to produce stocks for further experimental procedures. The use of one chicken can generate enough initial parasite material (oocysts) for the pre-screening of two compounds in the 24-well plate format. The use of the 96-well plate format will allow the pre-screening of 10 compounds per animal, this has the potential to reduce local animal use for the study of anticoccidial compounds by 80%.
This study was carried out in strict accordance with the Animals (Scientific Procedures) Act 1986 (United Kingdom Parliament Act). All animal studies and protocols were approved by the Royal Veterinary College Animal Welfare and Ethical Review Board (AWERB) and performed under the United Kingdom Government Home Office under specific project licence (PDAAD5C9D).
Eimeria tenella Wisconsin (Wis) strain (McDougald & Jeffers, 1976) was propagated chickens. Table 1 summarises the detailed information of the experiment and used animals following ARRIVE guidelines.
| Study design | |
| Sample size | |
| Inclusion/exclusion criteria | |
| Randomisation | |
| Blinding | |
| Outcome measures | |
| Statistical methods | |
| Experimental animals | |
| Experimental procedures | |
| Results |
Pastor-Fernandez et al. (2019) provides detailed protocols for oocysts isolation and excystation as well as for sporozoite purification. Sporulated oocysts were stored in water at 4oC for up to six months, the moment from which they start to lose viability for in vitro tests. Freshly purified sporozoites were used to infect cell monolayers immediately.
Standard protocols for cell culture maintenance were the same described for the standardisation of the in vitro model for compound-screening (Marugan-Hernandez et al., 2020). Briefly, Madin-Darby bovine kidney (MDBK) cells (NBL-1; ECACC-Sigma-Aldrich) were used as host cell cultures and maintained in a 37oC humidified incubator with 5% CO2 atmosphere. Cells were cultured in Advanced Dulbecco’s modified Eagle’s Medium (AdDMEM; Gibco) supplemented with 2% heat-inactivated foetal bovine serum (FBS; Sigma) and 100 U/mL penicillin/streptomycin (Fisher, Leicestershire, UK). Confluent cell monolayers (100%) were passaged twice a week to a new confluence of 70-80% by washing them in Ca2+- and Mg2+-free PBS and released with 3mL 0.25% Trypsin/EDTA (Gibco), incubated for three minutes, followed by neutralisation with 5 mL volume of complete medium. The cell suspension was centrifuged at 1,500 g for 10 minutes at room temperature after which the pellet was suspended in 5 mL of fresh medium. Cells were counted and seeded on T75 flasks (Thermo Fisher Scientific) at a density of 10×106 cells with AdDMEM at 15 ml of final volume or seeded on flat bottom 96-well microtiter plates (Thermo Fisher Scientific Nunc) at a cell density of 0.05×106 cells in each well in 100 μl of AdDMEM culture media for subsequent infection with sporozoites (see Optimisation of infection ratios section).
Optimisation of infection ratios
Cells were seeded on flat bottom 96-well microtiter plates (Thermo Fisher Scientific Nunc) at a cell density of 0.1×106 or 0.05×106 cells in each well in 100 μl of AdDMEM culture media (lower density than 0.3×106 per well in 500 μl used in 24-well plates) for subsequent infection after two hours with sporozoites at different sporozoites:cells ratios (1:1, 2:1, 4:1; Table 2). Three wells were used per conditions (A, B, C) and incubated at 41oC, 5% CO2. After 4 hours, infected monolayers were removed with 3 mL 0.25% Trypsin/EDTA (Gibco) and cells counted in a Fuchs-Rosenthal counting chamber. Empty cells were recorded as non-infected, cells with the presence of at least one sporozoites were recorded as infected. Two counts were done per well (Table 2).
| Ratio sporozoites:cells (no. sporozoites) | Wells | Non-infected cells | Infected cells | % Infected cells/count | % Infected cells/well | % Infected cells (average) | Standard deviation |
|---|---|---|---|---|---|---|---|
| 0.05×106 cells/well | |||||||
| 1:1 (0.05×106) | A | 21 | 16 | 43.2 | 36.1 | 39.0 | 7.6 |
| 27 | 11 | 28.9 | |||||
| B | 26 | 18 | 40.9 | 47.6 | |||
| 21 | 25 | 54.3 | |||||
| C | 26 | 13 | 33.3 | 33.3 | |||
| 22 | 11 | 33.3 | |||||
| 2:1 (0.1×106) | A | 12 | 23 | 65.7 | 60.2 | 60.7 | 7.2 |
| 19 | 23 | 54.8 | |||||
| B | 7 | 21 | 75.0 | 68.1 | |||
| 12 | 19 | 61.3 | |||||
| C | 22 | 21 | 48.8 | 53.8 | |||
| 14 | 20 | 58.8 | |||||
| 4:1 (0.2×106) | A | 7 | 14 | 66.7 | 68.1 | 73.2 | 5.2 |
| 7 | 16 | 69.6 | |||||
| B | 10 | 26 | 72.2 | 73.0 | |||
| 16 | 45 | 73.8 | |||||
| C | 11 | 35 | 76.1 | 78.5 | |||
| 9 | 38 | 80.9 | |||||
| 0.1×106 cells/well | |||||||
| 1:1 (0.1×106) | A | 22 | 30 | 57.7 | 59.5 | 57.3 | 2.0 |
| 22 | 35 | 61.4 | |||||
| B | 21 | 29 | 58.0 | 56.6 | |||
| 22 | 27 | 55.1 | |||||
| C | 26 | 34 | 56.7 | 55.7 | |||
| 34 | 41 | 54.7 | |||||
| 2:1 (0.2×106) | A | 17 | 43 | 71.7 | 63.5 | 61.1 | 7.3 |
| 17 | 21 | 55.3 | |||||
| B | 21 | 51 | 70.8 | 66.9 | |||
| 17 | 29 | 63.0 | |||||
| C | 22 | 34 | 60.7 | 52.9 | |||
| 33 | 27 | 45.0 | |||||
| 4:1 (0.4×106) | A | 15 | 33 | 68.8 | 75.8 | 80.0a) | 4.3 |
| 10 | 48 | 82.8 | |||||
| B | 6 | 28 | 82.4 | 79.9 | |||
| 7 | 24 | 77.4 | |||||
| C | 8 | 28 | 77.8 | 84.3 | |||
| 3 | 30 | 90.9 | |||||
Analytical standard preparations of the classical anticoccidial drugs amprolium hydrochloride (AMP), robenidine hydrochloride (ROB) and salinomycin monosodium hydrate (SAL) were purchased from Sigma (Sigma-Aldrich). Drugs were prepared at 10 mg/mL stock in dimethyl sulfoxide (DMSO; Merck) and dilutions for final working concentrations of 50 μg/ml, 20 μg/mL, 5 μg/mL, and 1 μg/mL were freshly prepared from the stock in AdDMEM just before incubation with sporozoites.
MDBK cells were seeded in four different 96-well plates (one per each harvested time point) at a density of 0.05×106 cells per well and incubated for four hours at 37oC, 5% CO2. Right after, sporozoites (0.2×106 per well) of E. tenella Wis strain were pre-incubated for 1 h at 41oC, 5% CO2 with each anticoccidial compound concentration; untreated sporozoites were used as controls for invasion and replication. After pre-incubation, sporozoites were centrifuged at 1,500 g for 10 minutes at room temperature and washed with PBS to rinse them free of drugs, then resuspended in 300 μl of AdDMEM (equivalent to 100 μl per well). Sporozoites were then added to MDBK monolayers seeded in 96-well plates (after the 4 hours incubation period) and incubated at 41oC, 5% CO2. All the wells were washed twice in AdDMEM to remover extracellular sporozoites. The first time point was harvested at two hours post infection (hpi) by washing twice with PBS, then adding RTL buffer (Qiagen) and storing the plate at -20oC until used for genomic DNA isolation. The other infected monolayers were collected and stored in the same way at 24, 44 and 52 hpi. Three wells were used per condition (for untreated and treated sporozoites and for each drug concentration at each time point). Two whole separated experiments carried out in different weeks were performed.
Genomic DNA (gDNA) was isolated from the samples stored in RTL buffer (Qiagen) using the AllPrep DNA/RNA 96 Kit (Qiagen) following manufacturer’s instructions using the vacuum manifold for processing 96-well plates (Qiagen).
CFX96 Touch R Real-Time PCR Detection System (Bio-Rad) was used to perform the quantitative PCR following the procedures described previously, using DNA-binding dye SsoFastTM EvaGreen Supermix (Bio-Rad) (Marugan-Hernandez et al., 2016). For parasite quantification, the number of haploid genomes (equivalent to single sporozoites or merozoites) per well (three wells/sample, technical replicates) was determined for each condition using gDNA specific primers for E. tenella 5S rDNA (Fw_5S: TCATCACCCAAAGGGATT, Rv_5S: TTCATACTGCGTCTAATGCAC) (Clark et al., 2008) and a standard curve of sporozoite gDNA extracted by the same methods (gDNA equivalent to 107 followed by serial dilution until 102). Once the run was completed, a baseline was calculated by Bio-Rad CFX Manager software (Bio-Rad) and applied to each sample to compare the quantification cycles (Cq values) obtained from different wells.
The number of sporozoites for each data point analysed by qPCR was automatically determined for each well by a regression analysis using Bio-Rad CFX Manager software (Bio-Rad). Three replicates were included per sample to allow single points showing a standard deviation of more than 0.5 to be excluded from the analysis without affecting the quantification, if all three curves were out of this range, qPCR was repeated for this sample.
In vitro inhibition percentage for each anticoccidial drug and concentration was calculated following the equation implemented by Thabet et al. (2017):
Comparison of replication rates after drug treatment were done by calculating slopes of the regression line (m) between 24 and 44 hpi, which were calculated following the universal formula:
For the miniaturisation of the model to a 96-well plate format, we screened out the optimal combination of MDBK cells and E. tenella sporozoites. Good monolayer confluences were achieved with 0.05×106 cells/well. An optimal rate of invasion and development without causing multiple sporozoite infection per cell was obtained with a 4:1 sporozoites:cell ratio (0.2×106 sporozoites/well). Parasite invasion and development was evaluated in a time course experiment followed by qPCR (Figure 1). Increasing amounts of parasite DNA were detected from 24 hpi onwards as described for the 24-well format, indicative of nuclear replication. The linear rate of replication was equivalent to the one described in a 24-well plate format (Figure 1), validating in this way the suitability of a 96-well plate format to track and evaluate sporozoites invasion and replication. Higher variability was found for the latest time point (52 hpi) which is explained by the rupture of schizonts which will release merozoites as has been observed before (Marugan-Hernandez et al., 2020); these merozoites are washed from the monolayers and therefore not contributing to the parasite numbers when analysed by qPCR. Fewer parasite number were detected for the 96-well plate format along the time course, which correlated with the lower initial numbers of sporozoites used for monolayers infection (0.2 million/well versus 1 million/well).
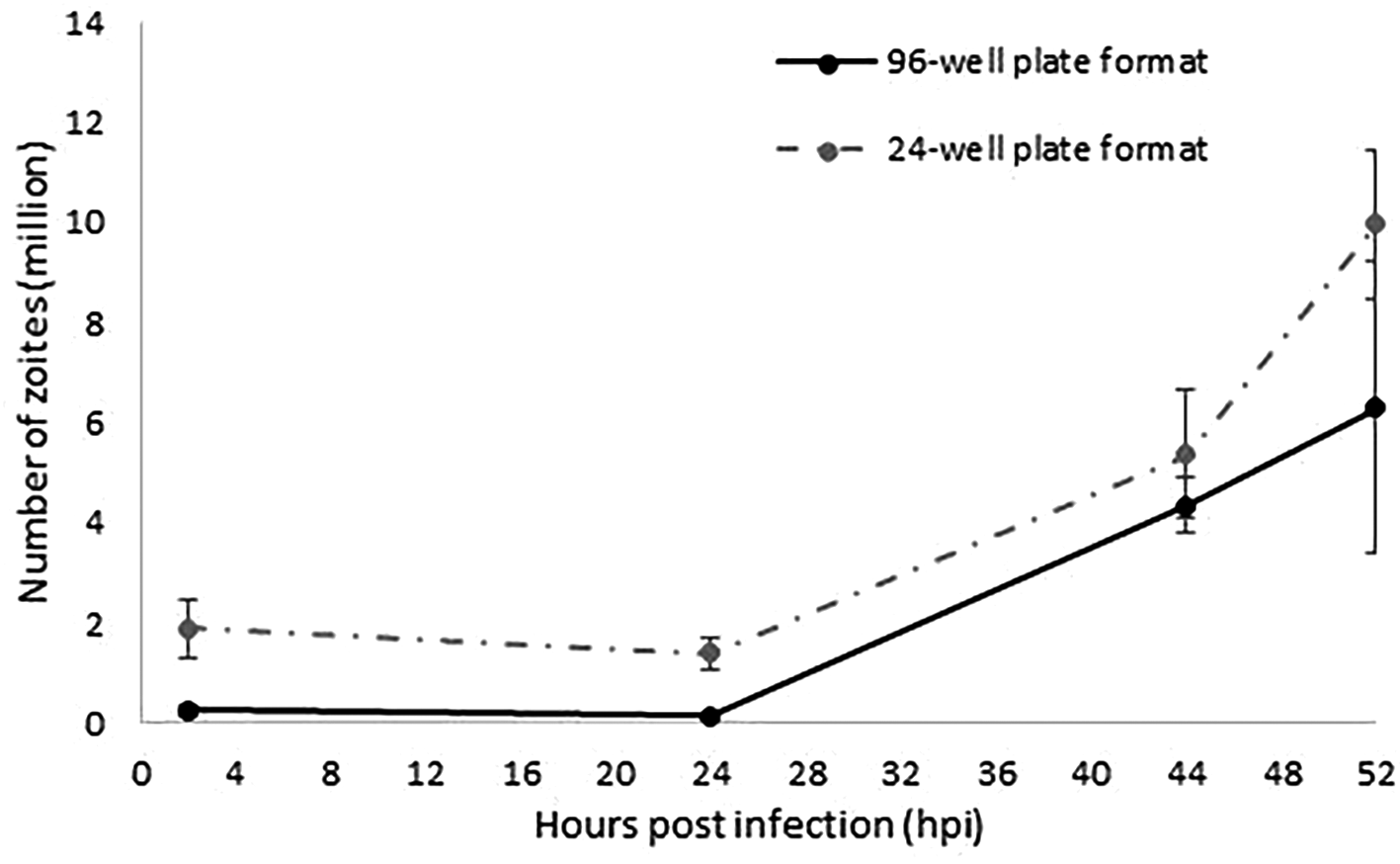
The continuous line represents the number of zoites quantified by qPCR in samples collected at 2, 24, 44 and 52 hpi as the average of the two different replicated experiments performed in separated weeks in a 96-well plate format. Discontinuous line represents data extracted from Marugan-Hernandez et al. (2020) equivalent to the number of zoites quantified by qPCR at the same time points as the average of the three different replicated experiments performed in separated weeks in a 24-well plate format. Error bars represent the standard deviation between repeated experiments for each plate format. Both types of plate format show an equivalent linear fashion growth after 24 hpi.
Pre-treatment of sporozoites with different concentrations of AMP, ROB and SAL, three drugs with reported anticoccidial activity, showed different degrees of inhibition of invasion and replication (Table 3). The pre-treatment with AMP at any concentration caused a moderate inhibition of invasion at 2 and 24 hpi (10-45%; average of 22%); however, those sporozoites which were successful at invasion then developed at the same rate as the untreated controls (slope of the regression curve was parallel to that exhibited by the untreated control). Pre-treatment with ROB had little effects on invasion (inhibition of 0-20%, average 5.6%); nevertheless, sporozoites did not replicate well once intracellularly (slope decreased compared with untreated controls, with sporozoite numbers decreasing for the highest concentration, 50 μg/mL). Pre-treatment with SAL exhibited effects in both invasion and development. Invasion was inhibited at higher levels than for AMP (10-60%; average 27%) and intracellular replication was severely impaired for the higher concentrations (50 and 20 μg/mL). In general, for every drug, higher inhibition of invasion and replication was observed for the higher concentration, although some variations were observed. These results evidence the suitability of the miniaturised model to detect inhibition of invasion and/or replication.
Each value represents the average of the percentage of inhibition±standard deviation or slope of two different experiments including each three wells per condition, calculated using the equations displayed in Data analysis section.
| Average | Concentration (μg/ml) | 2 hpi | 24 hpi | 44 hpi | 52 hpi | Slope (24-44 hpi)* |
|---|---|---|---|---|---|---|
| AMP | 50 | 22.05±21.71 | 34.22±34.22 | 8.17±8.17 | 47.03±15.18 | 282486 |
| 20 | 17.34±10.96 | 7.17±7.17 | 12.88±12.88 | 23.54±23.54 | 264669 | |
| 5 | 22.89±4.85 | 10.19±10.19 | 0.00±0.00 | 14.11±6.08 | 227343 | |
| 1 | 46.78±5.45 | 24.45±24.45 | 1.96±1.96 | 0.00±0.00 | 227636 | |
| ROB | 50 | 9.13±9.13 | 25.71±25.71 | 5.13±5.13 | 6.05±6.05 | -9013 |
| 20 | 0.00±0.00 | 7.91±7.91 | 97.81±0.07 | 48.49±48.49 | 100536 | |
| 5 | 0.00±0.00 | 9.07±9.07 | 45.43±23.7 | 40.99±31.24 | 194893 | |
| 1 | 0.00±0.00 | 0.00±0.00 | 5.42±3.62 | 0.00±0.00 | 89050 | |
| SAL | 50 | 62.42±1.79 | 19.02±19.02 | 52.34±13.91 | 18.23±18.23 | -3380 |
| 20 | 16.47±16.47 | 7.94±7.94 | 98.4±0.4 | 99.23±0.09 | 4664 | |
| 5 | 40.02±6.5 | 13.5±13.5 | 94.19±0.04 | 87.87±0.94 | 55507 | |
| 1 | 44.18±21.42 | 31.35±14.43 | 73.66±6.83 | 49.41±20.62 | 152050 |
In this study, the in vitro model developed to evaluate compounds with potential anticoccidial effects on sporozoites of E. tenella has been successfully miniaturised from a 24-well plate format to a 96-well format. Conditions were refined to create an optimal confluence of the monolayer supporting invasion rates of >70% in the new format. These conditions have proved to support schizonts and merozoite development as described before in the original model (Marugan-Hernandez et al., 2020). The tracking of the sporozoites invasion and development by qPCR in time course experiments have shown the same linear growth fashion than described before and the evaluation of traditional anticoccidial drugs to validate the model has also proved the suitability of this model to detect different levels of inhibition of invasion and/or development.
Eimeria parasites are self-limited monoxenous (single-host) parasites, and despite many efforts, there are no efficient in vitro systems supporting continuous replication or the completion of the life cycle; in consequence, research on this species depends on the use of animals. Examination of the literature shows that testing of novel anticoccidial compounds, mainly natural products, is in expansion. In 54 refereed publications between 2017 and 2021 a total of 19,786 chickens (~4,000 per annum) were used to evaluate novel compounds for anticoccidial effects. In addition to these chickens already mentioned, there are chickens used in unpublished studies from pharmaceutical/nutrition companies or those only providing negative results, numbers of which are harder to discover. Looking specifically at some 2021 publications, if pre-screening with in vitro models had been used by Fu et al. (2021), concentrations could have been adjusted to reduce animals from 630 to 480 (23.8%). Similarly, the use of in vitro models could have excluded one testing group for both Lima et al. (2021) and Herrero-Encinas et al. (2021), reducing animals from 900 to 720 (20%) and 400 to 320 (20%), respectively. If we extrapolate these data (an average of 20% reduction) to the total published studies in 2021, the use of our in vitro models could have avoided the experimental use of 792 chickens (out of 3,958) globally, or 3,958 (out of 19,786) in the last 5 years. Nevertheless, we still need some chickens to generate enough parasite materials to perform the in vitro pre-screenings. Therefore, the described modification to the in vitro model will have a direct impact on the reduction of animal use.
Testing one compound at four different concentrations (excluding untreated controls) requires 12 million of sporozoites in the 24-well plate format whereas only 2.4 million are necessary in the 96-well plate one. Efficiency of sporozoite excystation and purification from oocysts is set at 1:1 in our experimental settings. A single chicken can produce an average of 30 million oocysts without causing clinical symptoms. Therefore, with the use of one chicken, we could test at least ten compounds (plus untreated compounds) with the 96-well format, whereas only two compounds (plus controls) could be tested in the 24-well plate format. This supposes a direct 80% reduction in animal use for the evaluation of anticoccidial compounds locally. However, since we have collaborations with several research institutions and companies at different locations worldwide, this miniaturised model will also have a significant global impact on the number of chickens undergoing a mild severity procedure for the generation of E. tenella oocysts.
Another advantage of this miniaturisation is the high-throughput capacity to test many compounds simultaneously, while reducing materials, reagents and time to perform them. Up to seven compounds could be tested on a single plate per time point (including controls) using <10 mL of culture media, versus only two compounds per plate using volumes of >20 mL per plate in the 24-well plate format. Time is also significantly reduced by a streamlined protocol using 96-well plates and multichannel pipettes throughout the whole experiment, from cell culture to DNA isolation and qPCR analysis. These added benefits will also impact positively in the wider uptake of the model by other researchers since costs and time to achieved results will be significantly reduced.
DRYAD: Reduction of chickens use to perform in vitro pre-screening of novel anticoccidials by miniaturisation and increased throughput of the current Eimeria tenella compound-screening model, https://doi.org/10.5061/dryad.mw6m90605 (Marugan-Hernandez & Arias, 2022).
This project contains the following files:
- Experiment_1.xlsx
- Experiment_2.xlsx
- 1st_EXPERIMENT_-_2hpi_DMSO_-_ROB_20.pcrd
- 1st_EXPERIMENT_-_2hpi_ROB_5_-_24hpi_AMP_20.pcrd
- 1st_EXPERIMENT_-_24hpi_AMP_5_-_SAL_20.pcrd
- 1st_EXPERIMENT_-_24hpi_SAL_5_-_44hpi_ROB_20.pcrd
- 1st_EXPERIMENT_-_44hpi_ROB_5_-_52hpi_AMP_20.pcrd
- 1st_EXPERIMENT_-_52hpi_AMP_5_-_SAL_20.pcrd
- 1st_EXPERIMENT_-_52hpi_SAL_5-1___Controls.pcrd
- EXP_2._2hpi_DMSO-ROB_20.pcrd
- EXP_2._2hpi_ROB_5_-_24hpi_DMEM.pcrd
- EXP_2._24hpi_AMP_50_-_ROB_1.pcrd
- EXP_2._24hpi_SAL_50_-_44hpi_AMP_20.pcrd
- EXP_2._44hpi_AMP_5_-_SAL_20.pcrd
- EXP_2._44hpi_SAL_5_-_52hpi_AMP_1.pcrd
- EXP_2._52hpi_ROB_50_-_SAL_1.pcrd
- README.txt
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the 3Rs implications of the work described accurately?
Partly
Are a suitable application and appropriate end-users identified?
Yes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Parasitology, drug discovery & development, infection biology, immunobiology. Referee suggested by the NC3Rs for their scientific expertise and experience in assessing 3Rs impact.
Are the 3Rs implications of the work described accurately?
Yes
Are a suitable application and appropriate end-users identified?
Yes
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nutrition, Gut physiology, Poultry science
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 01 Oct 24 |
read | read | |
|
Version 1 04 Oct 22 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
Please enter your details to receive updates from the NC3Rs
We'll update you about the NC3Rs and the NC3Rs Gateway.
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)