Keywords
Linear alkylbenzene sulfonate, Microcystins, Brassica oleracea, Solanum tuberosum, combined effects
This article is included in the Agriculture, Food and Nutrition gateway.
This article is included in the Cell & Molecular Biology gateway.
Linear alkylbenzene sulfonate, Microcystins, Brassica oleracea, Solanum tuberosum, combined effects
Cases of harmful algal blooms (HABs) have been on the rise globally in the last few decades. Such increases have been linked to growing urbanization and the resultant increase in nutrients loads into the aquatic environment, higher atmospheric temperatures and salinity, all of which are worsened by human-induced climate change (Zhao et al., 2013; Howard et al., 2017; Díez-Quijada et al., 2018). South Africa is a semi-arid country with limited water resources and thus relies on surface waters for irrigation, domestic and industrial uses. The country mainly relies on man-made reservoirs for potable and irrigation water supplies and unfortunately, such surface water sources are increasingly being contaminated by cyanobacteria and cyanotoxins which can bioaccumulate in plant tissue when such water is used for irrigation. This makes the consumption of crops and vegetables irrigated with the contaminated water a potentially dangerous route for human exposure to different cyanotoxins.
There are numerous types of cyanotoxins that have been reported and documented, but the liver toxins, microcystins (MCs), are the most commonly occurring toxins in freshwater, hence the most widely studied. Contaminated drinking and recreational water are known to pose immediate human health threats, but recent studies have demonstrated food consumption as another exposure route, since cyanotoxins can accumulate in animal and plant tissues (Manubolu et al., 2018). Thus, drinking water and the consumption of contaminated fish, crops and other food supplements are now recognized as routes for human oral exposure (Díez-Quijada et al., 2018).
Due to the recognition of the potential health threats that could be posed by cyanotoxins, the World Health Organization (WHO) has set thresholds for MCs in both drinking water and food (Howard et al., 2017). WHO has set a provisional tolerable daily intake (TDI) of 0.04 μg kg-1 of body weight (b.w.) for MCs and 1 μg L-1 was set as the upper limit for MC-LR in drinking water (Díez-Quijada et al., 2018; Yao et al., 2019). In plants, MCs are also known to have numerous damaging effects such as reducing photosynthesis, causing necrosis of tissues, inducing oxidative stress, reducing productivity of crops, and causing economic losses (Campos et al., 2021).
Linear alkylbenzene sulfonates (LAS) on the other hand, belong to a group of anionic surfactants commonly used in domestic and industrial processes (Wang et al., 2015). Anionic surfactants are a common ingredient in detergents due to their simple synthesis and low cost (Pierattini et al., 2018). LAS is known to alter membrane permeability and in turn affect the toxicity and accumulation of other toxins such as cyanotoxins in organisms (Wang et al., 2012). LAS find their way into the aquatic environment through the discharge of untreated and treated wastewater. LAS elimination in the aquatic environment occurs via adsorption and biodegradation, but their degradation is very slow in anaerobic and anoxic environments and this leads to their accumulation under such conditions in water (Wang et al., 2012).
This makes hypereutrophic lakes and reservoirs ideal environments for the co-existence of toxic cyanobacteria since the excessive growth of cyanobacteria in eutrophic lakes consumes oxygen and their eventual death and degradation makes water bodies anoxic and anaerobic (Wang et al., 2012). Previous studies have reported enhanced MCs uptake by plants in the presence of LAS (Wang et al., 2012). Furthermore, Wang et al. (2015) also found increased production of MCs by Microcystis aeruginosa in the presence of LAS.
In South Africa, the Crocodile (West) Marico Water Management Area (WMA) which covers parts of Gauteng and Northwest Provinces, houses dams such as Hartbeespoort, Rietvlei, Roodeplaat, and Bospoort. For many decades these dams have been classified as hypertrophic (van Ginkel, 2004). Thus, the co-existence of pollutants such as LAS, cyanotoxins, and other pollutants is likely in these reservoirs and the ecotoxicological risk of cyanotoxins, such as MCs in combination with other stressors including LAS, on terrestrial food plants is of importance. It is particularly important because the water derived from these hypertrophic dams is mainly used for irrigation.
The aim of this study was to assess the effect of anionic surfactants (LAS) on the accumulation of three MC congeners (MC-LR, MC-RR and MC-YR) in Brassica oleracea (cabbage) and Solanum tuberosum (potato) plants using environmentally realistic concentrations of the pollutants. Water used in the study was collected from reservoirs in Crocodile (West) and Marico catchment, considering the prevailing hypertrophic conditions in the catchment.
A field survey was conducted from the 23rd to the 25th of June 2019 and again from the 14th to the 16th of September 2019 to collect field water to be used for the experiments. The water was collected from canals and farm dams from the Roodeplaat and Hartbeespoort dam sites. Total dissolved solids (TDS), EC, pH, and turbidity of the water were monitored in-situ and anionic surfactants, chlorophyll-a, microcystins (MCs), and cations were measured ex-situ. The water was kept frozen at −20oC until required. The LAS used in this study was sodium dodecylbenzene sulfonate (SDS) (CAS No. 25155-30-0; molecular weight 348.48 g mol-1; and chemical formula C18H29NaO3S acquired from BYMAZ Pty Ltd, Johannesburg, South Africa).
The B. oleracea seeds were purchased from NTK Agricultural Products & Services (S.A) and the S. tuberosum seeds were purchased from Livingseeds Heirloom Seeds (Pty) Ltd Midvaal, Gauteng (S.A). The S. tuberosum seeds were first washed with distilled water before being planted in 200 mm plant pots filled with non-contaminated soil. The B. oleracea seedlings were produced and pre-grown in plastic trays with non-contaminated soil. The soil used in this study was collected from the agricultural farm at the University of Venda. The farm lies in the low veld climate and has well-drained deep red soils mostly dominated by clay and falls in the Hutton classification, which is the same as the Rhodic Ferralsol (Mabasa, 2019). Regarding the main nutrients, phosphorus (P), potassium (K), total nitrogen (N) and organic matter, the soil contained 25.86 (mg kg-1); 184 (mg kg-1); 0.079% and 2.07%, respectively. All of which indicated healthy soils for plant growth. The soil was collected from a depth of 0-50 cm, and approximately 15 kg of the soil was placed into 350 mm plastic pots for the experiments and treated with half a cup of Protek General Fertilizer with N:P:K (%) 2:3:2 (14) before introducing the plants.
To investigate the effect of LAS on MCs uptake and accumulation in B. oleracea and S. tuberosum, plants were watered daily with dam water with a mean concentration ± standard deviation of MC-LR: 10.47 ± 3.879; 6.158 ± 4.127 for MC-RR, and 8.160 ± 2.544 for MC-YR. In order to maintain approximately constant concentrations of the LAS (3.4 mg L-1), the media were tested daily and refreshed accordingly. After 5 days and 20 days of exposure, the accumulations of MCs in edible parts of B. oleracea (leaves) were measured (Table 1). For S. tuberosum, the accumulated MCs in the tubers were assessed only after 20 days of exposure, since the seeds take longer to sprout. Leaves were also harvested from both plant species on the 20th day of exposure for total chlorophyll determination.
Chlorophyll content was measured according to Baskar et al. (2015). 50 mg of the leaves were crushed using a mortar and pestle, and then soaked in 10 mL of 95 % (v/v) ethanol, followed by incubation in the dark for 72 hours. This was then followed by centrifuging for 30 minutes at 2264 g, then collection of the supernatant and reading of the absorbance at 664.2 and 648.6 nm using a UV-vis spectrophotometer (SPECTROstar Nano, BMG LABTECH, Germany). Equations 1, 2 and 3 (below) were then used to calculate the chlorophyll-a and b, and the total chlorophyll content.
Total chlorophyll content was expressed as milligram per gram per fresh matter (FM).
Microcystins (MC-LR, MC-RR, and MC-YR) in plant tissues were determined using a triple quadrupole LC–MS/MS system (model 8045, Shimadzu Corporation, Japan). Toxin extraction was conducted using a modification of the method used by Manubolu et al. (2018). Accurately weighed, 100 g of plant material (leaves for B. oleracea and tubers for S. tuberosum) were freeze-dried for 48 hours at −48oC under a constant vacuum of 44 μmHg (Telstar Lyoquest Freeze Dryer, Terrassa, Spain). The freeze-dried material was then ground to powder using a mortar and pestle.
10 mL of 50% methanol solution was added to 1 g of each freeze-dried sample and sonicated for 5 minutes in a water bath (SCIENTEC Ultrasonic Cleaner, Model 705, South Africa) for 5 minutes. Upon sonication, the plant extracts were then centrifuged for 30 minutes at 2264 g. The whole process of sonication, centrifugation, and collecting the supernatant was repeated thrice and the supernatant was pooled to give approximately a 30 mL extract of each sample. The 30 mL extract was then cleaned up using solid phase extraction (SPE) with HLB (3 cc, 60 mg, Waters Oasis).
For SPE with HLB, the cartridges were first conditioned with methanol (6 mL), followed by ultrapure water (6 mL). The samples (± 30 mL) were slowly loaded onto the cartridges, followed by rinsing with 20% methanol. The cartridges were then eluted with 10 mL of 80% methanol. Lastly, the eluent was then dried at 50oC under a stream of nitrogen (N2) gas. The dried samples were reconstituted in 1 mL of 80% methanol prior to LC-MS/MS analysis.
Chromatographic conditions
Levels of microcystins (-LR, -RR and -YR) in the plant extracts were determined on a triple quadrupole LC–MS/MS system (model LCMS-8045, Shimadzu Corporation, Japan) with a Shim-pack Velox SP-C18, 2.7 μm, with dimensions 2.1 × 100 mm (Shimadzu, Japan). The injection volume was set at 10 μL and the mobile phases used were 0.1% formic acid (FA) in water (A) and 0.1% FA in acetonitrile (B). A flow rate of 0.4 mL min-1 and a 5-minute binary gradient was used with an elution profile of: 2% B (0.4 min), linear gradient to 70% B (3.1 min), 100% B (0.5 min), and, finally, 2% B (1 min).
The LC-MS/MS interface conditions were: 300oC interface temperature, 3 L min-1 for the nebulizing gas flow, 235oC DL temperature, 10 L min-1 for both drying gas and heating gas flow and interface voltage of 3.0 kV for electrospray in the positive (ES+) mode.
The final concentration of toxins in each sample was determined using equation 4:
Where = the concentration of the sample determined from the calibration curve (μg L-1).
Equation 5 was utilized to estimate the daily intake of cyanotoxins for an average size human (Bartos, 2020):
Where:
T = the concentration of toxins in the edible fractions of the cabbage (T, μg kg-1 fresh weight).
C = the daily consumption amounts of cabbage (C, kilograms per day).
W = weight of an average-sized human (W, 60 kg adult).
We assumed that the consumption of cabbage is similar to that of lettuce and used 85 g dry weight (DW) of cabbage and 148 g dry weight (DW) of potatoes based on the U.S. FDA (2017) suggested serving size (Bartos, 2020). An EDI of >0.04 exceeds the total daily intake limit set by WHO.
To compare the levels of accumulated MCs and the total chlorophyll of the various plant treatments, analysis of variance (ANOVA) and/or the Kruskal-Wallis tests were used at 95% confidence intervals (CI) using GraphPad InStat 3 (GraphPad Software, California, United States; RRID:SCR_000306). Levels of MCs are presented by their means ± the standard deviation (SD). Kolmogorov–Smirnov and Bartlett tests were used to test for normality and variance homogeneity at 95% CI. Data which passed this test was compared using ANOVA while data which did not pass the test was compared using Kruskal–Wallis at 95% CI. The Tukey-Kramer multiple comparisons test and Dunn’s multiple comparisons test were used as post-hoc assays for data which passed the normality tests and data which did not pass the normality test, respectively.
The dam water used to water the plants was alkaline, with a mean pH of 9.02 ± 0.29, had high EC and TDS levels (380 ± 16.52 μS cm-1 and 228 ± 7.51 mg L-1, respectively). The water also had a high cyanobacterial biomass (Chlorophyll-a 440.24 ± 328.147 μg L-1). The raw dam water had the following mean concentration ± standard deviation of MC-LR: 10.47 ± 3.879 μg L-1; 6.158 ± 4.127 μg L-1 for MC-RR, and 8.160 ± 2.544 μg L-1 for MC-YR. The pH of the dam water used for irrigation was above the 6.5–8.4 threshold for water intended for irrigation in S. A (DWAF, 1996). Even though the EC of the dam water was quite high, it was within the S. A (DWAF, 1996) guideline and the FAO (1985) (Ayers and Westcot, 1985) limits for irrigation water, which are set at ≤400 μS cm-1 and 700 μS cm-1, respectively. The levels of anionic surfactants in the water ranged from 0.13 to 3.4 mg L-1.
To identify and quantify MCs, two multiple reaction monitoring (MRM) transitions for MC-LR and MC-YR were selected and optimized, with the most abundant ionic product utilized for quantitation and the other for confirmation, whereas for MC-RR only one transition was used. For MC-LR and MC-YR, the single protonated molecular ions [M + H]+ were formed, as a result of the presence of one arginine moiety, which is the most preferred protonation site for these compounds (Zervou et al., 2017). The MRM transitions employed for MC-LR were 996.0078/996.00 and 498.5078/162.90; for MC-RR: 520.0078/134.90; for MC-YR: 1046.5078/1046 and 523.7578/127.00, with the first one being used for quantification and the second one for confirmation (for MC-LR and MC-YR).
With respect to all the three MCs monitored, the mass-to-charge ratio (m/z) signal 135 was the main ionic product. Like other polypeptides, MCs form sodium replacement ions which results in ion envelopes at each charge state apparent in mass spectra (Draper et al., 2013). For MC-RR the transition with a m/z of 520.0078 corresponds to the double charged protonated molecular ion [M + 2H]2+ precursor ions, as they contain two arginine residues in their molecular structure (Draper et al., 2013; Zervou et al., 2017).
Standard solutions at seven different concentrations (1, 2, 5, 10, 20, 50, and 100 μg L-1) were prepared using cabbage leaves extracts and potato tuber extracts and these were used to quantify the toxins in the plant samples (Figure 1). The MRM chromatograms of the quantification ions for the three MCs at a concentration of 100 μg L-1 are shown in Figure 2.
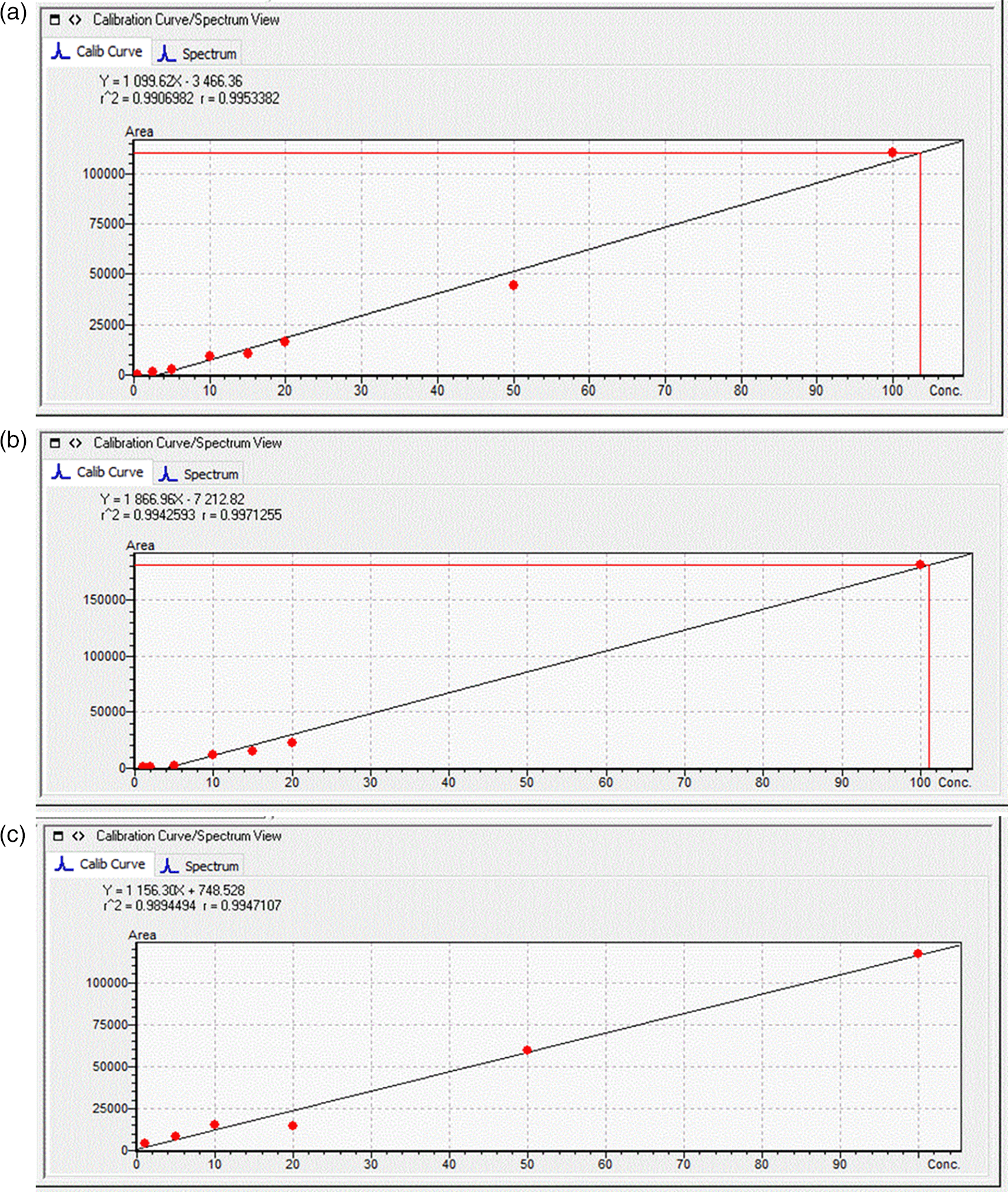
When exposed to the four different treatments for a period of 20 days, Table 2 shows the mean ± SE concentrations of MCs accumulated in the tubers of S. tuberosum. The accumulation patterns resembled the levels of MCs in the raw water samples, with plants exposed to raw dam water (T2 and T4) showing higher levels of MC-LR, followed by MC-RR then MC-YR. Statistically significant differences among the mean levels of accumulated toxins were reported for MC-LR and MC-YR, whereas MC-RR did show any statistically significant differences (ANOVA/Kruskal-Wallis Test, at 95% CI) among the four treatments. Higher levels of the toxins accumulated in plants exposed to raw dam water (T2) compared to the other three treatments. The presence of LAS in the raw dam water in T4 did not result in higher uptake and accumulation as anticipated.
Data labelled with a–d differed significantly (ANOVA/Kruskal-Wallis Test) at p < 0.05 in each row (n = 6).
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | p-value | EDI (mg kg-1 of body mass day-1) | |
|---|---|---|---|---|---|---|
| MCRR (μg g-1 DW) | 0.000253 (± 0.0001552)a | 0.004851 (± 0.0009748)a | 0.000535 (± 0.0001112)a | 0.00382 (± 0.001209)a | 0.0793 (n.s) | 0.007 |
| MCYR (μg g-1 DW) | 0.000554 (± 7.95E-05)a | 0.006404 (± 0.001069)a,b | 0.001167 (± 0.0003442)b | 0.005309 (± 0.0007399)a,c | 0.0007*** | 0.009 |
| MCLR (μg g-1 DW) | 0.016041 (± 0.008997)a | 0.200387 (± 0.05003)b | 0.022709 (± 0.009017)a,c | 0.150868 (± 0.0458)a,b,d | 0.0013 ** | 0.284 |
Except for MC-LR, the levels of MCs accumulated by the tubers did not reach levels high enough to exceed the TDI of 0.04 mg kg-1 of body weight recommended by WHO. Since MC-LR is normally the dominant congener in many waters dominated by the Microcystis and Aeruginosa genera, the raw dam water used was dominated by MC-LR, hence it was the only congener which exceeded the recommended TDI.
Regarding the accumulation of the toxins in B. oleracea leaves, Table 3 and Table 4 show the mean levels of MCs accumulated in the leaves of the plants after 5 days and 20 days, respectively. Based on the findings, a clear increase in the accumulation of the three MCs in the plants from the 5th day to the 20th day is observed. Statistically significant differences in treatments were observed for MC-YR and MC-LR after 5 days of exposure, with significantly higher accumulations observed in T2 followed by T4, compared to the other treatments (ANOVA/Kruskal-Wallis Test at p = 0.05).
Data labelled with a–d differed significantly (ANOVA/Kruskal-Wallis Test) at p < 0.05 in each row (n = 6).
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | p-value | EDI (mg kg-1 of body mass day-1) | |
|---|---|---|---|---|---|---|
| MCRR (μg g-1 DW) | 0.000583 (± 0.0005829)a | 0.011646 (± 0.006789)a | 0.000884 (± 0.000884)a | 0.012962 (± 0.008763)a | 0.5427 (n.s) | 0.018 |
| MCYR (μg g-1 DW) | 0.00059 (± 5.80E − 05)a | 0.005786 (± 0.0002905)b | 0.000603 (± 3.10E − 05)a | 0.005473 (± 0.0003488)b | <0.0001 *** | 0.008 |
| MCLR (μg g-1 DW) | 1.14E − 05 (± 6.82E − 06)a | 0.003271 (± 0.000625)b | 2.66E − 05 (± 6.20E − 06)a | 0.001076 (± 0.000435)a | <0.0001*** | 0.005 |
Data labelled with a–d differed significantly (ANOVA/Kruskal-Wallis Test) at p < 0.05 in each row (n = 6).
| Treatment 1 | Treatment 2 | Treatment 3 | Treatment 4 | p-value | EDI (mg kg-1 of body mass day-1) | |
|---|---|---|---|---|---|---|
| MCRR (μg g-1 DW) | 0.000155 (± 0.000155)a | 0.083923 (± 0.02975)b | 0.000683 (± 0.000683)a | 0.034973 (± 0.01751)a,b | 0.0143* | 0.119 |
| MCYR (μg g-1 DW) | 0.00063 (± 5.82E − 05)a | 0.006938 (± 0.000273)b | 0.000521 (± 1.41E − 05)a | 0.005134 (± 0.000344)c | <0.0001*** | 0.010 |
| MCLR (μg g-1 DW) | 2.17E − 05 (± 8.64E − 06)a | 0.005741 (± 0.000651)b | 1.41E − 05 (± 8.17E − 06)a | 0.002531 (± 0.000969)c | <0.0001*** | 0.008 |
Findings in Table 4 show that statistically significant differences were found among the treatments for all of the three congeners of MCs (ANOVA/Kruskal-Wallis Test at p = 0.05) in B. oleracea leaves upon 20 days of exposure to the four treatments. Similar to the patterns observed for S. tuberosum tubers and for B. oleracea leaves after 5-day exposures, higher levels of MCs accumulated in T2, followed by T4, compared to the other treatments. The findings imply that the presence of LAS in T3 did not have any impact on the uptake of MCs from the soil (without exposure to MCs in irrigation water) and that the presence of LAS in raw dam water in T4 did not enhance the uptake and accumulation of MCs by the plants.
Much of the work on the combined ecotoxicological risks of LAS and MCs has been done by Wang et al. (2011, 2012). According to Wang et al. (2011), LAS affects organisms by altering their membrane permeability, the activity of enzymes, and the structure of tissues in organisms (Wang et al., 2011). Unlike our findings, where the presence of LAS did not impact the accumulation of MC-LR in plants, Wang et al. (2011) reported higher accumulation rates when lettuce seedling were exposed to a combination of MC-LR and LAS compared to MC-LR alone.
Similar to our findings, where we found higher levels of MCs in potato tubers compared to cabbage leaves, Wang et al. (2011) reported higher levels in roots compared to other parts of the plants. In contrast to our findings, Wang et al. (2012) found enhanced uptake of MC-LR in duckweed even at the lowest concentration of 3 μg mL-1, which was comparable to the 3.4 μg mL-1 used in this study. The major difference between these experiments was the media in which the experiments were conducted, with Wang et al. (2012) having used aquatic plants compared to the terrestrial plants tested in this study.
In our study, the presence of microbes with the potential to degrade LAS in the soil could have been a major factor. According to Mao et al. (2015) at low concentrations, surfactants build up at the liquid to liquid or at the solid to liquid interface as monomers. Increasing their concentrations, eventually replaces the interfacial solvent, such as water, leading to decreased polarity of the aqueous-phase and a surface tension reduction. In high concentrations of surfactants, dissolved pollutants in the aqueous phase gain more mobility which is conducive for removal and uptake by plants and even degradation by microbes. Also, the properties of the soil and the surfactant itself influence the adsorption of a surfactant (Mao et al., 2015). In addition, previous studies have demonstrated that soils have the potential to temporarily make cyanotoxins unavailable for uptake by plants through chemical and physical modification, though this is dependent on the type of soil (Bartos, 2020).
The interaction and combination of LAS and other contaminants including microcystins and metal ions has been found to be both synergistic and, in some cases, antagonistic (Chai et al., 2020). Our findings did not suggest any synergistic nor antagonistic effects of LAS in combination with MCs in the water used. Consistent to our findings, Zhang et al. (2008) did not find increased uptake of Cadmium (Cd) by soybeans in the presence of LAS. Jensen and Sverdrup (2002) also did not find any combined effect of LAS and pyrene on the Folsomia fimetaria. According to Chai et al. (2020) synergistic or combined effects are influenced by a number of factors including the types of contaminants tested, the plant species, the concentrations tested, and the duration of exposure. In this study factors such as faster biodegradation of LAS by microbes and the low concentrations of LAS tested could all have affected the activity and toxicity to the plants of LAS, MCs, and other contaminants.
Even though MC-RR was of a lower concentration in the raw dam water compared to MC-LR, the findings of the current study have shown that it can accumulate in cabbage leaves to levels which can exceed the 0.04 mg day-1 kg-1 of body weight when plants are watered with contaminated dam water. This is of concern since this limit was reached after only 5 days of exposure to the dam water. However the fact that the EDI was not exceeded in the cabbage leaves after 20 days of exposure to the same dam water could be a reflection that the plants were finding ways of copying and bio-transforming the toxins as the exposure was prolonged. Xiang et al. (2020) established that the biotransformation and depuration rates of MC-LR in plants can sometimes exceed its uptake and there can also be higher degradation in soils, thus lower bioaccumulation from soil.
It is also important to mention that WHO has provisional TDI values for MC-LR only and not for other MC congeners and here we calculated EDIs for each of the three MC congeners monitored. Based on that, assuming that all the MCs have similar impacts on human beings, the combined EDIs for the MCs monitored here and those not monitored in this study can easily exceed the set TDI values.
Since exposure to increased levels of MCs in plants is known to induce oxidative stress, hinder the plants’ ability to produce chlorophyll, and inhibit photosynthesis (Campos et al., 2021), total chlorophyll levels in the two plant species were monitored to assess the potential effects of LAS and MCs on the plants. In addition to the two stressors (MCs and LAS), the dam water used in treatments 2 and 4 also had a high EC (380 ±16.52 μs cm-1) and TDS levels (228 ±7.51 mg L-1), indicating contamination with other salts. All these contaminants, for example MCs (Saqrane et al., 2008; Machado et al., 2017), high pH, high EC (Huang et al., 2017), and anionic surfactants (Pandey & Gopal, 2010; Wang et al., 2012) are also known to induce oxidative stress, reduce chlorophyll production, and affect plant growth.
Figure 3 (i) shows higher total chlorophyll content in the leaves of B. oleracea plants exposed to treatment 1 compared to other treatments. There were no statistically significant differences in the mean total chlorophyll content among the plants exposed to the four treatments after 20 days of exposure (p > 0.05). This implied that the levels of MCs, LAS, and other pollutants in the raw dam water used were not high enough to impact the synthesis of chlorophyll and other photosynthetic processes in the plants.
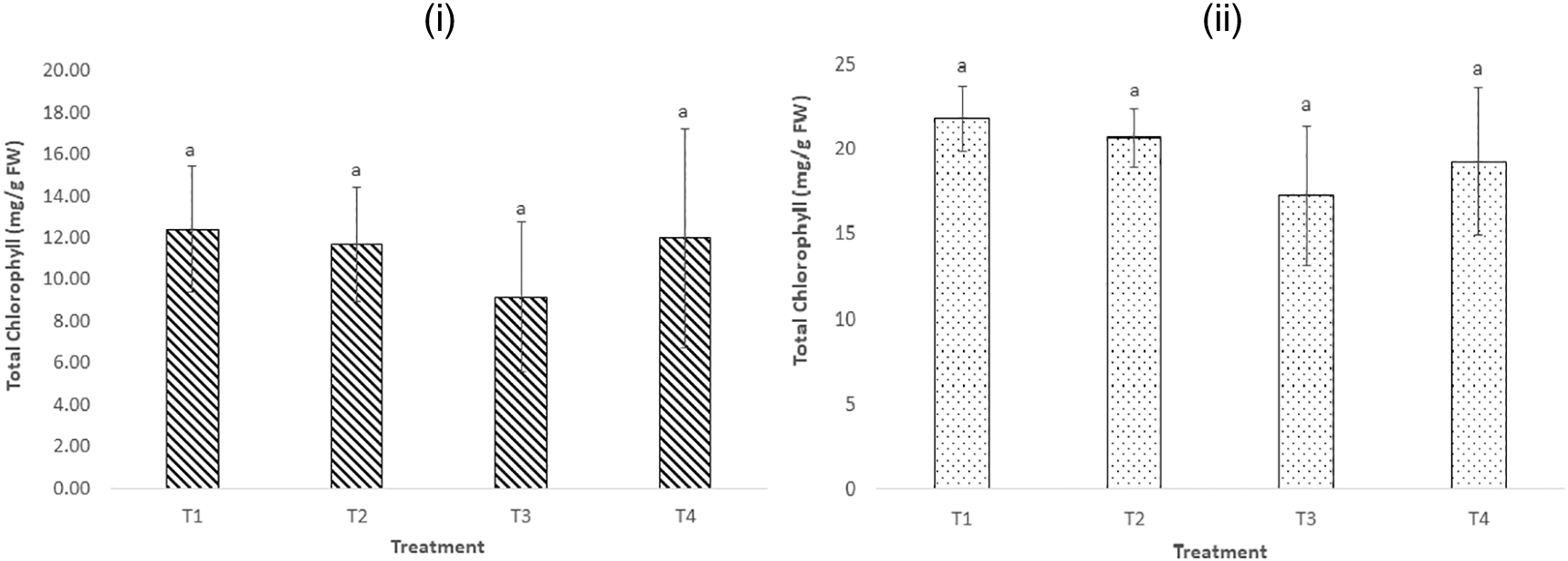
(n = 6). a indicates no significant difference among treatments (p > 0.05).
Concerning the total chlorophyll content of the S. tuberosum leaves, higher total chlorophyll levels were observed in plants exposed to treatments 1 and 4, but there were no statistically significant differences in the mean total chlorophyll levels among the four treatments (p > 0.05). The findings imply that exposure to environmentally relevant levels of MCs and LAS, as applied in this study, and the high EC in the raw dam water did not induce oxidative stress nor inhibit chlorophyll production in the plants. In addition, no significant visual impacts were observed on the plants exposed to the four treatments.
Based on the findings, the presence of the anionic surfactant (LAS) did not induce or promote the uptake of MCs by the two plant species. In addition, the presence of LAS and MCs in the irrigation water did not affect the total chlorophyll content and well-being of the tested plants. The study demonstrated that irrigation of terrestrial food plants with cyanobacteria-infested water from dams, such as Roodeplaat, can lead to MCs accumulating in the edible parts of the plants to levels that can exceed the set TDI of 0.04 mg kg-1 of body weight, as recommended by WHO. Long-term studies investigating the levels of cyanotoxins in irrigation water in areas such as the Crocodile (West) Marico WMA and their potential impacts on crop productivity and the dietetic acceptability of such plants for human consumption are recommended. Such studies will need to factor in the local climate, soil types, degradation rates of cyanotoxins in the soil, and also consider a variety of cyanotoxin classes for a proper risk assessment.
Pindihama G.K.: Conceptualization, Methodology, Data curation Roles/Writing - original draft; Writing - review & editing, Investigation; Methodology.
Gitari W.M.: Conceptualization, Methodology, Data curation Roles, Supervision; Funding acquisition; Investigation; Resources; Roles/Writing - original draft; Writing - review & editing.
Madala N.E.: Data acquisition/sample analysis, Writing - review & editing
The knowledge network for biocomplexity: Effect of linear alkylbenzene sulfonate on the uptake of microcystins by Brassica Oleracea and Solanum Tuberosum, DOI: 10.5063/F16M3589 (Pindihama, 2022).
This project contains the following underlying data:
- Data file 1: comparison of MC levels in the two plants
- Data file 2: total chlorophyll data for Brassica oleracea
- Data file 3: total chlorophyll for Solanum tuberosum
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors would like to acknowledge the Department of Nutrition (Faculty of Health Sciences) at the University of Venda for the use of the Shimadzu High Performance Liquid Chromatography Triple Quadrupole Mass Spectrometer (LCMS-8045).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
References
1. Máthé C, M-Hamvas M, Vasas G, Garda T, et al.: Subcellular Alterations Induced by Cyanotoxins in Vascular Plants-A Review.Plants (Basel). 2021; 10 (5). PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Plant stress biology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Ecotoxicologist with a good understanding of what is required in a scientific paper containing analytical chemistry data.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 26 Jan 24 |
read | read |
|
Version 1 12 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)