Keywords
anthropozoonosis, coronavirus, immunoassay, public health
This article is included in the Emerging Diseases and Outbreaks gateway.
anthropozoonosis, coronavirus, immunoassay, public health
The changes for this new version (third) of the manuscript are basic, following the recommendations of the referee regarding better explaining certain aspects of the justification of the study, methodology and improvement in the presentation of results (improvement of figure 3 and adding the new table 4).
See the authors' detailed response to the review by Diego A. Forero
Coronavirus disease 2019 (COVID-19) emerged in the Huanan Seafood Wholesale Market in Wuhan, China, in December 2019.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was the causal agent of this disease, which was declared as pandemic by the World Health Organisation (WHO) on 11th March 2020.2 The zoonotic origin of COVID-19 has been evidenced thanks to the high genomic similarity of SARS-CoV-2 with coronaviruses (CoV) recovered from bats3 and pangolins, the latter considered potential intermediate hosts of the virus.4 Betacoronaviruses such as SARS-CoV-2 belong to the family Coronaviridae. They exhibit linear single-stranded RNA of positive polarity5 and cause respiratory and gastrointestinal diseases in mammals.6 Even though humans are the most frequent route of transmission, it has been reported that cats (Felis catus) and dogs (Canis lupus familiaris) are susceptible to SARS-CoV-2 infection.7 This way, reverse zoonosis (anthropozoonosis) is viable through close contact with owners during acute infections.8
Since the beginning of the SARS-CoV-2 outbreak, different animal species have been implicated as possible intermediate hosts that could facilitate the virus transmission between species.9 This is the reason why the determination of these hosts has intensified, evidencing a number of reports involving wild, zoo, farm and pet animals.10 The zoonotic nature from which the transmission hypothesis has started, determines the importance of investigating animal species considered natural reservoirs of SARS-CoV-2.11 However, concern for the control and reduction of the spread of the virus has led to more vigorous investigation of the role that pet animals, such as dogs and cats, play in the spread of the disease. Although a cat-to-human transmission case was reported,12 it has been clearly defined that domestic canines and felines do not play a relevant role in the virus transmission to humans.8,13
On the other hand, human-animal transmission has been widely reported.14,15 This fact generates the need to investigate the implications for public and animal health, taking into account that animals are an epidemiological part of this pandemic.16 Various epidemiological and experimental studies, through serological detection of antibodies against SARS-CoV-2, neutralising antibodies, and detection of viral genome by reverse transcriptase polymerase chain reaction (RT-qPCR), have confirmed SARS-CoV-2 in pet animals around the world.17 Likewise, the occurrence of emerging variants has been described, as well as their influence on animals,18 for example, the Alpha variant (B.1.1.7) in dogs and cats with clinical signs of myocarditis,19 and the Delta variant (B.1.617.2) in dogs with clinical digestive and respiratory symptoms.20 Regarding the Omicron variant (B.1.1.529), concluded that the SARS-CoV-2 virus accumulated mutations within host cells in mice, giving rise to the Omicron variant that was transmitted to humans, indicating a ‘ping-pong’ (spillover and spillback) evolutionary trajectory between species.21 Transmission of SARS-CoV-2 Delta variant (AY.127) from hamsters to humans22 and animal-to-human transmission of SARS-CoV-2 within mink farms23 have also been reported.
Susceptibility to SARS-CoV-2 is determined by the affinity between the receptor-binding domain (RBD) of the viral spike (S) glycoprotein and the angiotensin-converting enzyme 2 (ACE2) of the host cell. Therefore, since vertebrates have conserved domains of ACE2, transmission of the virus between species becomes possible.24 Canines have lower susceptibility to SARS-CoV-2 infection in contrast to felines8,25 that exhibit greater respiratory pathology and efficient transmission of the virus to other felines through aerosols.26
In the context of the rapid evolutionary trajectory between species that SARS-CoV-2 has been developing, and taking into account the Report No. 13 of the World Organisation for Animal Health (OIE) of 31st May 2022, which reported 676 outbreaks in animals affecting 23 different species in 35 countries,27 the need for seroepidemiological monitoring in pet, wild and synanthropic animals becomes essential in order to broadly understand the adaptation, evolution and transmission of SARS-CoV-2.7
In Colombia, in December 2021, a lion exhibited symptoms of infection days after being in contact with a COVID-19 positive keeper.28 However, seroepidemiological studies of exposure to SARS-CoV-2 by pet animals have not been reported to date in the country. Since the dissemination of SARS-CoV-2 in dogs and cats is weak and short-lived, anti-SARS-COV-2 antibody detection studies are the best choice to determine the circulation of this virus in these companion animals. Of course, the indirect ELISA screening tests that detect immunoglobulin anti-RBD S1 SARS-CoV-2 are called for their greater accuracy in diagnosis.29 The goal of the present study was to determine the seroprevalence of anti-SARS-CoV-2 immunoglobulins (Ig) class G (IgG) in domestic dogs and cats and its epidemiological association with the frequency of COVID-19 patients in Villavicencio city, Colombia.
This research was endorsed by the Bioethics Committee of the Universidad de los Llanos, according to Minute 02 by consensus of April 6, 2021. In addition, all the owners of the dogs and cats involved in this study signed the respective informed consent.
This is a cross-sectional epidemiological study conducted in Villavicencio, Colombia. It consisted of applying a characterisation survey and taking blood samples from domestic canines and felines. The sample was estimated using the formula for size by proportions in finite populations, using the results obtained by Patterson et al.30 as a reference of p, with SARS-CoV-2 seroprevalences of 3.3% in dogs and 5.8% in cats from Italy. The population assessed in the present study corresponded to 68,651 domestic canines and felines in Villavicencio (47,573 canines and 21,078 felines), according to estimates from the report on anti-rabies vaccination of dogs and cats in Colombia.31 The confidence interval (CI) considered was 95% and the ‘Z’ value was 1.96 (1-α). The absolute precision considered was 0.15% (d = 0.0051).
The participants were selected based on their mandatory participation in the 2021 rabies vaccination campaign, carried out in Villavicencio (capital of Meta department) by the health secretary. A probabilistic sampling was conducted by randomly selected two-stage clusters of domestic dogs and cats from the eight communes (subdivisions) that compose the urban area of Villavicencio (Figure 1), which consisted of the random and proportional selection of individual dogs and cats, the sampling proportion of each cluster was determined according to the frequency of COVID-19 cases (RT-qPCR testing) in each commune32 (according to Table 1); for this, the EpiInfo v. 3.0 software, from the US Centers for Disease Control and Prevention (CDC) was used (https://www.cdc.gov/epiinfo/esp/es_index.html). The inclusion criteria considered domestic dogs and cats that had lived constantly in their homes for a minimum of two months before starting the present study. We determined as exclusion criteria, the animals that had consumed immunomodulatory medication (e.g., corticosteroid-type immunosuppressants) one week before the sampling were not included in the study.
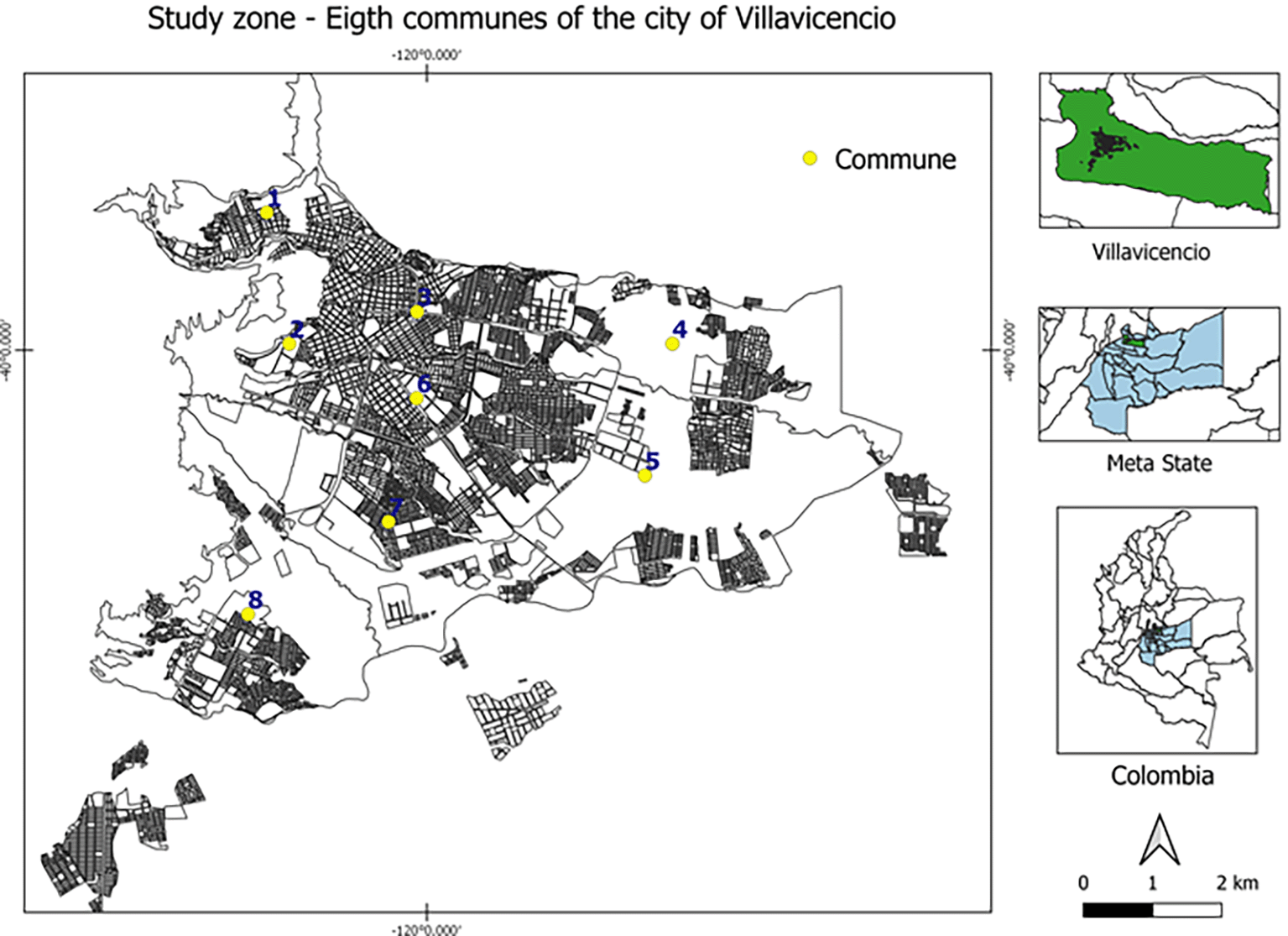
This figure is an original figure produced by the authors for this article.
Active human cases (RT-qPCR) in November 2020; data from the Villavicencio Municipal Health Secretary (2021).
| Commune | COVID-19* active cases (%) | Dogs | Cats | Total pet animals |
|---|---|---|---|---|
| 1 | 7.4 | 22 | 10 | 32 |
| 2 | 10 | 30 | 14 | 44 |
| 3 | 3.5 | 10 | 5 | 15 |
| 4 | 14.3 | 43 | 19 | 62 |
| 5 | 22.4 | 68 | 30 | 98 |
| 6 | 4.8 | 14 | 7 | 21 |
| 7 | 18.9 | 58 | 25 | 83 |
| 8 | 18.7 | 55 | 25 | 80 |
| Total | 100 | 300 | 135 | 435 |
A total of 435 blood samples were taken (300 domestic canines and 135 domestic felines). For this purpose, the authors of this study collected the blood from the jugular or cephalic vein, previous disinfection of the area with alcohol using a 21-gauge needle or vacutainer. Haemostasis was facilitated by applying pressure with sterile gauze in the sampling site for approximately 30 sec. The samples were centrifuged at 2000 g (Centrifuga Eppendorf 5424R) within three hours after being taken, and the sera were stored at -20 °C until analysis in a freezer (ABBA CVANF502B1). Table 1 shows the representative distribution of ‘n’ by commune (235 neighbourhoods) in Villavicencio.
Pet animals characterization was performed through a survey applied to the owners, following the model of a SARS-CoV-2 study that involved dogs and cats with COVID-19 patients in a metropolitan area.33 The characteristics of each pet recorded were: name; sex; age; species; breed; and owners’ names. This survey also inquired whether the individuals that cohabited with the pet animals (spontaneous communication) had histories of positive or negative RT-qPCR testing for COVID-19, and one of the survey questions was focused on the possibility for the owner to recognize if whether there were histories of clinical signs of the animals, such as signs in the upper or lower respiratory tract, or non-specific digestive signs (e.g., vomiting, diarrhoea, among others). Coordinates of the houses where the pets lived, were also recorded. The survey can be found as Extended data.57
IgG antibodies against the nucleocapsid protein (N) of SARS-CoV-2 in the sera of domestic dogs and cats were qualitatively determined using the indirect enzyme-linked immunosorbent assay (ELISA) (ID Screen® SARS-CoV-2, double antigen multi-species [IDvet, Grabels, France]) according to the manufacturer's instructions. For cats, the kit presents 63% of sensitivity and 96% of specificity. For dogs, the kit has 36% of sensitivity and 85% of specificity. Previous papers used the ID Screen® kit in their studies.34–37 Each plate contained 96 microwells sensitised with recombinant antigen of purified N protein of SARS-CoV-2, to which the following items were added: two negative controls (NC); two positive controls (PC); and 92 problem sera previously homogenised by vortexing. The optical density (OD) reading was performed using the Cytation 3 multimodal microplate reader (BioTek Instruments, Inc. Winooski, VT, USA) with a wavelength of 450 nm. In total, 435 problem sera samples and 25 pre-pandemic canine sera previously stored at -20 °C were analysed. Using the OD data of each well, the sample/positive control (S/P) ratio was calculated, which was expressed as a percentage using the following formula:
The test was validated when the mean OD value of the PC was greater than 0.350, and the ratio of the mean OD values of the PC and NC was greater than three. The samples were considered positive if the S/P ratio was greater than or equal to 60%, doubtful samples or samples in the gray zone had S/P ratios between 50% and 60%, and samples with S/P ratio less than or equal to 50% were considered negative.
The step-by-step protocol of this trial has been deposited under the title: Immunoassay of SARS-CoV-2 in dogs and cats V.1, DOI: dx.doi.org/10.17504/protocols.io.5qpvorn29v4o/v1 in protocolos.io (https://www.protocols.io/view/immunoassay-of-sars-cov-2-in-dogs-and-cats-v-1-5qpvorn29v4o/v1).
The frequencies of the data obtained in the survey and transformation of quantitative variables into categories for their subsequent analysis were estimated. The punctual seroprevalence (P) of SARS-CoV-2 in pet animals in Villavicencio was expressed as a proportion using the following formula, considering 95% CI:
The risk association measure odds ratio (OR), calculated by the binomial logistic regression model with 95% CI; was used in order to determine whether the frequency of active COVID-19 cases in humans by commune was related to SARS-CoV-2 seropositivity (exposure) of pet animals. Likewise, Spearman correlation was used to establish a possible relationship in the increase of cases in domestic animals at homes with COVID-19. Finally, using Kernel density analysis, the prevalence of COVID-19 in humans by commune was compared to anti-SARS-CoV-2 IgG seropositivity in domestic dogs and cats. A confidence level of 95% was used for all statistical calculations. Statistical estimates were made using the R 4.2 software with the packages dplyr, MASS, corrplot and epiDispaly, and the maps using the QGIS 3.10 software.
The overall seroprevalence of anti-SARS-CoV-2 IgG was 4.60% (95% CI = 3.2-7.4).57 Specifically, in canines the results indicated 3.67% (95% CI = 2.1-6.4), and in felines 6.67% (95% CI = 3.6-12.18) (Table 2). Twenty seropositive individuals (11 canines and 9 domestic felines) were detected through the enzyme immunoassay. In general, 22 animals with a history of respiratory signs (e.g., cough, runny nose, among others) were detected, of which 9.10% (95% CI = 2.53-27.81) were seropositive for SARS-CoV-2 (Table 3). Additionally, seven immunoassay results were classified as doubtful (gray area). Likewise, all 25 canine pre-pandemic sera were negative (Figure 2).
The following variables were taken into account: species, age, city communes and owners with positive or negative RT-qPCR testing.
| n | % | SARS-CoV-2 seropositivity | Crude OR | 95% CI | Adjusted OR | 95% CI | ||
|---|---|---|---|---|---|---|---|---|
| Species | Canines | 300 | 69 | 3.67% (11/300) | 1 | 1 | 1 | 1 |
| Felines | 135 | 31 | 6.67% (9/135) | 1.87 | 0.76-4.64 | 2.07 | 0.78-5.46 | |
| Age (Years) | 0-5 | 344 | 79 | 4.66% (16/344) | 1 | 1 | 1 | 1 |
| 6-10 | 78 | 18 | 3.85% (3/78) | 0.85 | 0.24-2.98 | 0.92 | 0.25-3.46 | |
| 11-15 | 13 | 3 | 7.69% (1/13) | 1.85 | 0.22-15.2 | 1.61 | 0.16-15.93 | |
| Communes | 1 | 32 | 7 | 0% (0/32) | 1 | 1 | 1 | 1 |
| 2 | 44 | 10 | 13.63% (6/44) | 4.17* | 1.52-11.49 | 5.84* | 1.1-30.88 | |
| 3 | 15 | 3 | 6.67% (1/15) | 1.48 | 0.18-11.86 | 1.97 | 0.15-25.69 | |
| 4 | 62 | 14 | 8.06% (5/62) | 2.13 | 0.75-6.12 | 3.13 | 0.57-17.36 | |
| 5 | 98 | 23 | 1.02% (1/98) | 0.17 | 0.02-1.29 | 0.37 | 0.03-4.2 | |
| 6 | 21 | 5 | 4.76% (1/21) | 1.02 | 0.13-8.01 | 1.96 | 0.17-23.06 | |
| 7 | 83 | 19 | 4.82% (4/83) | 1.07 | 0.35-3.3 | 2.02 | 0.36-11.45 | |
| 8 | 80 | 18 | 2.5% (2/80) | 0.85 | 0.18-4.67 | 1.04 | 0.21-12.21 | |
| Owners COVID 19 | COVID Test + | 25 | 6 | 4% (1/25) | 0.13 | 0.01-2.56 | 0.09 | 0-2.49 |
| COVID Test - | 410 | 94 | 4.63% (19/410) | 0.14 | 0.01-1.43 | 0.12 | 0.01-1.56 | |
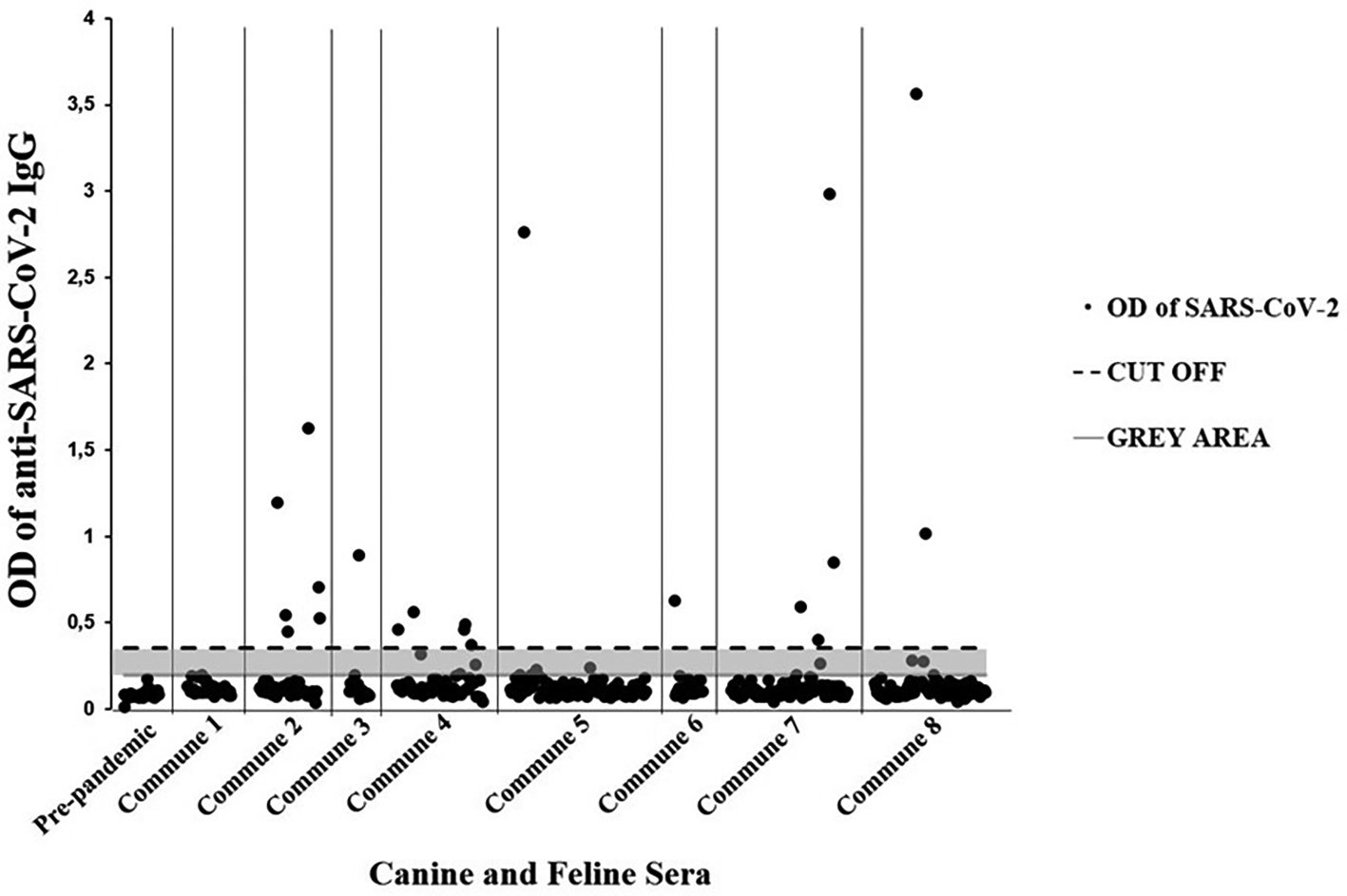
Ig = immunoglobulin class G; OD = optical density; SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2.
Regarding the eight communes, there was a general seroprevalence of 0, 13.63%, 6.67%, 8.06%, 1.02%, 4.76%, 4.82% and 2.5%, respectively (Table 2). In the map obtained through Kernel density analysis (Figure 3), it is observed that the density of cases was concentrated mainly in the west of the city. Communes with higher densities of SARS-CoV-2 seropositive animals were 2 and 4 in comparison to COVID-19 cases in humans, with a greater number of positive cases in communes 5, 7 and 8. Other visible sites of concentrations of seropositive animals, though with lower density, corresponded to communes 7 and 8. Finally, communes 1, 3, 5 and 6 had densities ranging from low to zero.
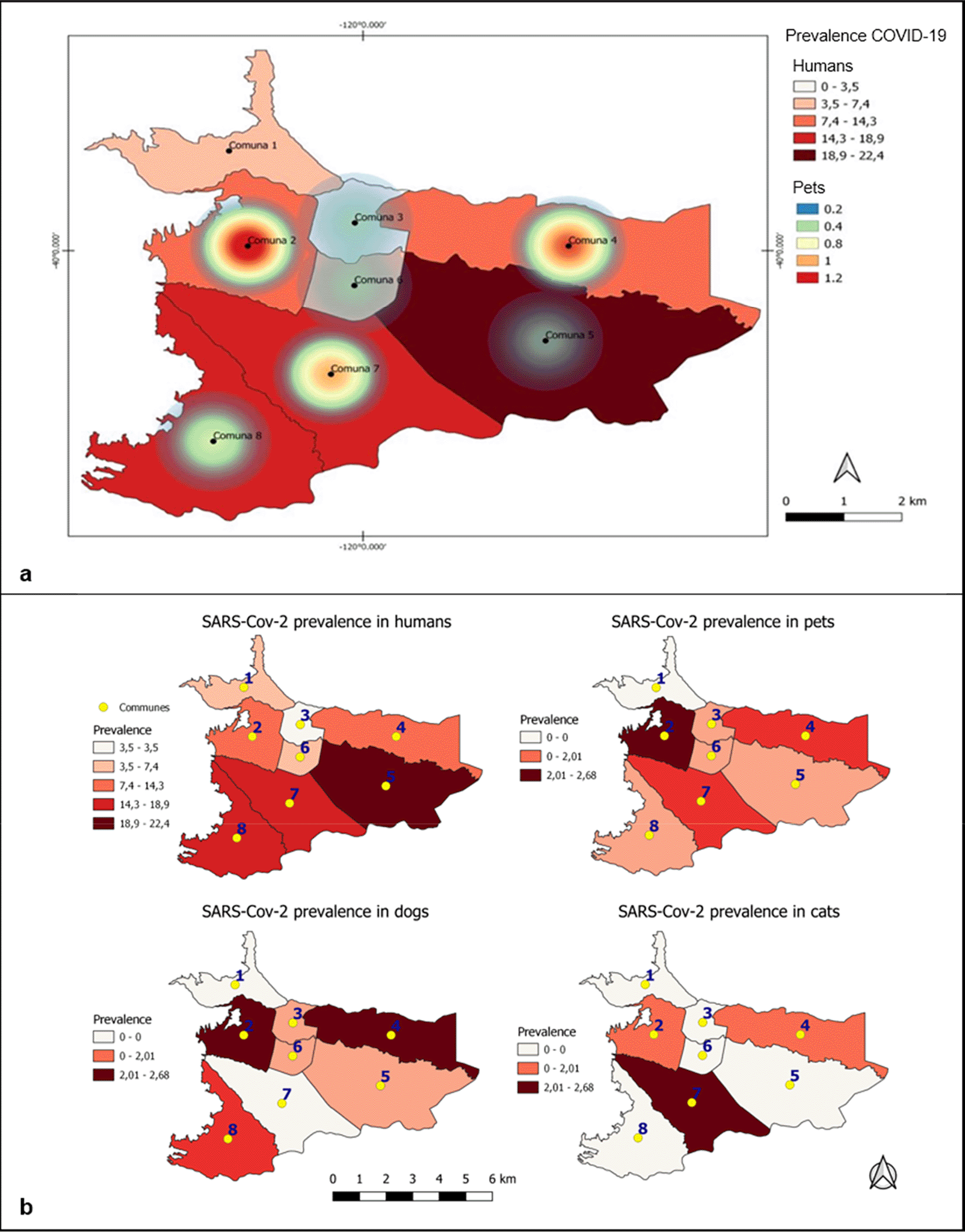
(a) Kernel density estimate of anti-SARS-CoV-2 IgG prevalence in dogs and cats in Villavicencio. The darkest colored shade represents the highest density of seropositive animals, and the lightest colors represent sites with the lowest densities. (b) Distribution of prevalences in humans, canines, felines and in general of these two domestic species according to each commune. Commune = Commune; COVID-19 = coronavirus disease 2019; IgG = immunoglobulin class G.
Regarding the SARS-CoV-2 exposure and the risk factors analysed, a statistically significant association between SARS-CoV-2 seropositivity and Commune 2 was found (adjusted OR = 5.84; CI 95% = 1.1-30.88). On the other hand, no significant statistical association was found (p >0.05) between anti-SARS-CoV-2 IgG seropositivity and the other items assessed (Table 2). Additionally, among the twenty seropositive animals, only one owner spontaneously confirmed to have positive RT-qPCR result for COVID-19.
Additionally, a Spearman correlation of p = 0.32 was found between the ratio of COVID-19 positive cases (RT-qPCR testing) of humans in November 2020 and domestic dogs and cats from the eight Villavicencio communes, result classified as a positive weak correlation. Finally, a strong positive correlation of 0.81 was found between the feline species and their SARS-CoV-2 seropositivity, as well as a positive correlation of 0.68 between the canine species and their SARS-CoV-2 seropositivity (Table 4).
| Humanos | Caninos | Felinos | Mascotas | |
|---|---|---|---|---|
| Caninos | 0.01 | 1 | -- | -- |
| Felinos | 0.33 | 0.17 | 1 | |
| Mascotas | 0.32 | 0.68 | 0.81* | 1 |
In the present study, the seroprevalence of SARS-CoV-2 in canines was 3.67% (11/300) and in felines 6.67% (9/135). Felines had more risk of becoming infected with SARS-CoV-2 that dog (adjusted OR = 2.07; 95% CI = 0.78-5.46) (Table 2) this tendency was no statistically different. In similar studies, Barroso et al.7 found SARS-CoV-2 seroprevalence of 4.7% in dogs and 21.7% in cats in Portugal, determining that, among seropositive animals, 50% had been possibly infected by human-animal transmission. On the other hand, 33.3% of seropositive cats had possibly been infected via the cat-cat route. Colitti et al.38 found a SARS-CoV-2 seroprevalence of 2.3% in dogs and 16.2% in cats in Italy, and Fritz et al.13 found a SARS-CoV-2 seroprevalence of 15.4% in dogs and 23.5% in cats from France. In all the studies mentioned, SARS-CoV-2 prevalence was higher in cats and its transmission was mostly related to exposure to humans when they were more seropositive and more susceptible to infection.27,38,39
As a result of the present study, a positive relationship between seropositivity and the age of the animals was observed.The older animals between 11 and 15 years exhibited this tendency predisposition, but it was not statistically different (adjusted OR = 1.61; 95% CI = 0.16-15.93) (Table 2). In this sense, a significant trend was found in the fatality and mortality rates of COVID-19 with advanced age in humans,40 given that there is a weakened immune system, underlying chronic diseases, multiple drug therapies, lack of attention and self-care, poor environmental hygiene, loneliness, and lack of adequate support from other family members in this population.41 These reasons could be considered with equal value in the case of animals, especially pet animals.42 On the other hand, Shi et al.25 reported that three-month-old canines exhibited low susceptibility to experimental infection, contrary to the results obtained in cats, since animals aged less than 100 days and up to nine months were highly susceptible to SARS-CoV-2 infection.
In the present study, no significant differences were found for respiratory and digestive symptoms of the animals sampled according to their SARS-CoV-2 seropositivity (X2 = 0.8206; p = 0.365) (Table 3). These results are similar to those reported by Pagani et al.43 and Shi et al.25 i.e., cats infected with SARS-CoV-2 were asymptomatic or highly susceptible to subclinical infections. Contrarily, in Germany, Keller et al.44 reported animals with mainly respiratory symptoms, describing the case of a cat with unresolved pneumonia, which was associated to the owner positive test for COVID-19. SARS-CoV-2-specific nucleic acid analysis was performed, revealing the complete genome and the presence of infection in that patient.
In both canines and felines, the highest seropositivity occurred in Commune 2 (13.63% [6/44]) (Table 2), which is located in the southwest of Villavicencio. Despite the fact that it is a commune with a low population (19,491 inhabitants),45 it has been reported with the highest number of inhabitants per house (6 inhabitants) in comparison to the other communes,46 suggesting that having more than one individual infected with SARS-CoV-2 in the same household increased the risk of infection in these pet animals.38 Likewise, this commune presented a positive association between the seropositivity of the animals sampled (adjusted OR = 5.84; 95% CI = 1.1-30.88) (Table 2) and the seropositivity of the owners, similarly, Colitti et al.38 found a positive association between COVID-19 positive owners and their felines’ SARS-CoV-2 seropositivity (OR = 2.5; 95% CI = 1.3-5.2), which may be related to the duration of the pets’ exposure to the infected owners, and the close contact of the felines with their owners, suggesting the development of antibodies in domestic animals as a consequence of viral transmission from owners.38,39,47 In the present study, the association between positive COVID-19 cases (RT-qPCR testing) in humans versus seropositivity in canines and felines from the eight communes of Villavicencio was weakly positive (Spearman's correlation of p = 0.32, Table 4). This significance may be influenced by the characterization of the survey, where due to social fear or ignorance some owners could indicate that they were not or had not been positive for the disease, while a strong positive relationship was expected, as in the case of the study conducted by Patterson et al.30 in Italy. On the other hand, the study conducted by Van Aart et al.48 showed that none of the felines had been infected with SARS-CoV-2 despite the fact that these were living with their positive COVID-19 owners. Therefore, these associations between species should be analysed considering different factors.
Animals and humans are susceptible to a large number of different coronaviruses, in fact, it has been shown that all pathogenic human coronaviruses have their origin in animals, which is why studies should focus on their role in the transmission of SARS-CoV-2.49 In the present study, a human-animal transmission was considered based on the results of Smith et al.50 in the United Kingdom, who ruled out that dogs and cats were reservoirs of infection for humans. However, we cannot be sure about the trasmission direction, which will only be confirmed through further studies. Ultimately, successful elimination of SARS-CoV-2 will only be possible by assessing and controlling transmission in all susceptible animal species, a one health approach that could prevent the re-emergence of the virus in the future.51,52 Although the 20 canine pre-pandemic sera reacted negatively to the immunodiagnosis, cross-reactions with ancestral coronaviruses in canines and felines are possible, but in low probability when are compared to commercial ELISAs with neutralizing antibody tests.53,54 The possible cross-reactivity and the need to verify if the reactive antibodies are neutralizing for SARS-CoV-2 are the main limitations of our study; likewise, it is advisable to carry out studies of “SARS-CoV-2 virus neutralization test from the serum bank obtained, since this is the gold-standard test for the Centers for Disease Control and Prevention (CDC).55
The present study provides the first positive results of anti-SARS-CoV-2 serological tests (ELISA) in domestic dogs and cats in Colombia, with information about the dynamics of virus transmission in Latin America and the world during the COVID-19 pandemic. As mentioned above, cats were more susceptible to natural SARS-CoV-2 infection than dogs, following similar dynamics described in other studies.7,38,56 The present study does not provide evidence that domestic canines and felines are sources of infection for humans; however, further studies focused on one health should not be ruled out52 in order to improve our knowledge about transmission, epidemiology and dynamics of SARS-CoV-2 and promote a better response to possible future pandemics.
Figshare: Seroprevalence of exposure to SARS-CoV-2 in domestic dogs and cats and its relationship with COVID-19 cases in the city of Villavicencio, Colombia. https://doi.org/10.6084/m9.figshare.21271137.v2.57
This project contains the following underlying data:
Figshare: Seroprevalence of exposure to SARS-CoV-2 in domestic dogs and cats and its relationship with COVID-19 cases in the city of Villavicencio, Colombia. https://doi.org/10.6084/m9.figshare.21271137.v2.57
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors are grateful to the support of the General Research Division of Universidad de los Llanos. Likewise, to professionals and other employees of the Villavicencio Municipal Health Secretary involved in the rabies vaccination program for domestic dogs and cats. An earlier version of this article can be found on SSRN: https://ssrn.com/abstract=4156064 or doi: http://dx.doi.org/10.2139/ssrn.4156064.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Diagnostics, Immunodiagnostics, Molecular Diagnostics, Research and Development of Diagnostics, Clinical Research of Diagnostics and Medical devices.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human health; molecular biology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human health; molecular biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Human health; molecular biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 10 Aug 23 |
read | read |
|
Version 2 (revision) 21 Mar 23 |
read | |
|
Version 1 17 Oct 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)