Keywords
Survival rate, medicinal cannabis, combined hepatocellular cholangiocarcinoma, cHCC-CC, palliative care, Northeastern Thailand
This article is included in the Oncology gateway.
Cholangiocarcinoma (CCA) incidence in Northeastern Thailand is very high and a major cause of mortality. CCA patients typically have a poor prognosis and short-term survival rate due to late-stage diagnosis. Thailand is the first Southeast Asian country to approve medicinal cannabis treatment, especially for palliative care with advanced cancer patients.
A retrospective cohort study compared survival among 491 newly diagnosed advanced CCA patients between September 2019 and June 2021. Of these, 404 received standard palliative pain management (ST), and 87 received medicinal cannabis treatment (CT). Patients were enrolled from four tertiary hospitals and two secondary hospitals in five provinces of Northeast Thailand. Cumulative survival was calculated by the Kaplan-Meier method, and independent prognostic factors were analyzed using Cox regression.
For ST patients, follow-up time was 790 person-months, with a mortality rate of 48.35/100 person-months. For CT patients, follow-up time was 476 person-months, with a mortality rate of 10.9/100 person-months. The median survival time after registration at a palliative clinic was 0.83 months (95% CI: 0.71–0.95) for ST and 5.66 months (95% CI: 1.94–9.38) for CT. Multivariate analysis showed CT was significantly associated with prolonged survival (HRadj = 0.28; 95% CI: 0.20–0.37; p < 0.001).
The medical cannabis increased overall survival rates among CCA patients. In this retrospective cohort, Medicinal cannabis treatment was associated with more prolonged survival among advanced CCA patients in Northeastern Thailand. While this association remained significant after multivariable adjustment, unmeasured or residual confounding factors may have influenced the observed outcomes. Although the association remained significant after adjustment, unmeasured or residual confounders may have influenced outcomes. Further prospective studies are warranted to confirm these findings and explore potential mechanisms.
Survival rate, medicinal cannabis, combined hepatocellular cholangiocarcinoma, cHCC-CC, palliative care, Northeastern Thailand
This version has been substantially revised in response to peer review. Key updates include expanded references (2019–2024) on medicinal cannabis, CCA/HCC epidemiology, and palliative care; standardized terminology; and detailed methodological descriptions to ensure reproducibility. The Conclusion has been refined to highlight the association between cannabis treatment and prolonged survival while noting the study’s observational limitations. The Limitations section has been expanded, and the study’s significance is now framed within the context of Northeastern Thailand. These changes enhance clarity, transparency, and the cautious interpretation of findings.
See the authors' detailed response to the review by Nuttapong Ngamphaiboon
See the authors' detailed response to the review by Selamat Budijitno
See the authors' detailed response to the review by Ueamporn Summart
Combined hepatocellular-cholangiocarcinoma (cHCC-CCA) is an uncommon and aggressive form of primary liver cancer that exhibits both hepatocytic and cholangiocytic differentiation within the same tumor.1 The global incidence rate is approximately 0.59 per 1,000,000 people,2 whereas Thailand reports significantly higher rates.3 Notably, Northeastern Thailand—particularly Khon Kaen Province—has the highest reported incidence of CCA worldwide at 118.5 per 100,000 population, exceeding the global rate by over 100-fold.3 Due to its asymptomatic nature in early stages, CCA is typically diagnosed at an advanced stage when metastasis has already occurred, resulting in limited therapeutic options, aggressive disease progression,4 and poor prognosis.5
Previous studies have shown the median post-diagnosis survival of CCA patients to be about 9 months (95% CI 7–11), with 1-, 3-, and 5-year survival rates at 43.4%, 21.5%, and 17.1%, respectively.6 Mean overall survival rate at 1-, 3-, and 5-year was 66.6, 41.5, and 32.7% for patients with transitional HCC-CC,7 with median survival time from diagnosis 4.3 months (95% CI: 3.3–5.1),8 and after supportive treatment was 4 months.9 Survival time was increased among CCA patients receiving surgery, an average of 29.38 months, best supportive treatment, 5.12 months, and 13.38 months for chemotherapy patients.10
Cannabis-based medicinal products are now legally available in several countries11 and are commonly used in palliative care to alleviate pain, reduce nausea, stimulate appetite, and improve quality of life in adults with cancer.12 Observational evidence also suggests that supervised medical cannabis is generally well-tolerated and associated with quality-of-life improvements over about six months of use.13,14 Thailand legalized medical cannabis in February 2019, the first country in Southeast Asia to do so15,16 and has since integrated cannabis-based options into palliative care services as an adjunct or alternative to standard care.17 In a recent retrospective cohort of advanced cholangiocarcinoma treated in northeastern Thailand, patients receiving cannabis-based treatment had a median overall survival of 5.66 months and an approximate one-year (12-month) survival of 29.98%, based on Kaplan–Meier estimates,18 highlighting a potential role alongside symptom management.
Survival data for cannabis-treated cholangiocarcinoma (CCA) remain limited, but some evidence suggests potential benefits. A U.S. inpatient study found that cannabis users with CCA had significantly lower in-hospital mortality than matched non-users (OR = 0.40; 95% CI: 0.16–0.97; p < 0.04).19 In Thailand, a prospective cohort reported improved functional status and quality of life at two and four months among CCA patients receiving cannabis-based treatment compared to standard care.13 Preclinical studies also indicate anticancer effects: cannabidiol (CBD) and cannabigerol (CBG) inhibited CCA cell proliferation and migration, inducing apoptosis and autophagy,20 while delta-9-tetrahydrocannabinol (THC) suppressed proliferation and promoted apoptosis via MAPK/MEK and Akt pathway inhibition.21 These findings, though preliminary, highlight the need for further clinical research in advanced CCA, where current survival outcomes remain poor.22
This study aims to analyze survival outcomes (SA) and identify factors associated with survival rates among patients with combined hepatocellular–cholangiocarcinoma (CHCC/CCA) diagnosed by their physicians as requiring palliative care, who then choose either cannabis treatment (CT) or standard treatment (ST; conventional medical care according to national clinical practice guidelines) after receiving information on available options. Retrospective cohort data were obtained from hospital electronic medical records (EMR) and cancer clinic databases across four tertiary and two secondary hospitals in five Northeastern provinces of Thailand. The results could provide preliminary evidence to support policy discussions and the development of evidence-based palliative care strategies under the national medicinal cannabis framework.
A retrospective cohort study was conducted with 491 patients—404 who received standard palliative care treatment (ST) and 87 who received medicinal cannabis treatment (CT). All patients were diagnosed with advanced cholangiocarcinoma (CCA) or hepatocellular carcinoma (HCC) by at least ultrasonography and managed with supportive care at a palliative care and/or cannabis care clinic between 1 September 2019 and 31 December 2020. Data were obtained from hospital electronic medical record (EMR) systems and cancer clinic databases from four tertiary hospitals and two secondary hospitals across five provinces of Northeastern Thailand: Roi Et Regional Hospital, Buriram Regional Hospital, Surin Provincial Hospital, Sawang Dandin Crown Prince Hospital, Panna Nikhom Hospital, and Pana Hospital.
Patients were eligible for inclusion if they were: Newly diagnosed with CCA or HCC between September 2019 and December 2020; Aged 18 years or older; and registered at either a palliative care clinic or a cannabis clinic.
Exclusion criteria included: Prior use of medicinal cannabis before study registration and incomplete medical records.
Independent variables included age at registration, gender, type of cancer treatment, and the period from diagnosis to registration. The primary outcome was post-diagnosis survival time, measured from the date of registration to the date of death or the study endpoint (30th June 2021). Patients alive at the end of the study or lost to follow-up were classified as censored cases.
Participants were followed from the date of registration until death or the study endpoint (30th June 2021). Follow-up was conducted through review of EMR entries, clinic visit records, and linkage to the national death registry.
Data on cancer stage, performance status, and pain score were not consistently available in the EMR and were therefore not included as covariates in the analysis.
The study protocol was reviewed and approved by the Maha Sarakham University Human Research Ethics Committee (Reference No. 204/2563, dated July 24, 2023), the Roi Et Regional Hospital Ethics Committee (Reference No. RE064/2563, dated 26 August 2023), and the Buriram Regional Hospital Ethics Committee (Reference No. GCP0066/2563, dated 4 Febuary 2023). Permission for data access and extraction was also obtained from hospital administrators and multidisciplinary teams at each participating hospital.
Descriptive statistics summarized patient characteristics. Categorical variables were reported as frequencies and percentages, and continuous variables as means with standard deviations (SD) or medians with interquartile ranges (IQR). Baseline differences between the standard treatment (ST) and cannabis treatment (CT) groups were assessed using chi-square or Fisher’s exact tests for categorical variables and independent t-tests for continuous variables.
Survival probabilities were estimated using the Kaplan–Meier method, with group comparisons made via the log-rank test. Independent prognostic factors were identified using Cox proportional hazards regression. The proportional hazards assumption was assessed using Schoenfeld residuals and log-minus-log plots.
A two-step approach was applied:
1. Univariable analysis identified variables associated with survival (p < 0.20).
2. Multivariable analysis included these variables to adjust for confounders and estimate adjusted hazard ratios (HR_adj) with 95% confidence intervals (CI).
Statistical significance was set at p < 0.05. Analyses were performed in Stata version 17 (StataCorp LLC, College Station, TX, USA).
Table 1 shows the study participants’ characteristics. Overall, most baseline characteristics did not differ significantly between the Standard Treatment (ST) and Cannabis Treatment (CT) groups. Significant differences (p < 0.05) were observed for chemotherapy, combined treatment, palliative care, time from diagnosis to registration (< 3 months), and survival status.
ST, standard palliative care pain management treatment group; CT, medicinal cannabis treatment group.
There were 491 patients (296 males and 195 females) with CCA and HCC; there were 404 in the ST group (242 males and 162 females) and 87 in the CT group (54 males and 33 females). The mean ages of the ST group were 66.60, and the CT group was 65.64 years. Most patients (43.38%) were 70 years of age. More than 71.53% in the ST group received cancer chemotherapy and combinations, and 49.42% of the CT group also received palliative care. The mean point of diagnosis with advanced CCA, HCC to registration was 8.65 months for ST, and 5.32 months for CT. Most patients (38.49%) were registered at the palliative and/or cannabis care clinic, and 94.60% (ST), 59.80% (CT) had died by the end of the study. The total follow-up time for ST patients was 790 person-months, with a mortality rate of 48.35/100 person-years. For the CT group, follow-up was 476 person-months, a mortality rate of 10.9./100 person-years for CT ( Table 2).
Abbreviations: ST, Standard treatment; CT, Cannabis treatment; HR, Hazard ratio; CI, Confidence interval; IR, Incidence rate.
Notes:
• All data were obtained from secondary sources (hospital electronic medical records.
• Median survival time is presented in months with 95% confidence intervals. Incidence rate is expressed per 100 person-months. Censored cases include patients alive at the study endpoint or lost to follow-up. Hazard ratios are adjusted for age, sex, type of cancer treatment, and period from diagnosis to registration using Cox proportional hazards regression.
• HR<1 indicates a reduced hazard of death, and specifically interpret the HR for medical cannabis as indicating prolonged survival.
Survival outcomes are presented in Table 2 and illustrated in Figure 1. For ST patients, the total follow-up time was 790 person-months, with a mortality rate of 48.35 per 100 person-months. For CT patients, the total follow-up time was 476 person-months, with a mortality rate of 10.9 per 100 person-months.
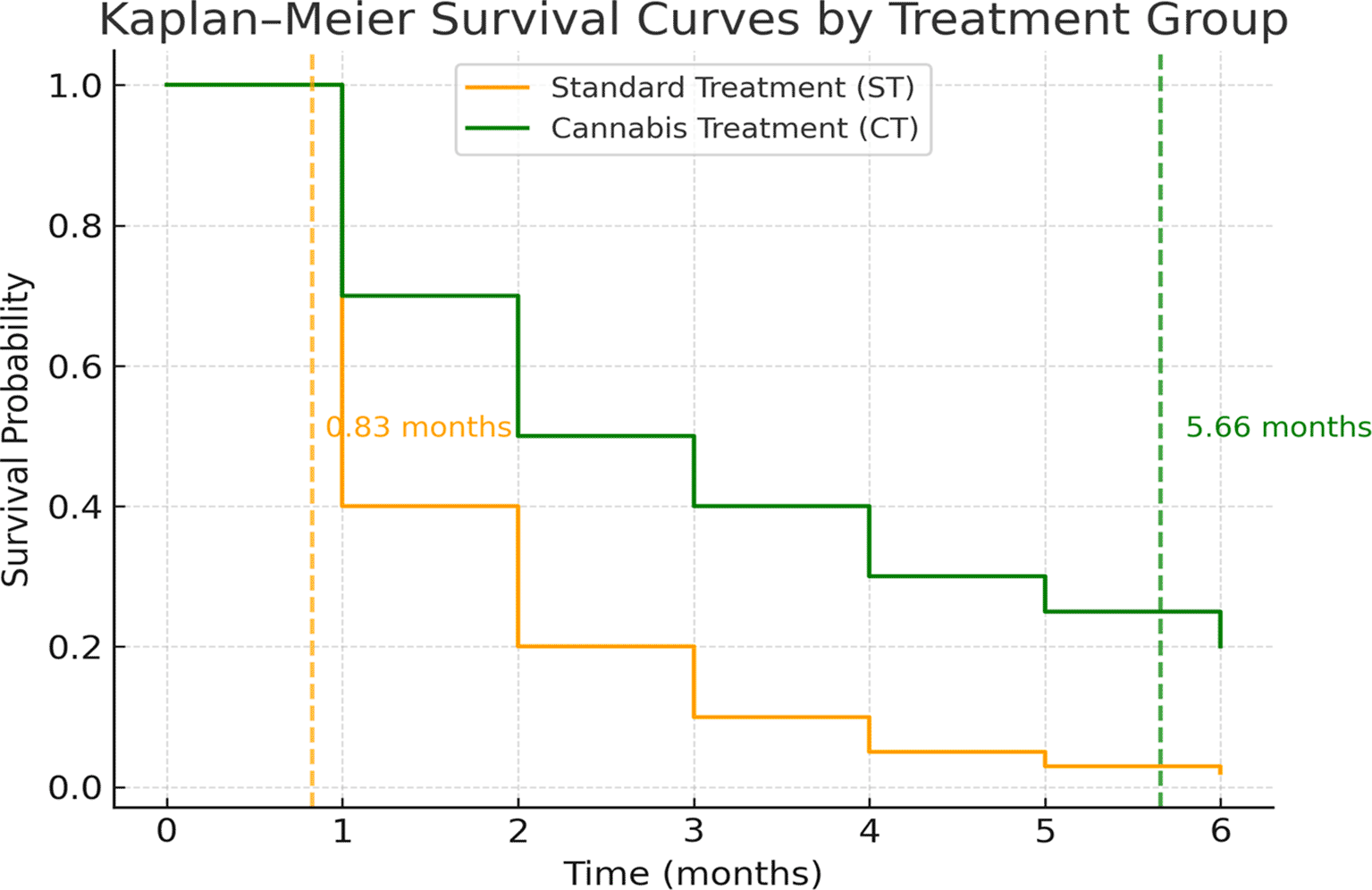
The median survival time was calculated from the date of registration at the palliative care or cannabis clinic to the date of death or the study endpoint (30 June 2021). The median survival time was 0.83 months (95% CI: 0.71–0.95) for the ST group and 5.66 months (95% CI: 1.94–9.38) for the CT group. Kaplan–Meier survival curves ( Figure 1) demonstrated significantly longer survival for the CT group compared with the ST group (log-rank test, p < 0.001).
In the Cox proportional hazards regression model adjusted for age, sex, and time from diagnosis to registration, receiving medicinal cannabis treatment was significantly associated with prolonged survival compared to standard treatment (adjusted HR = 0.28; 95% CI: 0.20–0.37; p < 0.001). Age, sex, and most types of prior cancer treatment were not significantly associated with overall survival. However, a time from diagnosis to registration of 3–6 months was associated with a higher risk of death compared to less than 3 months (adjusted HR = 1.31; 95% CI: 1.01–1.71; p = 0.044) ( Table 3).
In the present study, we examined the survival outcomes of patients with advanced cholangiocarcinoma (CCA) or hepatocellular carcinoma (HCC) receiving either standard palliative care treatment (ST) or medicinal cannabis treatment (CT) across tertiary and secondary hospitals in five provinces. Survival time was calculated from the date of registration at the palliative or cannabis clinic until death or censoring. The CT group demonstrated a markedly longer median survival time (5.66 months) compared with the ST group (0.83 months).
These findings are generally consistent with previous research in Northeastern Thailand, where patients receiving only supportive treatment had a median survival of 4.3 months after diagnosis.18 Moreover, patients diagnosed at an advanced stage were almost twice as likely to die (HR: 1.8; 95% CI: 1.1–2.9).23–25 However, our results contrast with Bar-Sela et al.,14 who reported shorter overall survival among advanced cancer patients using cannabis compared with non-users. This discrepancy may reflect differences in baseline characteristics, timing of cannabis initiation, disease stage, and access to other systemic treatments.
In the adjusted Cox regression model ( Table 2), receiving medicinal cannabis treatment was significantly associated with prolonged survival (adjusted HR = 0.28; 95% CI: 0.20–0.37; p < 0.001). However, the set of adjustment variables was limited to age, sex, type of cancer treatment, and time from diagnosis to registration due to the constraints of using secondary data.26 Important clinical parameters such as performance status, tumor burden, comorbidities, and detailed treatment history could not be included, and residual confounding is therefore possible.27
Baseline differences in care pathways may partly explain the observed survival advantage in the CT group. Many ST patients were referred to palliative clinics late in their disease trajectory, often after exhausting surgery, chemotherapy, or combination regimens.28 In contrast, CT patients—often older and treatment-naïve—were frequently registered directly at cannabis clinics in community hospitals following imaging or biopsy confirmation of advanced metastases.29 Some of these patients also received chemotherapy concurrently with cannabis, which may have contributed to extended survival.30
From a biological perspective, cannabinoids may improve survival indirectly by alleviating symptoms (e.g., pain, anorexia, nausea), enhancing nutritional intake, improving sleep, and enabling greater tolerance to systemic therapy.11,13,31 Preclinical studies also suggest potential anti-tumor effects via apoptosis induction, inhibition of angiogenesis, and suppression of tumor proliferation,28 although these effects remain to be validated in large-scale clinical trials. Taken together, the observed survival benefit in the CT group may be partly attributable to both patient- and treatment-related covariates, as well as the potential symptom-modulating and anti-tumor mechanisms of cannabinoids demonstrated in preclinical studies.11,13,15,18 This integrated interpretation underscores the multifactorial nature of survival outcomes in advanced CCA/HCC.
To our knowledge, this is the first multicenter Thai study to compare survival outcomes between ST and CT in advanced CCA/HCC patients treated with standardized, FDA-approved medicinal cannabis products under physician supervision.17–33 Our findings support the integration of medicinal cannabis into palliative care,33 especially in community settings with limited oncology resources.
Strengths of this study include the relatively large sample size, multi-level hospital participation, and the use of survival analysis adjusted for available covariates. Limitations include the retrospective design, reliance on secondary data,25 incomplete information on cannabis dosage/formulation/adherence, and the inability to adjust for important prognostic factors. Consequently, while the association between cannabis treatment and improved survival is compelling, causality cannot be established without prospective randomized controlled trials.26
N.P. contributed to the research design, data collection, and manuscript writing. P.P., A.W., contributed to data collection and revised the manuscript, and R. W contributed to research administrator, research design, data collection, review and revised the manuscript.
This study adhered to the principles of the Declaration of Helsinki and the International Council for Harmonisation (ICH) Good Clinical Practice Guidelines. It was a retrospective cohort study using secondary data from hospital medical record systems and reporting databases from oncology and cannabis clinics. All ethics committees waived the requirement for individual informed consent, as all patient data were anonymized before analysis. No direct patient contact, treatment modification, or additional intervention was performed. No animal experiments were conducted.
Given the retrospective nature of the study, the requirement for written informed consent was waived by the Maha Sarakham University Human Research Ethics Committee, the Roi Et Regional Hospital Ethics Committee, and the Buriram Regional Hospital Ethics Committee. All decisions regarding consent waivers were made in accordance with applicable regulations and ethical guidelines.
Patient data were available in the medical records room of the Roi-Et Regional Hospital, Burirum Regional Hospital, Surin Provincial Hospital, Sawang Dandin Crown Prince Hospital, Panna Nikhom Hospital, and Pana Hospital. The datasets generated and/or analysed during the current study are not publicly available because they are files in the medical records room in our hospital, but they are available from the corresponding author upon reasonable request.
Figshare: Data_survival_cannabis. https://doi.org/10.6084/m9.figshare.20101193.v1.34
Figshare: F1000_survival_table1_narisara_ranee. https://doi.org/10.6084/m9.figshare.20486913.v1.35
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to express their sincere gratitude to all patients and their families for their participation in this study. We are especially grateful to our colleagues at the Faculty of Medicine, Mahasarakham University, including the Hospital Center of Excellence Team, the palliative care clinic, and the cannabis clinic, for their invaluable assistance and encouragement throughout the course of this research. This study was financially supported by Mahasarakham University. We also thank Professor John F. Smith for his review and English language editing of the manuscript.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oncology
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical Oncology, biomolecular, Immunology, epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: surgical oncology, outcomes
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oncology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nursing and Public Health
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical epidemiology, pharmacoepidemiology, and social epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Bellera CA, MacGrogan G, Debled M, de Lara CT, et al.: Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer.BMC Med Res Methodol. 2010; 10: 20 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical epidemiology, pharmacoepidemiology, and social epidemiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Surgical Oncology, biomolecular, Immunology, epidemiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 03 Oct 25 |
read | read | |||
|
Version 2 (revision) 09 Apr 25 |
read | read | read | read | read |
|
Version 1 25 Oct 22 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)