Keywords
COVID-19, children, Critical Care, Paediatric Intensive Care Units
This article is included in the Emerging Diseases and Outbreaks gateway.
COVID-19, children, Critical Care, Paediatric Intensive Care Units
On January 30, 2020, the World Health Organization (WHO) reassessed the potential impact of COVID-19 on global public health and subsequently declared COVID-19 a public health emergency of international concern.1 This pandemic has caused a considerable number of hospitalizations among adults, especially in intensive care units, with many deaths were deplored, especially among the elderly population.1 Current research and data have proven that the clinical forms of paediatric cases are most often minor, moderate, or even asymptomatic,2 however, the disease can evolve into Acute Respiratory Distress Syndrome (ARDS), multi-organ dysfunction, and result in death in children.3 The first severe paediatric case published in China was of a one-year-old infant.4 Tunisian children were spared from severe forms during the first months of the pandemic. After two years of the evolution of the pandemic, publications concerned with severe paediatric forms, involving the vital prognosis, are scarce. The impact of comorbidities on the severity of infection is also controversial.
To our knowledge, this is the first publication concerned with severe and potentially fatal forms in a Tunisian paediatric population.
The objective of our study is to describe the clinical, biological, and therapeutic particularities of severe and critical SARS-CoV-2 infection among paediatric patients admitted in a Tunisian intensive care unit.
This research received ethical approval from the Committee of Medical Ethics and research of the University hospital of Farhat Hached (Sousse/Tunisia) (approval number CER-26-2022).
Personal information collected would be kept strictly confidential and used solely for the purposes of this study. Consent was waived by the ethical committee as this was a retrospective and descriptive study with medical records.
This is an observational cross-sectional study conducted in the paediatric department of Farhat Hached hospital of Sousse in Tunisia. Data were collected retrospectively from medical records. We covered the period of two years (from March 1st to February 28th, 2022).
Our hospital is a tertiary teaching hospital composed of 26 medical wards, four surgical wards, and nine laboratories. The paediatric department has a 42-bed capacity of hospitalisation, out of which 10 are dedicated to PICU.
Children were admitted through the local and regional emergency departments or regional hospital wards belonging to the centre region of Tunisia.
We included all children aged from one month to 15 years, who had revealed on admission or during the evolution, a severe or critical form of infection with SARS-CoV-2.
Criteria for performing SARS-CoV-2 testing evolved during the study period but predominantly required a febrile respiratory illness, or/and atypical symptoms (acute abdominal pain, diarrhoea …), especially among children with comorbidities and those who require PICU admission.
Confirmation was obtained either by the positivity of the nasopharyngeal reverse transcription-polymerase chain reaction (RT-PCR), and when outside the period of positivity for RT-PCR, using IgM and/or IgG antibodies positive against SARS-CoV-2.
We excluded patients whose diagnosis of Multisystem Inflammatory Syndrome in Children (MIS-C) was retained on admission.
The severity of COVID-19 infection was identified according to WHO classification5:
• Severe disease: SpO2<90% on room air, signs of pneumonia, or signs of severe respiratory distress.
• Critical disease: Requiring life-sustaining treatment, sepsis, or septic shock.
We collected different types of variables as detailed below:
• Demographic variables: Sex, age.
• Clinical variables: comorbidities, close contact tested positive for COVID-19, clinical symptoms at presentation, organ involvement, and dysfunction.
• Biological variables: SARS-CoV-2 rapid test result, Nasopharyngeal RT – PCR, IgM against SARS-CoV-2, and blood tests results.
• Radiological data: chest radiology, CT of the thorax, and echography result.
• Therapeutic variables: type of respiratory support, adjuvant treatment, hemodynamic support, and pharmacological treatment.
• Evolutionary variables: Acute Respiratory Distress Syndrome, Multisystem Inflammatory Syndrome in Children, and Healthcare Associated Infection.
During the study period, 125 children’s infected with SARS-CoV-2 were admitted to our department.31 Among these patients, 26 cases (20.8%) were identified as suffering from a severe or critical form of COVID-19. The median age in our series was six months [1-156 months]. 16 infants (61.5%) were under one year old and eight patients (30.7%) were school-age children and adolescents. There were two obese patients, at six and eight years old. Their body mass index (BMI) was 23 and 29 respectively. 17 (65.3%) patients had previous comorbidities. The median duration of symptoms before hospitalization was three days [one-10 days].
The most common symptoms at admission were fever in 23 cases (88.4%) and cough in 17 cases (65.4%). Polypnea, signs of struggles, and saturation of less than 90% on room air were observed in 21 (80.8%), 13 (50%), and 10 (42.3%) respectively.
Other signs were found as hypotension in four cases (7.8%), tachycardia in nine cases (34.6%), seizures in four children (23.1%) and gastrointestinal symptoms in 11 patients (42.3%) (Table 1).
| Characteristicsa | value |
|---|---|
| Sex | |
| Male n (%) | 18(69.2) |
| Female n (%) | 8(30.7) |
| Age | |
| ≤ 1 year n (%) | 16(61.5) |
| 1-5 years n (%) | 2(7.7) |
| ˃ 5 years n (%) | 8(30.7) |
| Comorbidities n (%) | 17(65.4) |
| None | 9 |
| Hypotrophy | 1 |
| Obesity | 2 |
| Cardiac | |
| Single ventricule | 1 |
| Interventricular communication | 1 |
| Operated coarctation of aorta | 1 |
| Congenital atrio-ventricular block | 1 |
| Neurological | |
| West Syndrome | 1 |
| Anoxo-ischemic | 1 |
| Genetic encephalopathy | 1 |
| Endocrine | |
| Type 1 diabetes | 2 |
| Hypothyroidism | 1 |
| Respiratory: Asthma | 2 |
| Immune deficiency: Ataxia telangiectasia | 1 |
| Haematological: sickle cell disease | 1 |
| Others | |
| Gastro oesophageal reflux disease | 1 |
| Malformative uropathy | 1 |
| Anophthalmia | 1 |
| Close contact tested positive for COVID-19 | |
| Absent n (%) | 16(61.5) |
| Familial n (%) | 10(38.4) |
| Clinical features at presentation | |
| Fever (°C), n (%) | 23 (88.4) |
| 38-38.5 | 8 |
| 38.5-39 | 9 |
| 39-41 | 6 |
| Respiratory signs | |
| Cough, n (%) | 17(80.9) |
| Tachypnea, n (%) | 21(80.8) |
| Signs of respiratory struggles, n (%) | 13(50.0) |
| SpO2 <90%, n (%) | 10(43.4) |
| Circulatory, n | |
| Tachycardia | 9 |
| Hypotension | 4 |
| Cardiac breath | 3 |
| Gastrointestinal, n | |
| Diarrhoea | 4 |
| Vomiting | 7 |
| Neurological, n | |
| Drowsiness | 7 |
| Hypotonia | 6 |
| Meningism | 1 |
| Bulging fontanelle | 1 |
| Seizure | 4 |
| Mucocutaneous signs, n | |
| Macular rash | 1 |
| Pseudo-varicella rash | 1 |
| Conjunctival hyperaemia | 1 |
During hospitalization, we collected 21 cases of respiratory distress (80.7%), six cases of shock (23%), four cases (15.3%) of heart failure (HF) including three cases occurring on congenital heart disease and one case of high abundance pericarditis complicated by tamponade in a 13-year-old child. Their evolution was rapidly favourable after emergency pericardial drainage, which brought back 700 ml.
Neurological distress was the reason for admission for three patients: one case of status epilepticus, one case of SARS-CoV-2 co-infection with Neisseria meningitides serogroup B, and one case of fatal encephalitis. Two children were admitted with Diabetic ketoacidosis (DKA), which was inaugural in one case. Besides, we have two cases of haematological involvement: an autoimmune haemolytic anaemia and an acute haemolytic crisis in a patient with sickle cell disease (Table 2).
The diagnosis was confirmed by RT-PCR for 24 patients (92.3%). The other two cases were confirmed by detection of IgM antibodies in the presence of suggestive symptoms. A rapid antigen detection test was maintained for fifteen children before confirmation by RT-PCR and it was recorded positive in 13 cases (86.6%).
Chest radiography showed bilateral diffuse alveolar-interstitial infiltrate in nine cases (among 12 performed chest radiography).
Ground glass opacities were present in the chest-computed tomography (CT) of 11 patients.
Specialized echocardiography was performed in 11 cases. It showed a large circumferential pericardial effusion of 35 mm with tamponade in one case (Figure 1).
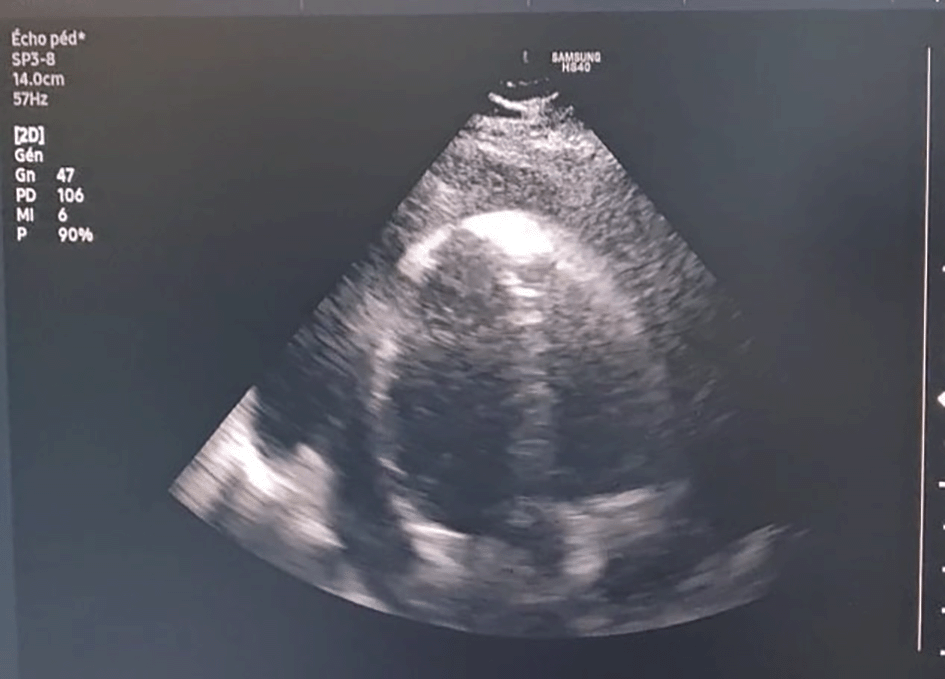
Additional laboratory and radiological findings are shown in Table 3.
Twenty four patients (92.3%) required some type of ventilator support. Twelve patients (46.15%) were managed noninvasively. One patient was put on non-invasive ventilation (NIV) at Bilevel Positive Airway Pressure (BIPAP) and then put on high-flow nasal cannula oxygen therapy (HFNC). Ten other children had received HFNC, immediately in five cases and after standard oxygen therapy in five cases.
Mechanical ventilation (MV) was initiated in six cases (23%). Intubation was done on the day of admission for three patients. For the other children, it was done during the therapeutic escalation on the fourth, twelfth, and twenty-third days.
Among those requiring MV, the indication was severe ARDS in three cases, requiring conventional ventilation and then the use of high-frequency oscillation ventilation (HFO), and nitric oxide (NO) (n = 3). The mean duration of MV was nine days [1-27 days].
Antibiotics and corticosteroids were administered to 22 (84%) and 11 (43%) patients respectively. Intravenous immunoglobulin therapies (Ig IV) were administered to five patients: one patient who had ataxia telangiectasia, one patient who had severe myocarditis, and three patients who had presented MIS-C criteria in the course of evolution.
No patient used specific SARS-CoV-2 treatments like Remdesivir or Hydroxychloroquine.
Intratracheal surfactant instillation at the dose of 50 mg/kg X 3/day was performed in an ARDS that escaped other therapeutic alternatives with an initial good response to treatment (Table 4).
| Type of medical care | n (%) |
|---|---|
| Respiratory support | |
| Standard nasal O2 therapy | 16(61.5) |
| High Flow Nasal Cannula (HFNC) | 11(42) |
| Non Intensive Ventilation (NIV) | 1(3.8) |
| Bilevel Positive Airway Pressure (BIPAP) MV | 6(23) |
| Other adjuvant treatment | |
| Curare | 1(3.8) |
| Nitric oxide (NO) | 3(11.5) |
| Hemodynamic supports | |
| Fluid bolus therapy | 8(30.7) |
| Noradrenaline | 3(11.5) |
| Dobutamine | 5(19.2) |
| Pharmacologic treatment | |
| Antibiotic | 22(84) |
| Corticosteroids | 11(42.3) |
| Heparin therapy | 10(38) |
| Preventive dose | 8(30.7) |
| Curative dose | 2(7.6) |
| Clonazepam | 2(7.6) |
| Intravenous immunoglobulin (Ig IV) | 5(19.2) |
| Surfactant | 1(3.8) |
ARDS was documented in four children (15.3%) of our sample and was severe in all four cases of them. One of these children was supported with NIV then HFNC, the second was initially supported with HFNC and he required escalation to invasive mechanical ventilation. The other two patients were supported at admission with invasive mechanical ventilation.
Deaths occurred in six children, so the mortality rate was 23%. Five of them had comorbidities as congenital heart diseases, West syndrome, hypotrophy, and probable immune deficiency. Concerning the case of probable immune deficiency, we performed an immune assessment which showed a profound and persistent lymphopenia on all lymphocyte lineages with a normal mitogen response to PHA and very weak to anti-CD3, a normal NBT test.
One infant was hospitalized with critical form, encephalitis, and multisystem organ failure and died the same day (patient 4).
The role of SARS-CoV-2 in the outcome of (Patient 1, Patient 2, Patient 5, and Patient 6) is uncertain since the length of hospitalization is extended and the children presented other complications. Additional information is shown in Table 6.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Age, months | 1 | 2 | 2 | 1 | 7 | 27 |
| Sex | Male | Female | Male | Female | Male | Male |
| Comorbidities | Probable immune deficiency | Hypotrophy | VSDa | - | Congenital heart disease | West Syndrome |
| Organ dysfunction | ARDSb | Heart failure | Encephalitis Sepsis | Heart failure | Status epilepticus | |
| Complication | MIS-Cc DVTd | HAIe | MVFf | MIS-C | MIS-C | |
| Duration of stay in PICUg (days) | 27 | 28 | 8 | 1 | 13 | 17 |
| Cause of death | MVFf | Septic shock | MVF | MVF | Shock | Shock |
In this study, we report the characteristics and clinical course of 26 severe and critically ill infants and children with COVID-19 hospitalized during the first two years of the pandemic at paediatric department of Farhat Hached Sousse Tunisia.
The first paediatric publications concluded that the disease was mild.2 Publications of severe and critical paediatric forms, involving the vital prognosis, are still rare. Thereupon, several studies were published in the west demonstrating the characteristics of children infected with COVID-19 and requiring intensive care.6–8 In our series, the percentage of children admitted to intensive care was 20.8% of the total number of children hospitalized for COVID-19. This percentage was higher than the rate reported in a series published earlier in China, which included 2,135 children. In the literature, the rate of admission to the PICU varies from 5.9 to 39% among hospitalized children.2,3,8
According to our findings, Chinese publications settled that the majority of serious and critical cases were seen in children under the age of one year.2
This has important implications and requires amplified monitoring in children less than one year of age. However, we noted that the age group of one to five years was the less affected. This result was observed in North America.6 Consistent with other series, our study found that school-aged children and adolescents exceed 30% of patients hospitalized for COVID-19 in PICU.6,8
We noted in our series the predominance of the male gender. One key discovery in understanding the mechanism of SARS-CoV-2 infection involves the role of the transmembrane serine protease 2 (TMPRSS2), MPRSS2 is a critical factor enabling cellular infection by SARS-CoV-2. Some authors put forward the hypothesis that the modulation of its expression by sex steroids could contribute to the male predominance of severe infection.9 A preponderance of the male gender was noted in the paediatric series of Chao et al. (67.4%)7 and Shekermedian (80%)6 but Tsabouri et al., concluded that male sex is not an independent risk factor for severity in children.10
Moreover, multicentre studies have described high rates of comorbidities in children hospitalized in PICU with COVID-19.11 Congenital heart disease was communal in our series, followed by neurological and endocrine aetiologies. These comorbidities were predominant in severe cases in the series of the literature.12,13 The hypotheses proposed to explain the severity of COVID-19 in children with congenital heart disease by: the destructive effect of COVID-19 on the heart,13 the poor prognosis of influenza and other viral respiratory diseases in these children,14 and the fact that many of these patients may have concomitant abnormalities in other organs, such as the lungs and kidneys.15 On the other hand, the severity of these forms in children who have neurologic disorders could be attributed to the decrease in muscle tone and strength, mobility alteration or structural conditions.16 Early studies have shown that individuals at high risk of metabolic dysfunction appeared at higher risk for complications, but the relationship between diabetes and COVID-19 is still not well understood. Instead, a new hypothesis has emerged, which suggests a bidirectional relationship between diabetes and COVID-19.17
As was expected, in our study, and in published studies, the most common presenting symptoms were fever followed by lower respiratory tract symptoms in more than 60% of children.8
Likewise, COVID-19 can progress to ARDS and multi-organ dysfunction. To emphasize, respiratory and cardiovascular involvement were predominant in our series. In our series, we noted four cases (15.3%) of ARDS. This was similar to that observed in paediatric COVID-19 patients admitted to intensive care units in Brazil (13%).8 In our series, only one child did not need MV and was supported with NIV and HFNC. In general, SARS-CoV-2 ARDS is described as an atypical form of ARDS. The main characteristic is the dissociation between relatively well-preserved pulmonary compliance and the severity of hypoxemia.18 In our series, we found that respiratory and hemodynamic distress were the main indications for ICU hospitalization. Fisler et al., have found that hypoxia, hemodynamic instability, pneumonia, or ARDS were the most frequent indications for PICU admission.3
Paying attention to the two cardiovascular manifestations of the patients of our series: Tamponnade and myocarditis. Pericarditis complicated by tamponade has been described even in children with COVID-19.19 The pathogenesis of tamponade secondary to COVID-19 is still unknown. Two mechanisms could be incriminated. The first one: cardiac affinity of the virus which could be explained by the direct binding of the S protein of SARS-CoV-2 to the human angiotensin converting enzyme 2 present in the human heart, allowing a cellular infection.20 The second mechanism: the cytokine storm triggered by an imbalanced response of type 1 and type 2 helper T cells.21
Myocarditis is a cardiac manifestation that was also observed in our study. The mechanism of cardiac injury is not well defined, but it might be caused by direct myocardial infection, hypoxemia, and indirect injury due to systemic inflammatory response. Myocardial necrosis and endothelial involvement were found in biopsies performed in post-mortem. Given that, pathological processes included direct myocardial injury through virus binding to ACE2, systemic inflammation, and altered myocardial demand-supply ratio.22
Since our series is unusual by the diversity of manifestations extrapulmonary, we have identified neurological symptoms, which ranged from non-specific or specific mild symptoms such as altered state of consciousness and seizures. For neurological distress, in the series of Qiu et al., refractory seizures were the main causes of admission to PICU.23 Two infants had encephalitis. An autopsy report has documented the presence of SARS-CoV-2 in the brain tissue of a COVID-19 patient. Thus, there is no definitive conclusion on the mechanisms of SARS-CoV-2 neuro-invasion. These proposed mechanisms include direct viral invasion, systemic blood circulation, or distribution of infected immune cells.24
One patient had a Meningococcal meningitis and COVID-19 co-infection.
Bacterial coinfection is more common in paediatrics, particularly for pneumococcus and meningococcus. The frequent carriage of the bacteria explains coinfection with meningococci at this age. However, the mechanism is not yet known. Bacterial co-infection with meningococcus was also reported in a 22-year-old woman.25
In our series, two children were hospitalized with severe DKA. A descriptive study carried out in Germany resolved that there was a significant increase in the frequency of diabetic decompensation in the area of COVID-19 in children newly diagnosed with type 1 diabetes.26 SARS-CoV-2 can trigger severe DKA for people with new-onset diabetes. However, at present, there is no perceptible evidence that SARS-CoV-2 induces type 1 diabetes.27
Management is essentially symptomatic. With the acquisition of experience in the management of COVID -19 patients, clinicians realized that the mortality on invasive ventilation was high and that when HFNC is used with precautions, it avoids intubation.28 Until now, the use of ONHD in case of ARDS complicating SARS-CoV-2 infection in children is exceptional. In a study carried out in the United States of America, seven children with ARDS were initially treated by HFNC, four had subsequently required mechanical ventilation.7 HFNC could have a central role in improving the disease. We need more data and guidelines. Patients treated with HFNC should be closely monitored to detect the need for therapeutic escalation and mechanical ventilation. Patients in our study did not receive Remdesivir or Tocilucimab. Remdesivir was not available in Tunisia and Tociluzimab did not have marketing authorization in paediatrics. We tried exogenous surfactant treatment for our 1-month-old patient with ARDS. In the literature, surfactant was effective in the treatment of ARDS in combination with other therapeutic measures.29,30
Mortality was marked high in our series (23%)compared with the initial paediatric reports.2,6–8
Our study has important strengths. First, it is the first Tunisian paediatric series of severe and potentially fatal cases of COVID-19 reported. Secondly, this series is representative of the centre region of Tunisia, as our hospital covers most of the Tunisian central region.
The main limitation of our study was its cross-sectional design, which does not allow prospective surveillance of patients. Secondly, there is a diversity of factors explaining the severity of COVID-19, which can be related not only to patient-related factors but also to extrinsic factors.
Paediatric patients represent a small percentage of all severe and critical SARS-CoV-2cases in the general population. Nevertheless, paediatricians must be vigilant. Our study highlighted the large spectrum of vital clinical presentations as well as the significant occurrence of potentially fatal paediatric cases.
Careful monitoring and early intervention are necessary for an infant less than one year of age and in the presence of comorbidities. We hope that this series will help manage other similar patients with the same conditions. Prevention measures are also necessary to prevent the spread of COVID-19, with the need to protect children who are vulnerable and have comorbidities.
Zenodo: Paediatric critical COVID-19: clinical features and outcomes during five waves. https://doi.org/10.5281/zenodo.7133628. 31
This project contains the following underlying data:
• Severe covid pediatric.sav (anonymised underlying data collected from patient records for this study)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Intensive Care
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
References
1. Verdugo P, Álvarez P, Aroca P, Montes V, et al.: Hematologic parameters and biomarkers predictors of severity in Multisystem Inflammatory Syndrome in children associated with SARS-CoV-2.Andes Pediatr. 2021; 92 (3): 382-388 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Pediatric Critical Care
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 25 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)