Keywords
Betadine, sap, Jatropha multifida L., Pseudomonas aeruginosa L., Infection, Antibacterial, Nosocomial, Opportunistic.
This article is included in the Plant Science gateway.
Today, people use plants to treat various types of diseases and improve human health. One of the medicinal plants is the Betadine plant (Jatropha multifida L.). Betadine plants have many functions, especially the sap, leaves, fruit and seeds. The compound contents in Betadine stem sap, which is efficacious as an antimicrobial, are saponins, tannins, flavonoids and labaditin. One of the bacteria that cause infection is Pseudomonas aeruginosa. These bacteria can cause opportunistic and nosocomial infections.
This study was a true experimental laboratory with a post-test only control group design. This study used Betadine stem sap extract with concentrations of 25%, 50%, 75%, 100%, gentamicin cream 10% as positive control, and dimethyl sulfoxide (DMSO) solution as negative control. This study used the Kirby-Bauer diffusion method and the bacterium Pseudomonas aeruginosa was grown on nutrient agar media, then incubated for 24 hours and calculated using calipers. Research data were analyzed using one-way ANOVA test.
The highest inhibition zone was group 50% (12.725 ± 0.2500 mm) while the lowest inhibition zone was group 100% (8.675 ± 0.5620 mm).
Betadine stem extract had antibacterial activity in inhibiting the growth of Pseudomonas aeruginosa bacteria, with the 50% concentration being the most effective in inhibiting the growth of Pseudomonas aeruginosa bacteria.
Betadine, sap, Jatropha multifida L., Pseudomonas aeruginosa L., Infection, Antibacterial, Nosocomial, Opportunistic.
We added new figure of Betadine plant with its morphology parts such as leaves, flower and fruit. We revised petri plates figures by typing text over the plates. We added the vocer specimen number of Jatropha multifida. This new version more in-depth interpretation of the result and the relation to the previous study. We also added the novelty of this reaserch that used different extract (Betadine sap) and bacteria (Pseudomonas aureginosa). We clearly mention step by step Betadine sap extract preparation. We clearly mention DMSO 100% solution as negative control. We added 3 new references for the vocer specimen number, Betadine figure and the relation of the previous study explanation.
See the authors' detailed response to the review by Khaga Raj Sharma
See the authors' detailed response to the review by Siti Nur Hazwani Oslan
In Padang, West Sumatra Province, people use Betadine stem sap (Jatropha multifida L.) to heal and eliminate external wound infections by applying Betadine stem sap on the injured body part. Indonesian people call this plant a Betadine plant because it has the same potential as an external wound medicine like Betadine in duration to heal an infected external wound.1–3
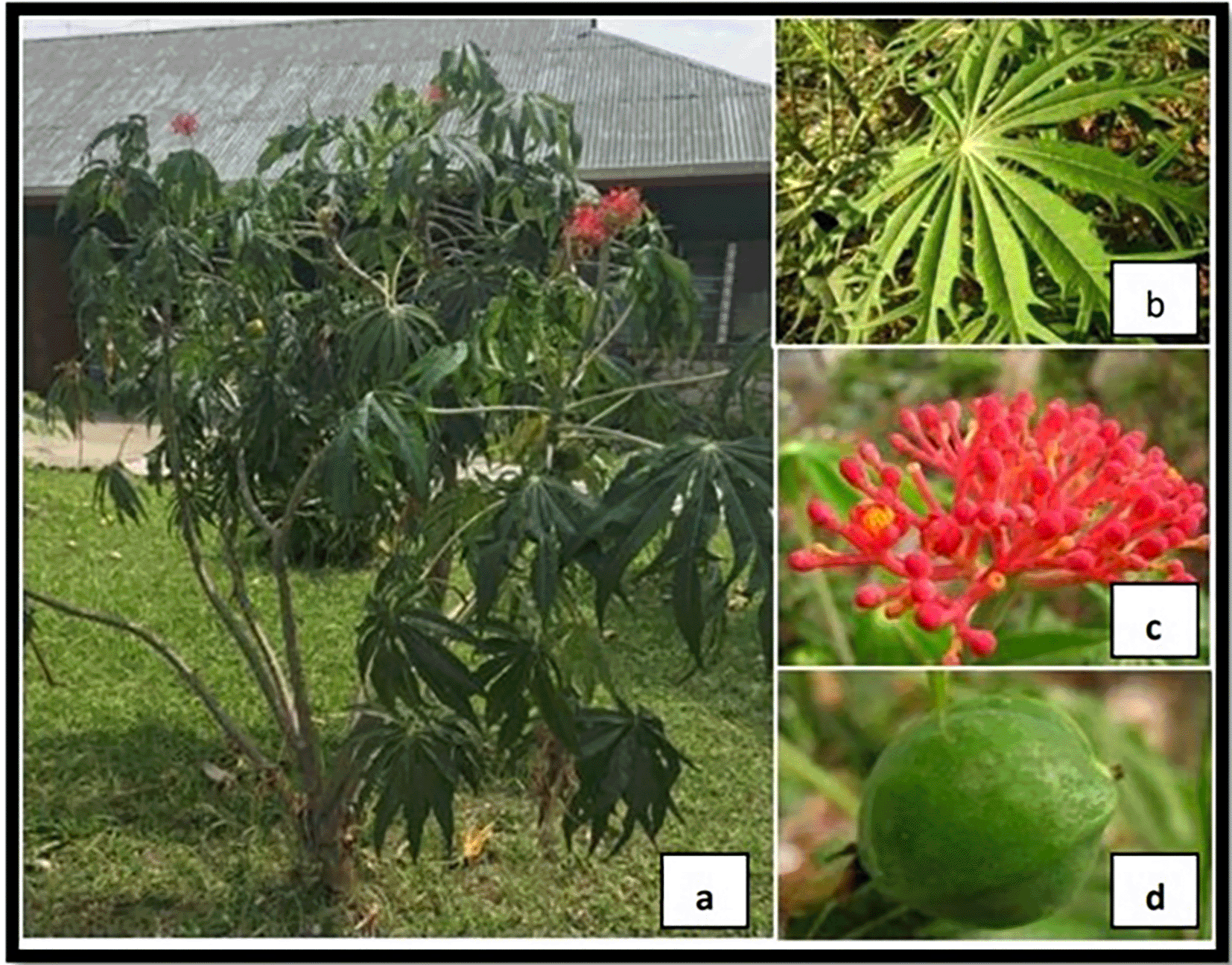
According to Ivan et al. 2019, the Betadine plant (Figure 1) can inhibit the growth of Staphylococcus aureus and Pseudomonas aeruginosa.4 Betadine plant sap consists of multifidol, biobollein, multifidone, multifidil, flavonoid, labaditin, saponin and tannin.5 In a study conducted by Aransiola et al. 2014 about the antibacterial and antifungal activity of Betadine plant sap, they found that the minimum inhibitory concentration (MIC) of Betadine plant sap gram negative bacteria (P. aeruginosa, E. coli, and Salmonella typhi) was 66 mg/ml. These results indicate that the antibacterial activity found in the Betadine plant sap is lower in gram negative bacteria because gram negative bacteria are more resistant to antibacterials.6
Based on WHO data in 2013, the mortality rate due to resistant bacterial infection is 700,000 people per year.7 P. aeruginosa is an anaerobic, nosocomial and opportunistic bacterium. P. aeruginosa bacteria live in humid environments such as surgical instruments, 74% in dental water and dental disinfecting units used for more than 24 hours. P. aeruginosa was isolated in maxillary osteomilitis and brain abscesses in patients who had dental infections. This bacteria can cause meningitis, endocarditis, pneumonia and sepsis with an average mortality of 12–25%. This bacteria is resistant to many antibacterial including amoxicillin. Antimicrobial ingredients that are effective against Pseudomonas aeruginosa are hard to find, so natural resources such as Betadine plants can be used to suppress bacterial growth.5,8,9
Based on the explanation above, the authors are interested in conducting research on “Antibacterial activity of Betadine stem (Jatropha Multifida L.) extracts on Pseudomonas aeruginosa bacteria growth by in vitro”.
This study was a true experimental study with a post-test only control group design. True experimental study measured or observed data after the treatment was given.
Laboratory of Traditional Medicine and Microbiology, Faculty of Pharmacy, University of Sumatera Utara, Medan, Indonesia.
In calculating the size of experimental research samples, we used the Federer formula:
where t = number of treatment and r = number of repetition. The study used six treatment group: group 1 (25% Betadine stem sap extract), group 2 (50% Betadine stem sap extract), group 3 (75% Betadine stem sap extract), group 4 (100% Betadine stem sap extract), group 5 (gentamicin as a positive control), group 6 (dimethyl sulfoxide or DMSO as a negative control)The number of repetitions (r) are four. So, 24 samples were obtained.
The dependent variable in this study was the sensitivity test growth of Pseudomonas aeruginosa that measured by the diameter of the inhibitory zone.
The independent variable in this study was the concentration of Betadine stem sap extract used, which were 25%, 50%, 75% and 100%.
There were six controlled variable such as the growth media used for the bacterium Pseudomonas aeruginosa was nutrient broth agar, the incubation temperature of Pseudomonas aeruginosa is 37oC, the incubation time of Pseudomonas aeruginosa was 24 hours, the isolation and culture technique of Pseudomonas aeruginosa bacteria, sterilization of tools, the materials and media used, and operator skills.
The vary betadine stem morphology such as diameters and length. The vary growth time of betadine stem, mature betadine stem has higher and browner sap. The vary nutrients of the soil and rainfall rate may contribute the different sap. Uncontrolled condition of laboratory storage such as power outage.
Autoclaves (Tomy ES 315, Japan), UV-VIS spectrophotometers (Orion Aquamate 8000, Germany), ovens (Memmert UN55, Germany), micropipets (Dragon Lab, China), Laminar air flow cabinet (Astec HLF I1200L), glass cuvettes, refrigerators (Thermo Scientific), incubators (Memmert, Germany), vortex mixers (Biosan, Latvia), glass tubes, glass petri dish, test tubes, inoculating loops, bunsen, measuring cups 20 ml, spatula, Vernier calipers (Electric Digital Capliper), analytical scales (Sartorious BSA323S-CW) and Erlenmeyer.
Betadine stem sap extract 100% concentration (the voucher specimen number FHI 109458, FHI 108012, FHI 96651, FHI 31400, FHI 109872),10 bacterial culture of Pseudomonas aeruginosa (ATCC® 140218, pure culture stock Faculty of Pharmacy, University of Sumatera Utara, Indonesia), DMSO solution 100%, gentamicin ointment 10% (IKAGEN®), aquadest, nutrient agar powder (Oxoid), nutrient broth agar powder (Oxoid), label paper, and 6 mm sterile paper discs.
First, the process of collected Betadine stem sap involved cut the stem and collected the sap in a sterile container. To purify the sap from impurities, a maceration method was used. Soaked 10 ml sap with 1 L DMSO 100% and continuous stirred for the first 6 hours, followed by occasional stirred for the next 18-hour. Then filtered to obtain the macerate. This macerate was evaporated using a rotavapor device at a temperature of 40°C to obtain a thick extract. Mixed the extract and DMSO 100% using a micropipette. This process resulted in extracts with concentrations of 25%, 50%, and 75%.
Next, all the instruments were sterilized with the autoclave at 121°C for 15 minutes and dried with an oven 170°C for 1–2 hours. Inoculating loop and pinset were sterilized with the bunsen flame. After that, took dilution of Betadine stem sap extract 100% with DMSO using a micropipet into a glass tube and measured with sartorious analytical scales (1 ml). The nutrient agar powder (28 g) was mixed with 1000 ml aquadest in an erlenmeyer flask with a spatula. This was heated for 2 hours at 100°C and after that saved in autoclave. Then, sterilized inoculating loops were used to take the isolated P. aeruginosa colonies and these were then placed into test tubes each containing the tilt media (contained 3 ml nutrient agar). The tube was put in an incubator at 37°C for 24 hours. Took P. aeruginosa colony using sterilized inoculating loops and than put into a tube that contain 10 ml nutrient broth agar. The tube was put on vortex mixers for 1 minute. Took 3 ml bacteria and put into glass cuvets, then put into UV-VIS spectrophotometers with 560 nm wavelength. The result must be 25% transmittance. In laminar airflow cabinet took 20 ml nutrient agar using measuring cups and 1 ml bacteria suspense using micropipets into glass petri dishes. The petri dishes were shaken slowly in a figure of eight movement until homogeneous. Paper discs were soaked with 25%, 50%, 75%, 100% Betadine stem sap extract, getntamicin and DMSO 100% for 3 minutes. Then these were placed on the surface of the nutrient agar and a little pressure was applied. The petri dishes were labelled with label paper and placed in the refrigerator for 1 hour. Then the petri dishes were put into an incubator for 24 hours at 37°C. The diameter of inhibitory zone was clear zone in the petri dish. Measured it with caliper.
From this study, the lowest inhibition zone was produced by the Betadine stem sap extract group 100% (8.675 ± 0.5620 mm), while the highest inhibition zone was produced by the 50% (12.725 ± 0.2500 mm) Betadine stem sap extract group (Figures 2 and 3). The Saphiro-Wilk test with a significance of p > 0.05 was used for the normality test and the data were found to be normally distributed (p > 0.05) (Table 1). The data was distributed normally, so statistical analysis was continued using a one-way ANOVA analysis test and post hoc LSD to see whether there was a significant difference in the diameter of the inhibition zone between group 25%, 50%, 75%, 100%, K+, and K-. The ANOVA test showed a significant difference (p < 0.05) in the effect of the inhibition zone diameter between group 25%, 50%, 75%, 100%, K+ and K- (Table 2). The LSD post hoc test was used to determine the significance of the inhibition of the growth of Pseudomonas aeruginosa between all groups in this study. The results of the LSD post hoc test are shown in Table 3.


| Treatment | Number of repetition | The average ± standard deviation | P-Value ANOVA |
|---|---|---|---|
| 100% | 4 | 8.675 ± 0.5620 | p = 0.000 |
| 75% | 4 | 8.925 ± 0.3948 | |
| 50% | 4 | 12.725 ± 0.2500 | |
| 25% | 4 | 12.275 ± 0.3304 | |
| K+ | 4 | 18.275 ± 0.4992 | |
| K- | 4 | - |
The study used Kirby-Bauer’s method.9 This method is fast, easy, simple and produces effective results to show the antibacterial activity of a substance.9 The solvent was DMSO which is able to dissolve polar and nonpolar compounds and does not have antibacterial activity so it does not change the results.12 Factors that influence the diffusion of extracts into the media are the extent of the concentration gradient, solubility, diffused molecular mass, temperature and density of the solvent.6
Inhibition zone group 25% (12.275 mm) smaller than group 50% (18.275%).13 This is because the group 50% had higher active ingredients than the group 25%. This is in line with previous research conducted by Brooks et al.3 and Anggita et al.14 in 2018 which states that the effectiveness of an antibacterial agent is influenced by the concentration of a given substance, the higher concentration has the higher active ingredient as an antibacterial, thus increasing the inhibition ability against microbes.
The inhibition zone group 75% the was 8.925 mm and group 100% was 8.675 mm.13 The decrease in inhibition zone diameter due to the minimum diffusion power of the dense viscous extract is because it had a high solution density. The group 75% had minimum penetration power because the minimal amount of solvent. The group 100% had minimum penetration power because there was no solvent. Molecules moved slowly because it is more difficult to pass through denser media so it takes longer and results in smaller inhibitory zones.9 This is similar to a study conducted by Komariah et al. 2013, where there was a decrease in antibacterial activity along with an increase in the concentration of the extract due to the higher concentration, the viscosity would increase so that the extract would be more difficult to diffuse into the agar media.15
The antibacterial activity of the Betadine stem sap extract is related to its antibacterial content, namely phenols, tannins, saponins and labaditin.3 Large molecule of phenol are able to activate essential enzymes in microbial cells even at low concentrations.16
Phenol compounds contained in Betadine stem sap are flavonoids and tannins.12 Flavonoids work by inhibiting nucleic acid synthesis, inhibiting cytoplasmic membrane function and inhibiting energy metabolism. Flavonoids can damage the outer membrane and cytoplasm of gram-negative bacteria, disturb the exchange of nutrient and metabolite and inhibit the energy supply for bacteria.17,18
Tannins can cause lysis of bacterial cell membranes due to the differences in bacterial cells osmotic pressure. Tannins damage cell membranes by breaking the phosphate group H+ so the phospholipid molecule breaks down into glycerol, carboxylic acid and phosphoric acid. Saponins are also contained in Betadine stem sap. Its mechanism of action is by interfering with bacterial cell membrane stability, causing bacterial cell lysis, damaging the cell membrane and releasing various important components of microbial cells, namely proteins, nucleic acids, nucleotides and others.17,18 According to a study conducted by Barbosa et al. 2019, Betadine stem sap contains labaditin which is effective in gram-positive bacteria but less effective in gram-negative bacteria because of the more complex structure of gram-negative bacterial cell walls.19
This study similar to Wayan et al. research 2015 that used betadine leaves extract for Staphylococcus aureus growth inhibition. The consentration group that they used was 25%, 50%, 75% and 100%. The group 25% was 11.2 mm, the group 50% was 13.8 mm, the group 75% was 11.8 mm, and the group 100% was 7.2 mm. Their result showed lower antibacterial activity than this study, however both showed there was a decrease inhibition zone in group 75% and 100%.9
The result of this study was vary form Darmawi et al. 2013 that used betadine stem sap for Staphylococcus aureus growth inhibition. The consentration group that they used was 25%, 50%, 75% and 100%. The group 25% was 13 mm, the group 50% was 13.5 mm, the group 75% was 14.6 mm, and the group 100% was 15.7 mm. The vary of the result due to swab method bacteria cultivation. Darmawi et al. used swab method for bacteria cultivation, that made the bacteria grewn only on the surface.20
From the four group of Betadine stem sap extract that were tested, the strongest antibacterial activity found the group 25% and group 50%.13 However, when compared with the positive control antibiotic gentamicin, Betadine stem sap extract was not more effective than gentamicin. This is due to the fact that many gram-negative bacteria contain lipids and little peptidoglycan. The outer membrane of the Pseudomonas aeruginosa is a bilayer that serves for the selective defense of compounds entering and exiting cells. The outer membrane consists of phospholipid (inner layer) and lipopolysaccharide (outer layer). This makes it difficult for active compounds to enter the cell so antibacterial activity of Betadine stem sap extract is smaller than gentamicin.19,21
The novelty from this research is that not only betadine stem leaf extract can be used as an antibacterial material, but also betadine stem sap extract. Betadine stem sap extract is not only effective on gram-positive bacteria, but also effective on gram-negative bacteria.
This research proves that Betadine (Jatropha multifida L.) stem sap extract has antibacterial activity on inhibiting the growth of Pseudomonas aeruginosa in vitro. This result is the first step in the possibility of utilizing papaya leaf extract as an alternative natural ingredient for antibiotics in dentistry with the need for a series of tests such as clinical trials, toxicity and side effects so that this research can be utilized by the public.
Based on the results, Betadine stem sap extract has an antibacterial activity on Pseudomonas aeruginosa growth in vitro. The most effective concentration to inhibit the growth of Pseudomonas aeruginosa is the group 50%, with the largest inhibitory zone diameter of 12.725 ± 0.2500 mm. This study has limitations, so further research is recommended to investigate using concentrations below 50% and using dilution methods to obtain the MIC (minimum inhibitory concentration) and MKC (minimum kill concentration).
Figshare: Antibacterial Activity of Betadine (Jatropha multifida L.) Stem Extract on Pseudomonas aeruginosa Growth In-Vitro. https://doi.org/10.6084/m9.figshare.20359653.v2. 13
This project contains the following underlying data:
• Data Inibition Zone of Each Repetition of Betadine Extract.csv (the file contains plain data of each concentration of Betadine stem sap extract with four repetitions).
• Test of Normality.csv (The file contains normal data distribution).
• Descriptives.csv (The file contains median, variance, standart deviation, minimum, maximum, range, interquartile range, skewness and kurtosis each concentration of Betadine stem sap extract).
• Case Processing Summary.csv (file contains of summary valid and missing antibacterial activity each consentration).
• Oneway Anova Test.csv (The file contains of data which is distinguish the antibacterial activity of each concentration of Betadine stem sap extract against Pseudomonas aeruginosa bacteria).
• Post Hoc Test.csv (The file contains of data which is show the significant difference between each concentration of Betadine stem sap extract).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
An earlier version of this article can be found at: https://repositori.usu.ac.id/handle/123456789/25701?show=full.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microalgae; functional food; Food microbiology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products chemistry, extraction, isolation, characterisation of various natural products and to perform the biological activities, molecular docking, green synthesis of nanoparticles and comparison of biological activities.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microalgae; functional food; Food microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products chemistry, extraction, isolation, characterisation of various natural products and to perform the biological activities, molecular docking, green synthesis of nanoparticles and comparison of biological activities.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 02 Apr 24 |
read | read |
|
Version 1 27 Oct 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)