Keywords
Ginkgo Biloba, KOOS, Knee osteoarthritis
This article is included in the Plant Science gateway.
Ginkgo Biloba, KOOS, Knee osteoarthritis
The major update in the new version is the adding of statistics (p-value) of the difference of patient characteristics in two arms ( Table 1).
Other information was added upon reviewer request:
-More details in abstract about number of patients involved in the study
- Mention the dosage form of Ginkgo that used was capsule
- KOOS scoring method
See the authors' detailed response to the review by Ali Azeez Al-Jumaili
ADL: Activities of daily living
EGb-761: standardized Ginkgo biloba extract
GB: Ginkgo biloba
GBE: Ginkgo biloba extract
KOOS: Knee injury and Osteoarthritis Outcome Score
OA: osteoarthritis
QOL: quality of life
Osteoarthritis (OA) is a degenerative joint disease characterized by deteriorating articular cartilage. Since the knees and hips are considered weight-bearing joints, osteoarthritis is widely spread in these locations.1 OA-related symptoms such as swelling, discomfort, and function loss are often primary reasons to visit a specialist.2,3 Risk factors for OA include aging, female gender, genetic susceptibility, and nutritional status. The prevalence of knee osteoarthritis is around 16.0% in patients aged 15 years and older and 22.9% in those aged 40 years and older.4 85% of people older than 75 years old were found to exhibit some symptoms of OA.5 Regarding gender variations, females display greater incidence of knee osteoarthritis than in males.4 In addition, obesity was reported to increase the load-bearing tension on the hip and knee joints. In fact, the risk of OA increases by 10% for every kilogram over the ideal weight.6 Painkillers are the most effective treatment available for patients with OA However, the long-term use of these compounds can impose toxic effects on the digestive7 and cardiovascular systems.8 An estimated 16,500 Americans with rheumatoid or osteoarthritis die each year from using non-steroidal anti-inflammatory drugs (NSAIDs). If the effects of NSAIDs on the gastro-intestinal tract were counted separately, they would be the 15th leading cause of death in the United State (US).9 At equipotent doses, the efficacy of various NSAIDs is similar, but there is clear individual diversity in the therapeutic responses to these medications. Some people may respond better to one treatment than other10 Dietary supplements were also considered, including glucosamine, chondroitin, vitamin D, and fish oil. At two years follow-up, high-dose fish oil showed a higher improvement in WOMAC score than vitamin D, but the other supplements showed no significant changes.11 Traditional Chinese medicine has been using the ginkgo tree for a long time. It is one of the oldest living tree species in the world.12 Standardized preparations (EGb-761) have 24% ginkgo flavonoid glycosides, 6% terpene lactones, and less than 5 parts per million ginkgolic acids.13 GBE is popular in Europe and the United States. In clinical research, 120-240 mg doses of GB demonstrated neuroprotective characteristics and other beneficial circulatory effects in elderly people, including cerebral insufficiency and cognitive consequences and peripheral circulatory impairment, particularly intermittent claudication, vertigo, and tinnitus.14 Due to the synergistic impact of flavonoids and ginkgolides, GB has been shown to possess strong antioxidant and anti-inflammatory properties.15,16 Oedema was significantly reduced with GB in animal trials; however, a greater reduction in oedema was seen when GB is combined with the NSAIDs like indomethacin, rofecoxib, and celecoxib, than with NSAIDs alone.17 Ho et al. study revealed that the cartilage was collected from OA patients who undergo total knee or total hip joint replacement surgery and prepare chondrocyte from it. Results indicated that EGb-761 displayed a dose-dependent inhibition of interleukin-1-induced Nitric Oxide production. The same study concluded that the phosphorylated types of c-Jun N-terminal kinases (JNK) were efficiently decreased by treatment with EGb-76.7 EGb-761 could also effectively reverse the chondrocyte/cartilage damage in an OA rat model.18 It is highly suggested to include non-drug strategies in the treatment of OA to prevent or reduce disease progression.19 Some patients may not get enough control of symptom despite the availability of various treatment options, while others will experience harmful consequences from the available therapeutic interventions20 and long-term use of NSAIDs. Significantly, non-specific medicines accelerate OA structural progression21 by increasing knee joint load after reducing the pain.22 Based on the above, this study aims to assess the efficacy and safety of the Ginkgo biloba extract in patient with knee OA.
This study is a part of a Master’s degree thesis. The study was registered in and approved from the Medical Ethics Committee in the University of Kufa, college of medicine with reference: (MEC_13). The study protocol was registered in ClincialTrials.gov (identifier: NCT05398874, 1st June 2022). Trial registration to ClinicalTrials.gov took place after the trial was completed due to a logistics issue in contacting the authorized personnel.
Participants were asked to sign a printed informed consent that explained the aim of study.
This was a randomized double blinded clinical trial. Patients were recruited from one private orthopaedic clinic in Al-Najaf Government in Iraq from November 1st 2021 to June 1st 2022. The doctor of clinic defined the patients that can be enrolled in the study and informed them about aim of study and take oral and written consent from them. Then the researcher randomized patients into two groups using simple randomization (Block size 4 (2A, 2B), allocation:1:1). The doctor and patients did not know in which group they were categorized. Group A received EGb-761 120 mg capsule (Natrol/USA) twice daily while group B received placebo (starch) capsule. Both GB and placebo capsules were given to the patients free of charge in similar containers. Patients in both groups administered their standard therapy of paracetamol tablet 1 gram twice daily plus diclofenac sustained release capsule 100 milligrams once daily. The treatment continued for eight weeks as demonstrated by the CONSORT flow diagram in Figure 1.
Out of 145 patients assessed for their eligibility, 103 patients were selected to be enrolled in the study. However, 65 patients only (35 group A, 30 group B) completed four weeks of treatment, while 60 patients (33 patients in group A and 27 patients in group B) completed eight weeks of treatment. In each visit, patients were asked to mention any side effects that may be related to GB.
Patients included in this study were diagnosed with grade 2 or grade 3 Knee OA according to Kellgren and Lawrence (K&L) classification system23 and they were aged between 38-75 years old. The exclusion criteria were patients with an allergy or contraindication to NSAIDs or GB or paracetamol, those with renal or hepatic problem, pregnancy or lactation and those who have any cardiovascular or neurological diseases.
Clinical assessment was done before starting treatment, after two weeks, four weeks, and eight weeks by KOOS questionnaire (Arabic version that is available at http://koos.nu) which is self-administered, however, the researcher was available for any clarification KOOS includes 42 items divided into 5 subscales. These are pain, symptoms, ADL, sport and recreational activity, and finally QOL associated with knee activity. Each question has 5 choices, and each choice has specific score range from 0 (no trouble) to 4 (excessively trouble). These scores are entered in specific Microsoft Excel format that available at http://www.koos.nu. A number range from 0 to 100 is the results of each subscale. Where 0 refer to very bad situation and 100 refer to good.24,25
Descriptive statistics are presented in the form of numbers and percentages for the categorical variables. Means and standard deviations are reported for numerical variables. Mixed ANOVA method was used to measure the difference in the outcome within same groups (Group A and group B) across different time points in addition to measure the difference between groups at each time point.
IBM SPSS 28 for windows software was used in the analysis, and a P-value < 0.05 is considered as statistically significant.
A total of 60 patients, groups A and B were 33 and 27 respectively, completed the eight weeks follow up. Females were 46 patients of the total participant while males were 14 males. The mean ages of patients in groups A and B were 54.2 and 58.0 years, respectively. All the adverse effects occurred during the study period were recorded (Table 1).
The mean KOOS score for each of the five subscales at different follow up periods as well as the main effects for the time and groups are shown in Table 2.
| KOOS | Group | Baseline | Treatment duration | Mean±SD | Mean difference | P-value |
|---|---|---|---|---|---|---|
| Pain score | A | 38.52±13.36 | 2 weeks | 53.03±16.37 | -14.52 | <0.001* |
| 4 weeks | 55.76±16.67 | -17.24 | <0.001* | |||
| 8 weeks | 57.94±15.16 | -19.42 | <0.001* | |||
| B | 38.89±12.12 | 2 weeks | 42.52±11.97 | -3.63 | 0.845 | |
| 4 weeks | 44.48±10.33 | -5.59 | 0.157 | |||
| 8 weeks | 46.74±14.51 | -7.85 | 0.040* | |||
| Symptoms score | A | 54.39±16.55 | 2 weeks | 68.00±16.33 | -13.61 | <0.001* |
| 4 weeks | 71.48±15.73 | -17.09 | <0.001* | |||
| 8 weeks | 73.61±16.84 | -19.21 | <0.001* | |||
| B | 49.04±13.08 | 2 weeks | 58.81±17.34 | -9.78 | 0.004* | |
| 4 weeks | 58.78±17.23 | -9.74 | 0.004* | |||
| 8 weeks | 58.89±19.00 | -9.85 | 0.015* | |||
| Activities of daily Living score | A | 40.36±12.15 | 2 weeks | 52.06±13.12 | -11.70 | <0.001* |
| 4 weeks | 58.15±14.95 | -17.79 | <0.001* | |||
| 8 weeks | 59.64±13.46 | -19.27 | <0.001* | |||
| B | 37.96±13.01 | 2 weeks | 41.30±10.13 | -3.33 | 0.584 | |
| 4 weeks | 43.19±10.47 | -5.22 | 0.147 | |||
| 8 weeks | 46.74±12.57 | -8.78 | 0.005* | |||
| Sports score | A | 21.36±10.99 | 2 weeks | 35.30±11.92 | -13.94 | <0.001* |
| 4 weeks | 39.09±14.33 | -17.73 | <0.001* | |||
| 8 weeks | 39.70±16.63 | -18.33 | <0.001* | |||
| B | 18.89±12.58 | 2 weeks | 26.11±12.04 | -7.22 | 0.019* | |
| 4 weeks | 24.81±12.44 | -5.93 | 0.217 | |||
| 8 weeks | 27.59±13.82 | -8.70 | 0.029* | |||
| Quality of life score | A | 28.30±7.439 | 2 weeks | 33.58±11.36 | -5.272 | 0.063 |
| 4 weeks | 39.82±13.86 | -11.515 | <0.001* | |||
| 8 weeks | 43.61±15.37 | -15.303 | <0.001* | |||
| B | 24.85±8.38 | 2 weeks | 31.30±10.90 | -6.444 | 0.03* | |
| 4 weeks | 32.85±12.88 | -8.00 | 0.016* | |||
| 8 weeks | 39.07±13.59 | -14.22 | <0.001* |
Table 2 demonstrated that there are several significant differences among different times of treatment, between groups (Group A vs B) and interaction between time and groups. Within the same group, indeed, the pain scores in group A (53.03±16.37, 55.76±16.67 and 57.94±15.16) have significantly increased at all follow up weeks two, four and eight, respectively, when compared with the baseline (38.52±13.36). In a different manner, the pain score in group B (46.74±14.51) has only increased significantly after eight weeks of treatment. As shown in Figure 2, significantly higher pain scores were observed in group A in comparison to that in group B at weeks two, four and eight of treatment with GB.
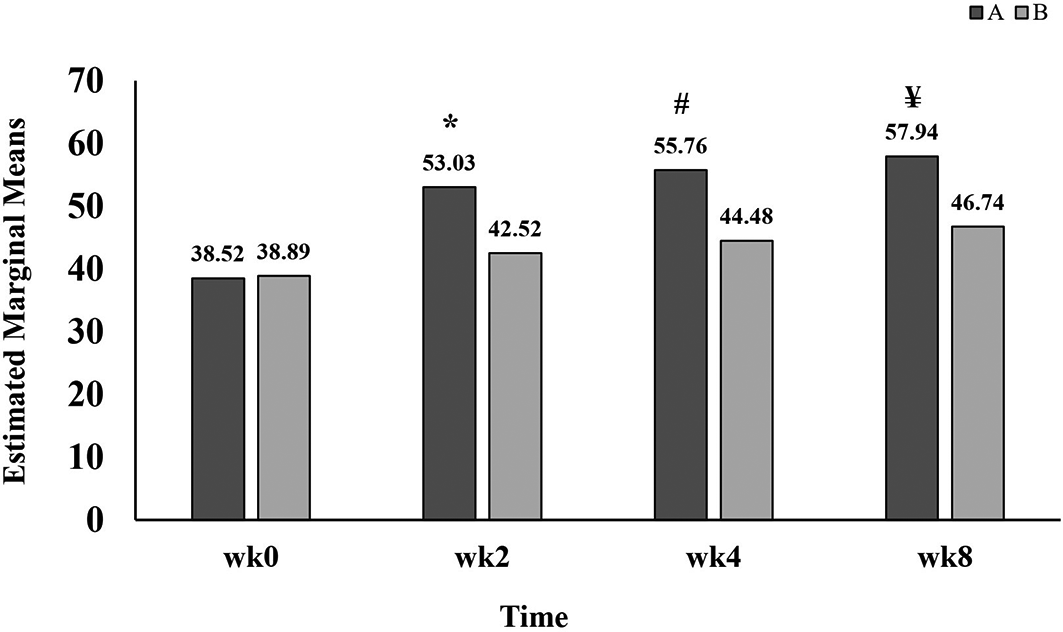
A refers to group A, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus GB.
B refers to group B, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus placebo.
wk refers to week;
* refers to p=0.007;
# refers to p=0.003;
¥ refers to p=0.005.
Data listed in Table 2 further showed important differences within different times, between groups and interaction between time and groups. Within group A, symptoms scores (68.00±16.33, 71.48±15.73 and 73.61±16.84) recorded at weeks two, four, and eight of treatment with GB, respectively, were all significantly higher than that at the baseline score (54.39±16.55). Within group B, the symptoms scores (58.81±17.34, 58.78±17.23 and 58.89±19.00) at weeks two, four and eight of treatment with GB, respectively, were also significantly higher than that at the baseline time (49.04±13.08). Comparison between groups (A and B) has been clearly demonstrated in Figure 3 which revealed a statistically significant improvement of symptoms scores in group A versus that in group B after two, four and eight weeks of treatment with GB.
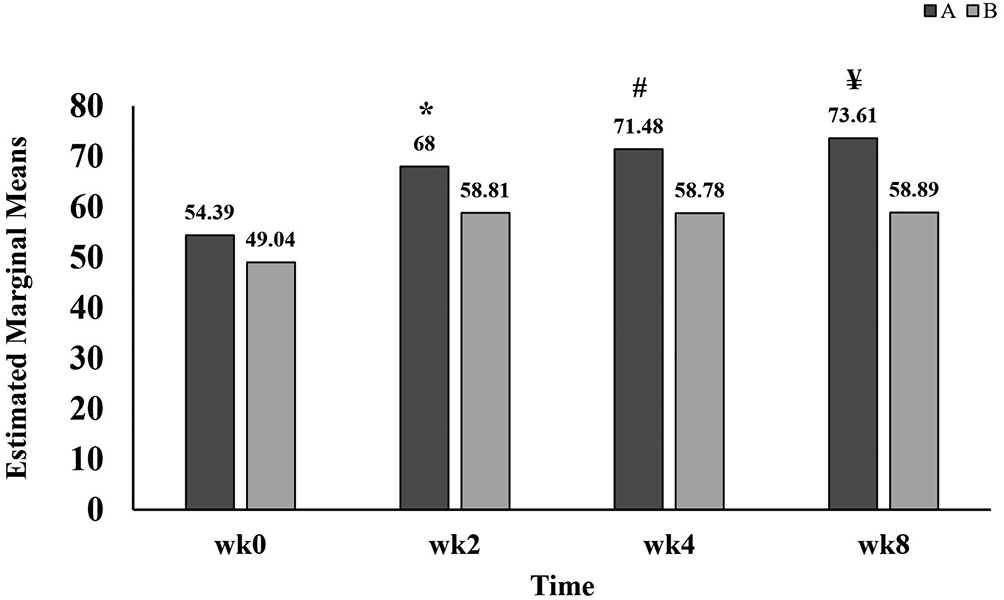
A refers to group A, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus GB.
B refers to group B, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus placebo.
wk refers to week;
* refers to p=0.039;
# refers to p=0.004;
¥ refers to p=0.002.
As listed in Table 2, there are significant differences within different times, between groups and interaction between time and groups. ADL score in group A has dramatically increased at weeks two, four, and eight (52.06±13.12, 58.15±14.95 and 59.64±13.46) when compared with baseline score (40.36±12.15). While in group B, a significant increase in ADL score has recorded after 8 weeks of treatment with GB (46.74±12.57) in comparison with the baseline score (37.96±13.01). However, no significant differences were found after two and four weeks of treatment. Moreover, Figure 4 displayed that ADL score of group A were significantly higher than group B at all times of follow up (two, four, and eight weeks).
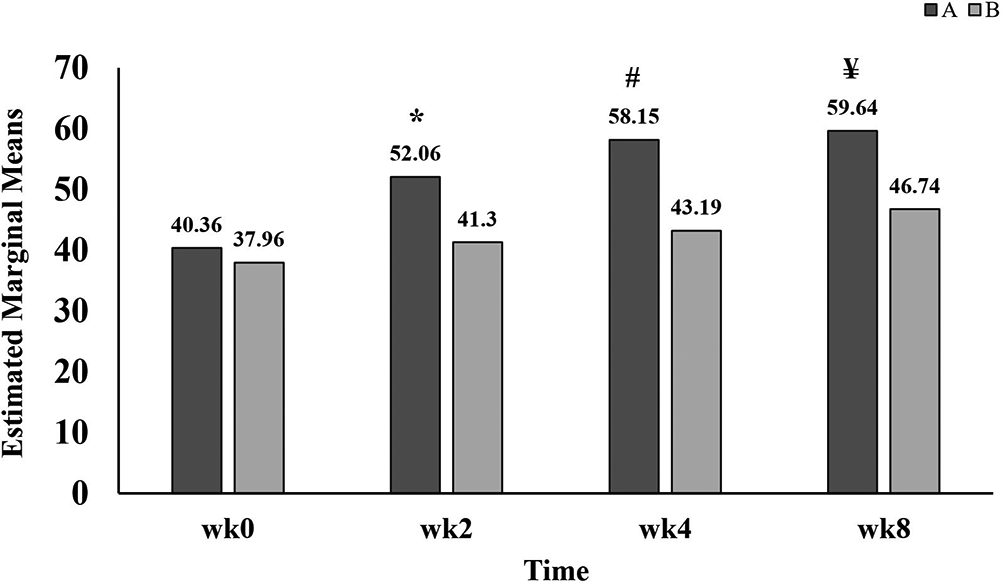
A refers to group A, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus GB.
B refers to group B, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus placebo.
wk refers to week;
* refers to p<0.001;
# refers to p<0.001;
¥ refers to p<0.001.
As noted in Table 2, there was significant elevation in sport scores of group A (35.30±11.92, 39.09±14.33 and 39.70±16.63) at all times of follow up, weeks two, four and eight, respectively, when compared with week 0 (21.36±10.99). On the other hand, group B showed significant improvement only at weeks two and eight (26.11±12.04 and 27.59±13.82). Intergroups comparison (group A versus B) was demonstrated in Figure 5, which showed that sport score in group A was significantly higher than group B at all times of follow up.
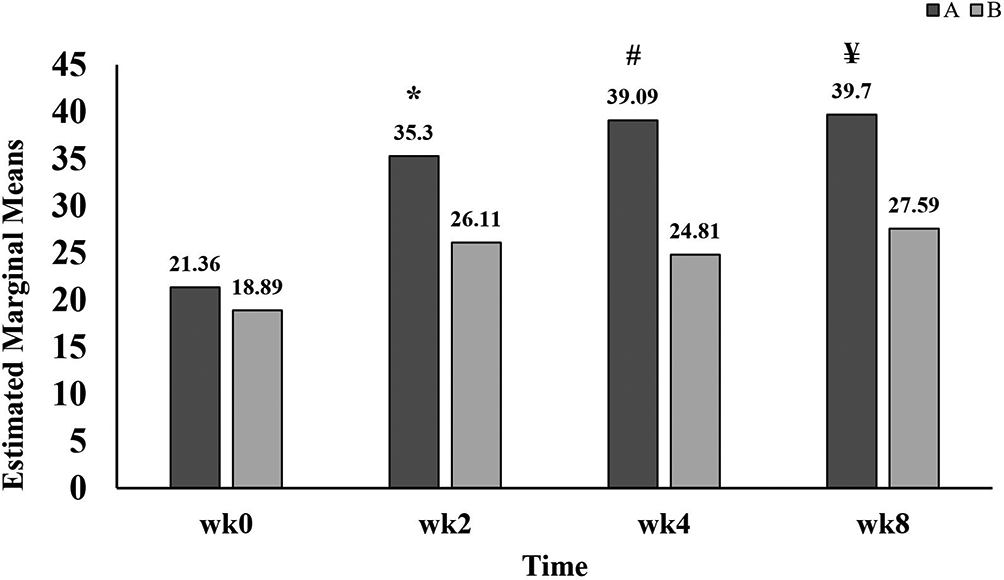
A refers to group A, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus GB.
B refers to group B, patients administered standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus placebo.
wk refers to week;
* refers to p=0.004;
# refers to p<0.001;
¥ refers to p=0.004.
Data listed in Table 2 showed that QOL scores were improved significantly in group A after weeks four and eight (39.82±13.86 and 43.61±15.37, respectively) of treatment with GB as compared with the baseline score. In group B, QOL scores at weeks two, four and eight (31.30±10.90, 32.85±12.88 and 39.07±13.59) were significant higher in comparison with the baseline score (24.85±8.38). Notably in Figure 6, it was found that although QOL scores in group A were higher than that in group B, these data were statistically not significant at weeks two, four and eight of treatment with GB.
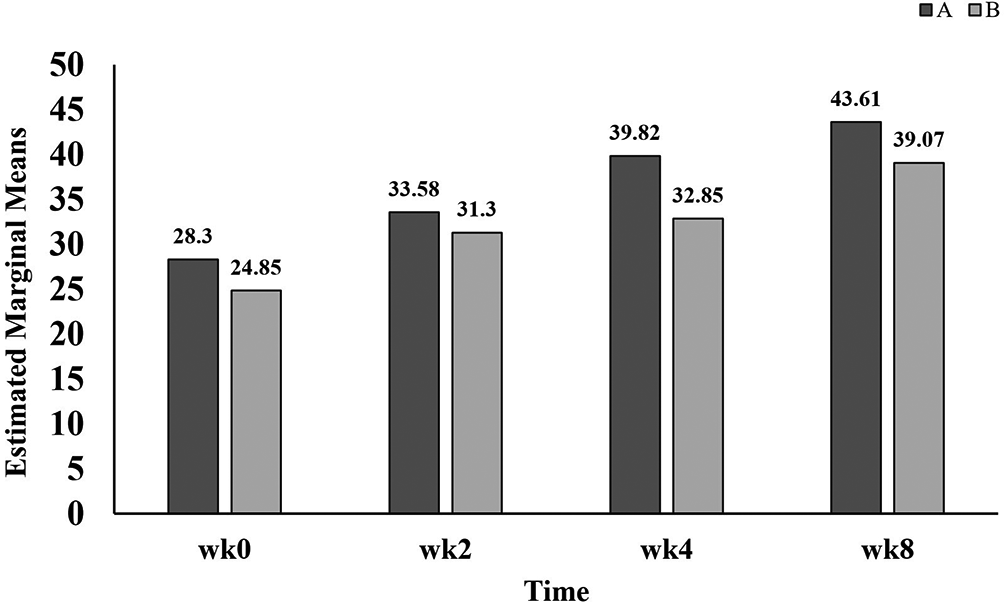
A refers to group A, patients administer standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus GB.
B refers to group B, patients administer standard treatment (diclofenac 100 mg sustained released and paracetamol 1 g twice daily) plus placebo.
wk refers to week.
Ten patients experienced adverse effects during this clinical trial as listed in Table 1: eight patients in group A and two in group B with no significant difference bwtween groups (p=0.08). All of the recorded effects were related to the digestive system. All these effects were mild and tolerable, and no patient discontinued the trial due to these mild effects.
This study aims to assess the efficacy of the GBE in patient with Knee OA based on KOOS. The KOOS is a knee-specific tool designed to evaluate patients' perceptions of their knee and related issues.25 The most obvious symptom of osteoarthritis is pain.26 KOOS evaluates the pain intensity through questioning the different daily activities in the last weeks from the visit. According to the results, the pain score in patients administered GB was improved more quickly, after two weeks of treatment with 240 mg/day GB, than those patients in the second group (group B) who administered the conventional therapy with placebo. These findings were in line with other similar previous studies. Clinical research conducted by Al-Rekabi in 2014 reported that twice-daily Glucosamine/Ginkgo biloba (500/50 mg) administration improved all scores of KOOS components significantly when compared with Glucosamine/Chondroitin (500/400 mg).27 GB was shown to block the enzymes cyclooxygenase-2 (COX-2) and 5-lipoxygenase, which are responsible for the conversion of arachidonic acid to prostaglandins (PGs) and leukotrienes, respectively.16,28 Since diclofenac was given orally to both groups in this study, patients in both groups reported improvement in symptoms such as morning stiffness and knee swelling. The KOOS symptom score was determined by asking the patient series of questions regarding experiencing swelling, clicking, or restriction in their ability to bend or straighten their knee. In spite of the fact that there was clear evidence of improvement in both groups, the ginkgo group showed significant difference throughout the course of the study period. This may be related to the analgesic and anti-inflammatory activities of NSAIDs which are typically obtained within weeks.29 Paracetamol may not be as effective as NSAIDs in individuals with pain and inflammation who are experiencing symptoms.30 NSAIDs are known for their anti-inflammatory impact through various mechanisms, including the COX enzyme, interleukins, chemokines, and others.31 When given orally, EGb-761 reduced thermal hyperalgesia and showed dose-dependent efficacy comparable to or greater than diclofenac in rat model of pain. Experimental results showed that the antihyperalgesic effect of GB can effectively alleviate the inflammatory pain associated with acute injury.32 It is well recognized that persons with knee OA may have difficulty to participate in physical activities due to knee pain.33 Limitations in walking, stair climbing, and squatting are common patient complaints that greatly interfere with activities of daily living and recreation22 which were improved significantly in group A of this study.
Inflammation affects central and peripheral nociceptive input, and it increases receptor sensitivity to nociceptive input by increasing prostaglandins and inflammatory cytokines.26 In a two-month follow-up study of 80 patients with knee OA, the combination of GB with celecoxib was associated with a higher decrease in serum malondialdehyde (MDA), matrix metalloproteinase (MMP)-1 concentration, erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) levels. Additionally, this combination resulted in a very significant increase in total antioxidant status (TAS) when compared to celecoxib alone.34
The failure of nonsurgical therapies to modify health related QOL in knee OA patients is not surprising given their inability to alleviate physical manifestations of OA.22 Individuals with osteoarthritis have a low perception of their quality of life in functional capacity, functional limitation and pain.35 During the study period of this study, QOL improved with time with but with no significant differences between study groups. Furthermore, coexisting disorders, altered nociceptive sensitivity, and psychosocial factors have been demonstrated to affect the clinical presentation and treatment response of patients.36
Finally, no patient in this study reported serious adverse effects, and all the recorded adverse effects were related to the gastrointestinal system. Similarly, these records were in consistent with that reported by a previous meta-analysis study.37
Patients recruited in limited number from one center may not reflect the effect on the general population. Moreover, the effects of ginkgo biloba cannot be predicted for long-term use because the trial lasted only eight weeks. Limited financial resource is also a barrier to involve more patients from different settings. Also, the study did not control the other confounders such as age, gender, and severity of the disease.
Standard extraction of Ginkgo biloba improved the KOOS in term of pain, symptoms, sport, ADL, and QOL in patients with knee OA significantly when add to the standard treatment along the study period. In contrast to the other scores, only QOL showed no significant difference between group A and B. All of the reported adverse events were mild gastrointestinal in the origin and were tolerable.
Figshare: Evaluation of clinical efficacy of Ginkgo biloba extract in the treatment of knee osteoarthritis: a randomized clinical trial, https://doi.org/10.6084/m9.figshare.21259335.v2.38
Figshare: Protocol for ‘Evaluation of clinical efficacy of Ginkgo biloba extract in the treatment of knee osteoarthritis: a randomized clinical trial’, https://doi.org/10.6084/m9.figshare.21259335.v2.38
Figshare: CONSORT checklist for ‘Evaluation of clinical efficacy of Ginkgo biloba extract in the treatment of knee osteoarthritis: a randomized clinical trial’, https://doi.org/10.6084/m9.figshare.21259335.v2.38
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmaceuitical science, Targeted drug delivery, Nanoparticles, natural product, PDT, nanomedicine.
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacy Practice and Clinical Pharmacy
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 14 Apr 23 |
read | read |
|
Version 1 31 Oct 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)