Keywords
Risk factors, different endemicity areas, housing effect, case-control, malaria.
This article is included in the Emerging Diseases and Outbreaks gateway.
Risk factors, different endemicity areas, housing effect, case-control, malaria.
Malaria is a disease that is not solely transmitted by itself, instead, it requires a specific vector to successfully inject itself to the host body. Theoretically, this is based on a causal concept of epidemiology that the occurrence of a diseases depends on three primary factors: (i) the host, (ii) the agent, and (iii) the environmental factors. Studies in the Amhara region, the northwestern part of Ethiopia, suggested an insight into critical factors comprising malaria risk. In general, three factors have been recognized to be the key factors deriving malaria risk: climate variables, entomological parameters, and human population dynamics. The impact of climate variables is inevitably accounted for as a potential circumstance in malaria transmission, as it known to be an enhancement factor of malaria transmissibility due to increasing vector capacity by providing potential sources of breeding places, increasing mosquito longevity, and feeding rates.1–11 It is plausible that the interconnection of co-factors such as environmental constraints affecting the entomological and parasitological constituents enhance the transmissibility of malaria. However, a broad range of influences could drive the transmission pattern of malaria from either humans or the vector.10,12 Therefore, instead of controlling the impact of climate change, upgrading public health interventions and socioeconomic conditions might better affect malaria.13
Besides climate change, whose alterations on various aspects have been well documented, another essential variable driving malaria transmissions are the entomological parameter and human population dynamics. Biological and behavioral variations of mosquito species, such as vector-habitat relationships; factors affecting vector population abundance; host-seeking behavior; and the emergence of vector resistance to insecticides, have been known to affect the transmission pattern of malaria.7,14–16 Endophilic mosquitoes tend to feed and rest indoors, thus poorly constructed dwellings and close proximity with vector breeding sites along with human’s behavior attracting mosquitoes such as unprotected sleep and placing their livestock in the house intensify the chance of mosquito contact.17 However, there is an observed behavioral change in the feeding habits of mosquitoes from endophilic to exophilic and its feeding time from late evenings to early evenings.18,19 It is also possible that vector control intervention could change the natural behavior of mosquitoes from endophilic to exophilic which is caused by avoiding control strategies such as insecticide exposure which are usually utilized inside human dwellings.18,20,21 On the other hand, the human population dynamic also becomes a potential source of malaria transmission by increasing the likelihood of spreading the disease mainly through import cases. The imported cases can either be from recent migration or short-term travel.22,23 A study report from Ethiopia in the 1980s found that a large population movement affects high transmission rates of malaria.24 This large movement of the human population might have a close relationship with agricultural work,25 and these immigrant workers are more likely to live in non-permanent houses, which are vulnerable to mosquito bites, and occasionally sleep outside, as well as having inadequate information about malaria risk.22,26 A difference in practical agricultural activities as suitable habitats of vector mosquitoes may be the predisposing factor of malaria transmission.20 Additionally, an untraceable small number of mobile sub-population groups might delay the malaria elimination strategy due to its higher risk of infection or might even re-introduce malaria in previously eliminated areas.22
Moreover, besides evidence from Ethiopia, plenty of studies have associated several other risk factors influencing malaria transmission, mostly published in recent years.27–38 In the late’90s, it was known that the older the patient, the less the incidence of malaria as well as less knowledge of malaria prevention. Several other associated factors were included such as exposure to forests and receiving previous antimalarial treatment.27,39 Bed nets could not be a very effective protective measure in a setting such as the environment in which this study was done; environmental intervention may be better applied.27 In pregnant women, the associated factors of malaria infection are lack of education, and non-possession of insecticide-treated nets (ITNs) followed by a decrease of parasite density as age increased.28 Children under the age of five years were also particularly at risk of being infected by malaria parasites, especially in sub-Saharan Africa.29 The associated risks of this particular at-risk population are mostly sociodemographic related factors such as the main floor and main wall material of the house and availability of electricity. However, indoor residual spraying (IRS) significantly reduced a child’s risk of malaria, with additional information that older children have a higher risk of malaria, notwithstanding that their risk decreases with increases in cluster altitude and their caregiver’s education level.30 Another study showed an exciting method to discover the associated factors of malaria infection. Pinchoff et al.,31 used a case-control approach based on positively detected incidence by a rapid diagnostic test (RDT) with a sophisticated statistical method. They found that, in multivariate model generalized by generalized estimating equations (GEE), the odds of being RDT positive are highest in five-17 years old (8.83 odds compared to 18 years old (or more)) and do not vary between seasons. Additionally, there is an interaction between age and report of symptoms, with an almost 50% increased odds of reporting symptoms with decreasing age category. Instead of using a case-control approach, Elijah Chirebvu et al.,32 uses a more convenient method over which the history of malaria infection is an independent variable and found that the correlated factors of malaria are household income, late outdoor activities, time spent outdoors, travel outside of the study area, non-possession of ITNs, hut/house structure, and homestead location from bodies of water. In addition, the proximity of a health facility and low vegetation cover are advantageous protective factors.
Another interesting study by Kazembe et al.,33 used a spatial regression analysis to estimate risk factors. These findings based on regression estimation found that the children who visited rural areas have six times the risk of being infected by malaria parasites, as with previous findings the higher the age of the children, the more the likelihood of being infected notwithstanding that the risk reduces as individuals gain a higher sociodemographic status. Proximity to a garden, river, or standing water are not associated but act as a cofactor of increased risk. Furthermore, this study showed that a spatial cluster of households of the infected patients affects the risk of transmission which may be explained by the variability of the environmental factors. A group of researchers,34 using a secondary database on a nation-wide scale with a regression model, found that wealth status is the first socio-economic factor which mostly contributed to the difference of malaria risk among African children. They did not find any demographic factor among the associated variables. On the other hand, sex of child and river or water body proximity are not associated with the risk of being infected with malaria. The country of resident and temperature could be a cofactor in the analysis with supplementary information of negative associations between population density and malaria incidence. One thing that should be noted, is that the study completed a comparison study of differing malaria risk and found there are several differences in associated variables between low and high-risk countries.34
In Indonesia, such risk factors have not been extensively discovered. Several studies were attempting to determine the associated factors of malaria. Based on active and passive surveillance assessing three common species of malaria in Aceh, a study35 found that the related factors are male (AOR 12.5), adult (OR 14.05), visiting the forest within the previous month regardless of the reason (OR 5.6), and working place located in the forest with overnight stays (OR 7.9). In Papua a study36 adopting the Bayesian hierarchical logistic model found that rural Papuans, as well as those who live in poor, densely forested, lowland districts, are at higher risk of being infected of malaria with the additional information of nine areas on the island having higher-than-expected malaria risks. Environmental factors such as the distance of the resident to forest areas, altitude, and rainfall are also associated with malaria. These environmental factors were also found to be strongly varied spatially in different regions.37 Additionally, a case-control study in the Purworejo district has found that not sleeping under a bed net and not closing doors and windows from 6 p.m. to 5 a.m. are associated with higher risks of malaria.38 With that limited information on malaria risk factors and the fact that there are such varying associated variables, the current study has an objective to uncover the risk factors with a broad categorical variable including individual and environmental factors. To strengthen the differences between low and high-countries as well as spatial effect of malaria, this study also included a comparison of risk factors between different endemicity areas. Additionally, to prove the risk of the sociodemographic factor, a series of entomological observations that include house type materials and condition was also included.
The local community and house owners gave permission for conducting this research in their surroundings and properties. This study was approved by the ethics commission of Hasanuddin university, Indonesia with ethical approval number: 663/H4.8.4.5.31/PP36-KOMETIK/2016. Written informed consent was obtained from all participants and mosquito collectors.
The design of the current study was case-control with a 1:2 ratio. There were four stages in this study; field malaria sampling, assessment of malaria risk factors, entomological survey, and mosquito species identification and plasmodium detection. Field malaria sampling was done for the purpose of assigning cases and controls in accordance with researchers’ criteria. The assigned cases and controls were then examined using a structured questionnaire to assess the associated malaria risk factors. A series of entomological surveys was then conducted in order to understand the effect of house type on malaria infection. There were three types of houses included in this study, namely; malaria houses (it was a non-permanent house where malaria was present at least once in the duration of one year back from the point this research started); non-malaria houses (it was a non-permanent house where malaria was absent in the duration of one year back from the start of this research); and permanent houses (it was a well-constructed house where all parts of the house closed properly). A series of human landing catch (HLC) observations were performed on these three types of houses every day for three weeks. Additionally, weekly screening on these three types of houses was carried out to monitor malaria incidence in each house type. Finally, the collected mosquito and blood samples were transferred to the laboratory for species identification and plasmodium detection.
Participant recruitment
Field malaria screening was done using tympanic temperature, rapid diagnostic test (RDT) and microscopic slide examination. People who tested positive for malaria by RDT, microscopic examination, or a combination of the two were identified, and those who met the eligibility criteria were designated as cases. Those who did not meet the eligibility criteria as cases were assigned as controls.
Eligibility criteria
Since we used a total sample, all positively detected malaria people were included in this study unless they refused or were unwilling to complete all the study protocols. In the case of children, the guardians were asked for their willingness and ability to participate in this study.
Methods of selection
The selection of controls was by criteria of an absence of malaria infection for at least a one-year period. To avoid geographical bias in controls that may lead to different vectorial capacities, the controls were selected based on their closest location by distance to the selected cases.
Field malaria sampling was conducted in two localities from the western and eastern part of Indonesia, namely Jambi province and Sumba Island as part of Nusa Tenggara province. The sampling activity was from 1st February until 31st October 2018. According to the national data of the Ministry of Health of Indonesia in 2016, Jambi province has an annual parasite index of 0.14 per 1,000 inhabitants, and Nusa Tenggara province has 5.41 per 1,000 inhabitants.40
Malaria screening was initially undertaken based on tympanic temperature. The screening was performed daily from 1 February 2018-31 May 2018 in Jambi, and from 1 June 2018-31 October 2018 in Sumba. Following the STROBE reporting guidelines, this study investigates the relationship between exposures and a health outcome. The exposure in this study was set as the risk factors, which were divided into two risk factor categories; individual and environmental exposures. A person who had a tympanic temperature of more than 37.5 was selected to be tested for the possibility of malaria infection. A finger prick for both microscopic slide and filter paper was taken after tympanic temperature screening. The slide was examined by two independent microscopists. Following up on the results of microscopic examination, positively detected malaria patients were treated with dihydroartemisinin+piperaquine tablets based on body weight and age as recommended by the Ministry of Health, Republic of Indonesia.41 All patients in our study sites were closely monitored to observe the medical condition of the patients due to malaria and potential re-infection.
Risk factors of malaria were examined by structured questionnaire comprised of both individual and environmental variables.80 The structured questionnaire was developed and created by the researcher. The questionnaire that has been created was then tested for content validity by two independent reviewers. Initially, an unpublished systematic review has been done to discover all possible risk factors associated with malaria. The risk factors were then categorized as two observational points; ‘individual’ and ‘environmental’. The ‘Individual’ section in the questionnaire is comprised of demographic and behavioral variables such as level of education, possession of mosquito nets, and spending nights in the forest. The ‘environmental’ section in the questionnaire consists of observable environmental risk factors such as the absence of gauze and closed ceilings in the house, the existence of shrubs, and the existence of livestock near the house. A day after malaria infection had been confirmed in each respondent, the researchers visited the respondent’s house to assess malaria risk factors using the structured questionnaire. The ‘individual’ section was obtained by interviewing the respondent using the questionnaire. The questionnaire was originally made in the Indonesian language, and thus the interview process with participants used the Indonesian language. All participants were able to complete the interview process. The researchers only assisted in filling out the form based on the answers provided by the participants. As soon as the interview was over, the researchers then assessed the possible risk factors in and around the house following the questionnaire. There are 12 environmental and 15 individual variables examined in the current study. The environmental factors are as follows: without gauze or barrier on ventilation, the existence of shrubs, no predator fish in the stagnant water, the presence of livestock inside household, the presence of any livestock nearby house, household wall material, the presence of puddle/stagnant water, the presence of rice field, house floor construction is not permanent, the presence of a ceiling of the house, hanging clothes inside the house, and the presence of a pool of water. On the other hand, the individual factors are: night activity outdoor, possession of bed nets, using mosquito repellant, using any kind of insecticide/pesticide, education level, previous antimalarial drug consumption, salary less than one million-rupiah, type of occupation, contact with malaria patient, high mobility, sex, age, visited a forest from previous month for any reason, working place is in the forest and requiring overnight stay.
The entomological survey of the current study was done for the purpose of observing the possible difference of mosquito bites between malaria, non-malaria, and permanent houses. A malaria house is defined as any non-permanent house that had a malaria infection at least once in the one-year period before the research started. A non-malaria house is defined as a non-permanent house that had no malaria infection in the one-year period before the research started. A permanent house is defined as any permanent house, properly closed, near malaria and non-malaria houses. A village with the highest incidence rate among our study sites was chosen to be the location of the entomological survey. According to the above-mentioned definition of three types of houses, the researchers then assigned the houses that match the definition. The data used for house selection was from routine screening by the local health office. A purposive sampling was performed to pick up malaria houses. The criteria used for this purposive sampling is the location with the highest malaria incidence and density. Non-malaria houses were selected by the nearest location to the malaria house to avoid distance bias. Due to limited number of permanent houses in study sites, purposive sampling was done and the nearest permanent houses to malaria house were selected. Malaria and non-malaria houses were the same house type, as non-permanent or not well-constructed houses. Because of the observational measurement of malaria cases, this entomological survey will support the finding of the malaria risk factors in which is often correlated with human dwellings. This survey was initially started by a week of pre-observational human landing catch (HLC) and then followed up by up to three weeks of a comparative observational HLC survey between the three types of houses. Pre-observational HLC was done to objectively select the location with the appropriate number of Anopheles species. To avoid disparity of mosquito species and abundance and indeed biases, the distance of the three kinds of houses has been set up not to exceed two km. The result of the initial screening was used to differentiate between malaria, non-malaria, and permanent dwellings. Non-malaria and permanent houses were defined as houses with an absence of malaria infection for at least one year prior to the screening. Additionally, to confirm the presence or absence of malaria infections, weekly screening was conducted throughout the study. If a malaria infection was detected in non-malaria and/or permanent houses, then the house will be excluded completely and a new household will be selected and included in the study. After initial screening, the selected houses were numbered and picked randomly for weekly HLC. In detail, a weekly schedule was made by shuffling the house number each day. Each day had four houses to be enrolled in. At the end of each week, a new shuffle was made with the same strategy repeatedly until the end of the research period. There were two houses per house types per day (two for malaria, two for non-malaria, and two for permanent houses). Two houses per group were necessary because it required human bait inside and outside the house to encompass both endophilic and exophilic mosquitoes. There were 12 houses in total per house types (six days of collection per week). However, with three repetitions (three weeks), the total sample was 36 houses per house types. Shuffling and repetition were done to avoid a disproportionate number of mosquitoes per house.
Mosquito species from the HLC survey were detected by an entomologist from Eijkman institute for Molecular Biology, Jakarta, under dissecting microscopy following a previously published species identification key.42 To confirm the species from an entomologist, randomly chosen samples were subjected to molecular examination using Internal transcribed spacer 2 (ITS2) primers. Afterward, to detect the presence of Plasmodium in the mosquito saliva, a mitochondrial based primer was used according to previous publication. For this molecular examination, we used MytaqTM HS Red Mix. The PCR mix contains purified DNA samples (1 μl per sample), double distilled water (10.7 μl), primers (0.4 μl) and MytaqTM HS Red Mix (12.5 μl). The PCR condition is as follows: 95° one minute of initial denaturation, 95° 15 seconds of denaturation, 54° (ITS2) and 48° (mitochondrial DNA for Plasmodium) of annealing, 72° 10 seconds of extension, 72° 15 minutes of final extension, and 4° for holding. The amplified product of PCR was then visualized in Biorad Gel documentation XR imaging system. The successful amplified product was then purified using ExoSAP-IT cleanup reagent to remove primers and dNTPs. The PCR reaction was run with Big-Dye terminator RR Mix and purified to remove dye-ddNTPs. Eventually, the samples were sent to the Biochem sequencing facility. After samples were successfully sequenced, the results were delivered and analyzed.
Chi-square X2 and logistic regression were used to determine the relationship of each variable with malaria bivariate and multivariate, respectively. Additionally, a general linear mixed analysis was carried out to discover the potential difference in terms of risk factors variable between Jambi and Sumba by summing all associated variables into a total variable with conditioning the number of cases and controls (prospective cases from Sumba and all cases from Jambi). IBM SPSS v20.0 (Chicago, SPSS Inc.) was used to run the statistical analysis both bivariate and multivariate. Logistic regression was done for univariate analysis to find the strongly associated independent variables with the risk of malaria infection. level of significance of p < 0.05 was determined for the association threshold. In order to find a different in associated variables, GLM (generalized linear model) analysis was applied. The GLM analysis was set to equate starting from the number of cases and control and the only associated variables that the sites share in the same manner. Kruskal-Wallis was used to determine the difference in each house type of HLC survey. For the visualization of the data, we used Graph Pad Prism 7. For molecular data, the result of Sanger sequencing was then sent to the National Center for Biotechnology Information (NCBI) website to blast with the genomic data bank.
Following the STROBE reporting guidelines for case control study, several potential sources of bias have been identified and addressed. First, to avoid an uneven distribution of risk factors between cases and controls, we have added a comparison of 1:2 between cases and controls. Second, the questionnaire was carefully arranged and validated to avoid low reliability and validity. Third, in order to minimize the imbalanced number of vectors in the HLC site, we set up the HLC site so as not to exceed 2 km. Fourth, to prevent bias in HLC houses, a malaria weekly screening was conducted, and if the screened house was malaria positive, it was then excluded and replaced. Fifth, to prevent bias in HLC houses, a strict randomization protocol was undertaken.
This research had a study duration of four months in Jambi, the western part of Indonesia, and another four months in Sumba Island, the eastern part of Indonesia, from February to October 2019. The total of 157 cases of both locations were successfully collected during the field sampling time.80 The proportion of case and control was following a 1:2 ratio. Therefore, out of 158 cases, there were 328 controls with a percentage of 32.3% and 67.7%, respectively. The basic demography of each location is presented in Table 1. The proportion of sex between Jambi and Sumba have a slightly similar pattern of male and female. The age strata from the two sites are identical at six-24 years. However, Sumba has more cases in children (0-5 years).
| Jambi | |||
|---|---|---|---|
| Proportion of case-control | Case | 48 | 30.9 |
| Control | 105 | 69.1 | |
| Sex | Male | 58 (29) | 37.9 (60.4) |
| Female | 95 (19) | 62.1 (39.6) | |
| Age | 0-5 | 9 (9) | 5.9 (18.8) |
| 6-24 | 34 (23) | 22.4 (47.9) | |
| 25-80 | 109 (16) | 71.7 (33.3) | |
Several individual factors from both Sumba and Jambi have been associated with malaria incidence (Tables 2 and 3). In Jambi, night activity outdoor (OR = 0.32; CI: 013-0.79), history of visiting forest areas in the previous month (OR = 0.35; CI: 0.15-0.84), and working place is located inside the forest (OR = 0.17; CI: 0.07-0.43) were protective factors against malaria infection. The individual risk factor for malaria infection in Jambi were not having a bed-net for sleeping (OR = 2.09; CI: 1.04-4.18), low level of education (OR = 1.01; CI: 0.29-3.45), occupation (P value = 0.000), and contact with malaria-infected patient (OR =. 3.37; CI: 1.62-7.01). The observed environmental factors that are associated with malaria in Jambi are the existence of shrubs around house areas (OR = 28.00; CI: 6.45-121.59), the existence of puddles or stagnant water around the house area (OR = 2.49; C I: 1.05-5.98), the presence of livestock nearby the house area (OR = 6.36; CI: 2.94-13.79), and the proximity of houses to forestry areas (OR = 10.84; CI: 3.97-29.58). There were eight associated individual variables with malaria from Sumba. The risk factors of malaria in Sumba are not having a bed net for sleeping (OR = 2.55; CI: 1.52-4.29), low level of education (OR = 6.09; CI: 2.12-17.47), never consumed antimalarial drug (OR = 4.16; CI: 2.09-8.28), if they ever had contact with malaria person (OR = 17.33; CI: 8.04-37.32), frequent traveling outside of the residential area (OR = 5.22; CI: 2.32-11.74), if they ever visited the forest in a previous month (OR = 1.96; CI: 1.03-3.73), and requiring an overnight stay in the forest (OR = 2.88; CI: 1.22-6.81). Additionally environmental risk factors associated with malaria are existence of shrubs surrounding house (OR = 20.99; CI: 8.24-53.46), existence of puddles or stagnant water surrounding the house area (OR = 39.98; CI: 13.86-115.32), existence of livestock inside house (OR = 3.24; CI: 1.70-6.18), existence of livestock nearby house (OR = 9.44; CI: 2.87-31.07), non-permanent house wall (OR = 5.22; CI: 1.81-15.06), non-permanent floor construction (OR = 20.79; CI: 2.81-153.79), house is in a close proximity to rice fields (OR = 14.69; CI: 0.75-286.96), and not having a ceiling of the house (OR = 19.72; CI: 1.18-330.29). Since Jambi and Sumba have different endemicity level, the generalized linear model (GLM) was applied to discover if any difference in risk factor variables from both sites. Due to any difference in the number of cases of both locations, only prospective cases from Sumba were included in the analysis. Prospective cases are those who enumerated within five to seven days after being confirmed by a rapid diagnostic test or the result from two independent microscopists or combination of both. The result of GLM indicates that there is a difference in risk factor variable from both Jambi and Sumba (P value = 0.002) (Table 4).
| No | Variable | Case (Total) | Control (Total) | P-value | OR (CI) |
|---|---|---|---|---|---|
| 1 | Bed net possession | 0.000 | 2.55 [1.52, 4.29] | ||
| No | 39 (109) | 40 (223) | |||
| Yes | 70 | 183 | |||
| 2 | Education | Uneducated49 | Uneducated49 | 0.000 | 6.09 [2.12, 17.47] SHS, diploma and bachelor are the reference |
| Primary school46 | Primary school76 | ||||
| JHS [10) | JHS56 | ||||
| SHS [4) | SHS36 | ||||
| Diploma1 | |||||
| Diploma (0) | Bachelor5 | ||||
| 3 | Antimalarial drug consumption | 0.000 | 4.16 [2.09, 8.28] | ||
| Yes/ever | 25 (108) | 15 (222) | |||
| No | 83 | 207 | |||
| 4 | Number antimalarial drug taken | 0.000 | - | ||
| 0 | 85 | 211 | |||
| 1-3 | 19 | 12 | |||
| >3 | 5 | 0 | |||
| 5 | Contact with malaria person | 0.000 | 17.33 [8.04, 37.32] | ||
| Yes | 101 (109) | 94 (223) | |||
| No | 8 | 129 | |||
| 6 | Visited forest in a previous month | 0.038 | 1.96 [1.03, 3.73] | ||
| Yes | 95(109) | 173(223) | |||
| No | 14 | 50 | |||
| 7 | Requiring overnight stay | 0.012 | 2.88 [1.22, 6.81] | ||
| Yes | 13 (109) | 10 (223) | |||
| No | 96 | 213 | |||
| 8 | Existence of shrubs | 0.000 | 20.99 [8.24, 53.46] | ||
| Yes | 104 (109) | 111 (223) | |||
| No | 5 | 112 | |||
| 9 | Existence of puddle or stagnant water | 0.000 | 39.98 [13.86, 115.32] | ||
| Yes | 46 (109) | 4 (223) | |||
| No | 63 | 219 | |||
| 10 | Existence of livestock inside house | 0.000 | 3.24 [1.70, 6.18] | ||
| Yes | 96 (109) | 155 (223) | |||
| No | 13 | 68 | |||
| 11 | Existence of livestock nearby house | 0.000 | 9.44 [2.87, 31.07] | ||
| Yes | 106 (109) | 176 (223) | |||
| No | 3 | 47 | |||
| 12 | Type of house wall | 0.000 | 5.22 [1.81, 15.06] Permanent construction is the reference | ||
| Made by wood | 4 (109) | 68 (223) | |||
| Made by cement (permanent construction) | 1 | 37 | |||
| Made by bamboo | 104 | 118 | |||
| 13 | House is in a close proximity to rice field | 0.013 | 14.69 [0.75, 286.96] | ||
| Yes | 3 (109) | 0 (223) | |||
| No | 106 | 223 | |||
| 14 | House floor construction | 0.000 | 20.79 [2.81, 153.79] | ||
| Permanent | 1 (109) | 36 (223) | |||
| Non-permanent | 108 | 187 | |||
| 15 | Ceiling in the rooftop | 0.002 | 19.72 [1.18, 330.29] | ||
| Yes | 0 (109) | 18 (223) | |||
| No | 109 | 205 |
| Study sites | Variable’s name | Num DF | F value | P value |
|---|---|---|---|---|
| Jambi | Risk factors | 1 | 9.865 | 0.002 |
| Sumba |
In order to discover the effect of house type with malaria infection, a series of entomological observations were carried out as described in the methods section. A total of 2,435 Anopheles mosquitoes were successfully collected from both sites. Out of the total collected Anopheles, 2.9% (71) is from Jambi, and the rest 97.1% (2,364) is from Sumba. Jambi was dominated with Anopheles balabacensis (79%), followed by other species; An. Maculatus (18%), An. barbirostris (1.41%) and An. sinensis (1.41%). Two species accounted to 40% and 58% of the total mosquitors catched in Sumba, An. aconitus and An. Sundaicus, respectively. The other species found were An. maculatus (1.06%), An. subpictus (0.17%), An. barbirostris and An. vagus (0.084%) and An. farauti and An. leucosphyrus (0.04%). No plasmodium was detected either from salivary nor abdominal part of the mosquitoes over 250 randomly selected mosquitoes from both Jambi and Sumba.
There is a significant difference between the total number of mosquitoes collected from Jambi and Sumba (P value ≤ 0.0001) (Figure 1). As explained in the methods section, this entomological observation characterized the houses into three types: malaria houses, non-malaria houses, and permanent houses. There is no significant difference in each house types from Jambi (P value = 0.1856). Although malaria houses from Jambi have the highest mean collected mosquitoes (0.64) than the other house types, permanent house types (0.34) have a higher mean of collected mosquitoes than the non-malaria house (0.29) (Figure 2). On the contrary, there is a significant difference between malaria-houses vs non-malaria houses (P value = 0.0143) and permanent houses (P value = 0.0351) in Sumba as presented in Figure 3. However, no difference was observed between non-malaria houses and permanent houses (P-value ≥ 0.9999). Additionally, if both sites are combined (Figure 4), only malaria-houses and non-malaria houses have a significant difference in the number of collected mosquitoes (P value = 0.0301). Permanent house type is slightly higher in the mean number of collected mosquitoes compared to non-malaria houses (5.6 and 5.032, respectively).
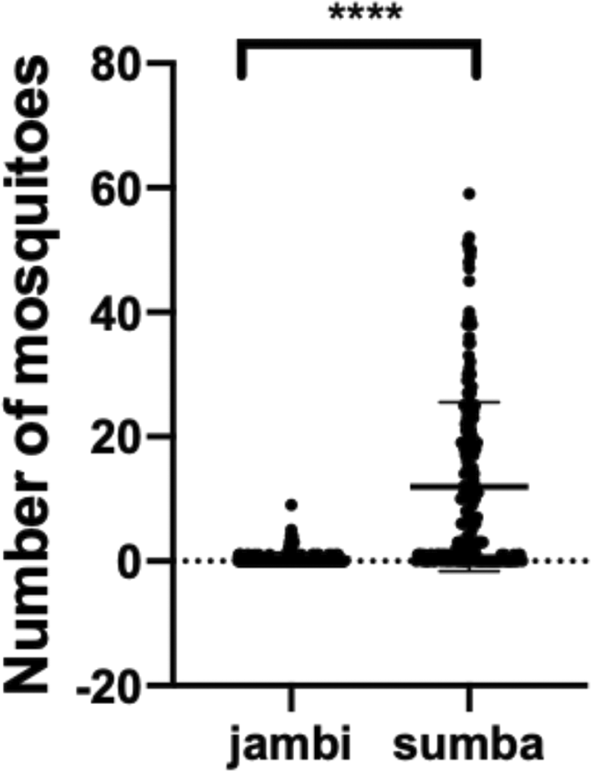
(P value ≤ 0.0001).
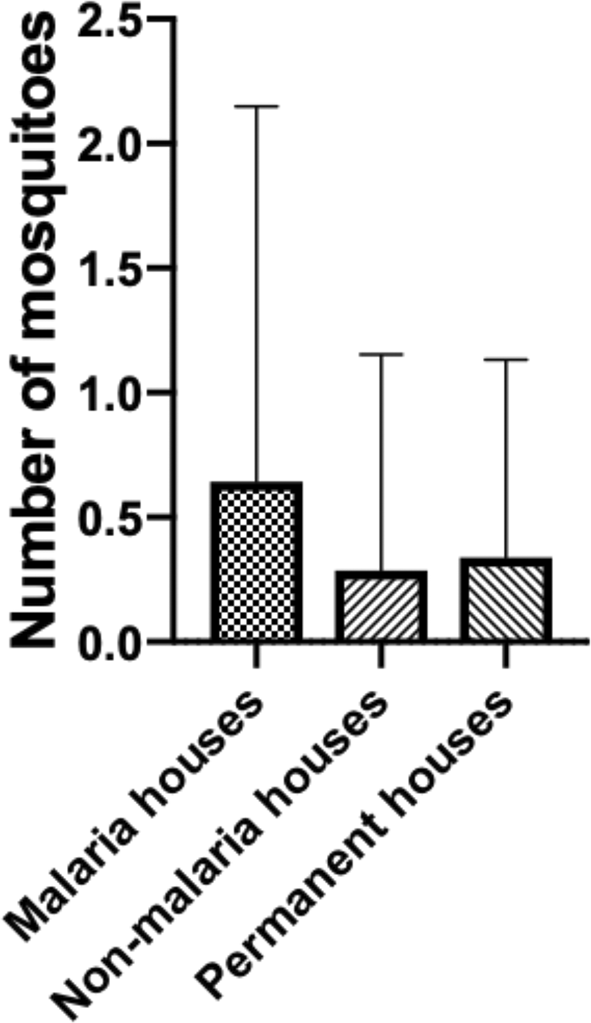
(P value = 0.1856).

Malaria-houses vs non-malaria houses (P value = 0.0143) and permanent houses (P value = 0.0351).
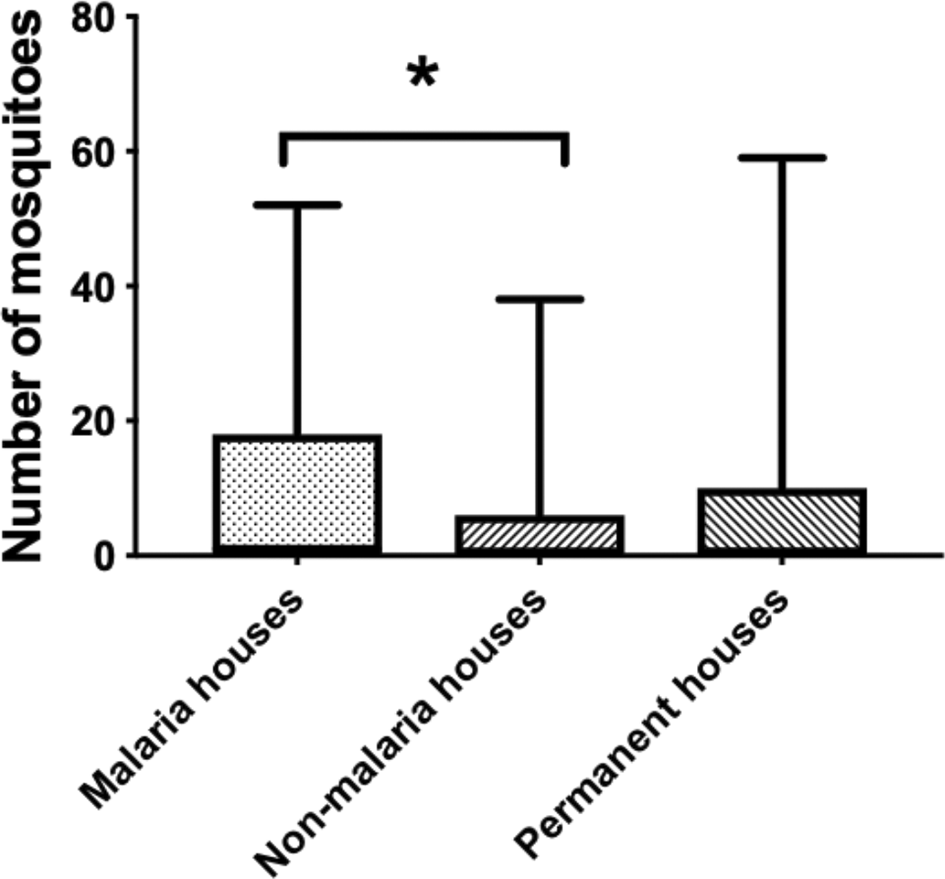
Malaria vs non-malaria houses (P value = 0.0301).
During the entomological observation, weekly malaria screening was conducted to ensure the presence or absence of malaria in each house types as well as to discover the incidence rate of each house types. Malaria has detected only one in the first week from Jambi. Otherwise, 10 cases were detected during three weeks of observation from Sumba (Table 5). If the number of cases is transformed into an incidence rate per collection method per year, then malaria houses from Jambi have 1.4 incidence rate per year and null for other types of houses. However, considering the difference in the endemicity level in Sumba, malaria houses have 8.7 incidence rate per year while non-malaria and permanent dwellings have the same rate of 2.9. Additionally, based on the calculated odds ratio, the odds of malaria houses compared to other house type is 3.77 (CI: 0.76-18.81) while non-malaria versus permanent houses have the odds of 1 (CI: 0.14-7.30).
Based on the Indonesian basic health profile, Jambi was categorized to have low cumulative incidence, while Sumba as part of Nusa Tenggara province has high cumulative incidence.40 By transforming the total number of collected cases in each location, Jambi and Sumba, then both sites have a high cumulative incidence of malaria (5.4 and 15.7, respectively). There is a discrepancy of classifying endemicity level between national data and the collected data from the current study. This phenomenon partly can be explained by the different denominators of the data, as national data considers a total number of populations in a provincial level rather than each sub-district level. Considering the fact that malaria varies greatly between sub-district and district and is not uniformly distributed, this may be the case.43,44
One of the interesting findings of the current study is the different patterns for the source of infection between Jambi and Sumba. Night activity outdoor, history of visiting forest areas from the previous month, and working place is located inside the forest are protective factors in Jambi, while the history of visiting forest areas and requiring an overnight stay inside a forest are risk factors in Sumba. This finding suggests that most of the case from Jambi was infected in the residential areas and forest areas were the source of malaria infection from Sumba. This finding underline that visiting forest areas are not always a risk for malaria infection as previous research has found.32,35,36,39 The phenomenon may be explained by the relationship of human and mosquito infection over which may be caused by uneven distribution of mosquito bites across the human population. For example, a study showed that in several areas a core group of the human population receive a substantial proportion of mosquito bites.45 Additionally, another finding indicates that a group with more individuals experiences a lower rate of mosquito bites.46
Jambi and Sumba share the same individual risk factors, namely no possession of bed net for sleeping, low education level, and if they ever contacted a malaria infected patient. It is common that bed nets and low education levels are a risk factor for malaria as previously discovered.28,32,38 The effective impact of bed nets has been extensively described in previous studies.47,48 Although defining the coverage of the bed net use is problematic.49,50 The effect of education on malaria infection has also been found from the previous study.28 Studies have demonstrated that the poor performance of children at school is a risk of malaria, and if knowledge of prevention is adequately elevated it will lower the incidence of malaria.51,52 While other researchers found that the performance of education may be temporary and not prolonged.53 Additionally, most of the cases had contact with the other cases being compared to control suggesting that they may have an infection during the interaction process. Considering the proximity of the distance of houses as neighboring, cases may also sleep in the same house or be involved in a late conversation or another way of interaction in which mosquitoes could bite them simultaneously.
There are several differences in individual risk factors between Jambi and Sumba. Occupation is statistically significant to be the risk factor of malaria in Jambi. This finding is in line with the previous discussion where most of the controls are a farmer that require them to go to the forestry areas. Most of the cases have an occupation that needs them to reside in the housing area such as a midwife, workshop worker, or odd jobs. On the other hand, in Sumba, having never consumed antimalarial drugs and traveling outside the residential area are the risk factors of malaria infection. Considering the effectiveness of the current antimalarial drugs, the higher number of antimalarial drug consumption in cases is since the majority of the cases may have re-infection that requires them to be prescribed with frequent antimalarial drug. It was described that re-infection is a common situation in a high transmission area.54 The risk of traveling outside residential areas with malaria infection is in line with a previous study.32 The participant of the current study may have traveled in neighboring villages in which infection rate are high. There are several studies that have demonstrated the risk of traveling into a high infection rate area.55,56 A reporting system need to be established to identify import cases from neighboring villages or areas.57,58
There are several same environmental factors between Jambi and Sumba, i.e., the existence of shrubs and puddles/stagnant water surrounding the household area and the presence of livestock near the house area. Studies have found that bushes are a risk factor in a densely forested area and can encourage mosquitoes to breed.31,59,60 Since case and control resided in a densely forested, basic biological attributes of the vector may play a role. It was shown that shrubs promote the malaria transmission capacity by providing an abundant source of sugar for the male mosquito while lack of sugar source contributes to lower insemination rate to females.61,62 Moreover, as observed in An. gambiae, Anopheles mosquitoes are distributed among dense growths of brush.63,64 They may rest in the shrub prior to get ready to bite. The existence of puddles/stagnant water was found to be a potential breeding place where Anopheles mosquito could oviposit.65–68 Although, evidence has suggested that no or low Anopheles larvae density is found when water is identified as turbid as puddles, drains, or swamps.69 As previously described, the existence of livestock nearby the house area increases the chance of mosquito contact.17 It was previously found that the presence of livestock at the household level can significantly alter the local species composition, feeding and resting behavior of malaria vector.70 However, the net impact of livestock-associated variation in malaria vector ecology on malaria exposure risk was unknown.70 In addition, the pattern of host attraction and biting behavior of Anopheles mosquito in Indonesia has not been yet extensively studied and only limited to one locality.71 Anopheles mosquito can be attracted to livestock even with the primary vector of malaria, because of their biting preference as zoo-anthropophilic species.72 Furthermore, placing livestock inside the house is also significantly correlated with malaria in Sumba, suggesting a different cultural behavior of tethering livestock inside. Our study demonstrates the importance of controlling malaria using livestock-based intervention or using any zoo-prophylactic agent as described elsewhere.73,74
House construction has been associated with malaria in Sumba such as non-permanent house walls, non-permanent floor construction, and not having a ceiling of the house. Such factors are in line with the previous report regarding the associated demographic factor of malaria infection underlying the importance of human dwelling construction.17,29 However, this finding may not be the case since there is a difference with entomological finding as discussed below. Additionally, the proximity of the house to forest areas and rice fields are the risk factor for malaria in Jambi and Sumba, respectively. As discussed previously, housing location in proximity to lower vegetation cover is the protective factor for malaria.32 Jambi and Sumba have different agricultural activity. Most of the people from Jambi work on rubber and palm plantations that require a large area of land, a single person could only handle five-10 hectares. On the contrary, Sumbanese people are mostly working on cashew or rice which requires a relatively limited space of land. Agricultural activities have been shown to be a predisposing factor for malaria in which suitable habitat of the vector may take place.20,25 It was previously described that rice field agro-ecosystems contributed significant vector populations.75,76 However, with the same densely forested areas, the source of infection is different between the two sites as noted in the above discussion.
Previous studies have found differences in associated variables between low and high-risk countries.34 As well as environmental factors, these are also varied across spatially different regions.37 In order to strengthen this fact, in the current study, we selected a different annual parasite index area for comparison. Based on GLM, there is a significant difference in the risk factor between Jambi and Sumba. The GLM analysis was set to equate starting from the number of case and control and the only associated variables that the sites share the same manner. It suggests that the frequency of risk factor variables between Jambi and Sumba is in a different state following its differing annual parasite index (API). Additionally, considering the fact of the different number of associated individual and environmental variables between Jambi and Sumba suggests that a high API area like Sumba has more diverse risk factors than low API area.
Finding from the current study and the other indicates that housing construction is associated with malaria infection.77 Others recommended that improved housing is a promising intervention for malaria.78,79 However, our entomological observation found that housing construction does not necessarily lead to decreased risk of Anopheles bites. Only malaria houses were found to be significantly different with non-malaria or permanent and non-malaria houses. Permanent house types had a higher mean number of collected Anopheles mosquito than non-malaria house regardless of the sites. This finding is also supported by malaria infection rate of the house types that malaria house type is higher than the two types of houses while non-malaria and permanent houses share the same number of infection rate. This phenomenon can be best explained by the existence of risk factors other than only housing types such as the presence of livestock, shrubs, puddles/stagnant water, or housing localities. As long as the other environmental risk factors are not controlled then the housing improvement program may not be effective as stated in the previous findings.78,79
Despite the findings given from this study, there are some limitations that need to be addressed in the future. The number of samples is not equal between the two locations where Jambi is substantially low. Due to an extremely low number of cases, a convenience sampling technique was performed, thus leaving inadequate analysis for Jambi. It is also necessary for future research to increase the number of samples of houses for mosquito density in each house type. Finally, to find the best association model, future research needs to consider matching analysis when performing a case-control study for malaria risk factors.
In the current study and the others have demonstrated that risk factors play a notable role in the malaria infection. In summary, there is information on our findings for malaria control strategies1; visiting forested areas is not always a risk factor for malaria as a source of infection may differ between location,2 livestock-based intervention or using any zoo-prophylactic agent is inevitably effective to avoid mosquito attraction regardless of the area,3 improving dwelling strategy may not be successful before controlling other environmental factors, and4 risk factors are site-dependent suggesting that applying risk factor management need to consider the endemicity status of an area.
Zenodo: Risk factors and housing effect on malaria infection: A case-control study. https://doi.org/10.5281/zenodo.6960903.80
The project contains the following underlying data:
• Jambi gabungan.pzfx (It contains data on the number of mosquitoes collected per house type in Jambi)
• Jambi vs sumba T-test.pzfx (it contains on total number of mosquitoes collected regardless of the house type in both Jambi and Sumba)
• Sumba gabungan.pzfx (It contains data on the number of mosquitoes collected per house type in Sumba)
• Sumba-jambi gabungan 2.pzfx (it contains combined data on the number of mosquitoes collected per house type in Jambi and Sumba)
Zenodo: Risk factors and housing effect on malaria infection: A case-control study. https://doi.org/10.5281/zenodo.7040054.80
The project contains the following underlying data:
• Supplementary questionnaire.docx (English questionnaire used in this research).
• Supplementary questionnaire.docx (Indonesian (originial) version of the questionnaire used in this research).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Zenodo: STROBE checklist for ‘Risk factors and housing effect on malaria infection: A case-control study’. https://doi.org/10.5281/zenodo.7040054.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Tropical diseases, malaria, epidemiology, clinical trials
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 07 Nov 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)