Keywords
Acute Pancreatitis, CT severity index, Modified CT severity index, clinical outcome parameters, Ranson’s criteria, hospital stay, multisystem organ dysfunction syndrome, sepsis.
This article is included in the Manipal Academy of Higher Education gateway.
Background: Acute pancreatitis (AP) has unpredictable severity. Its management is based on initial assessment of disease severity. It ranges from mild interstitial to severe necrotic form; the latter is associated with poor prognosis. Contrast-enhanced computed tomography (CT) of the abdomen is the gold standard in early detection of pancreatic necrosis and in assessing the severity of AP. Two CT grading systems exist to assess the severity of AP: CT severity Index (CSI) and modified CSI (MCSI). This study compares the usefulness of these two systems in predicting the severity and clinical outcome in AP in comparison with Ranson’s criteria and clinical outcome parameters.
Methods: This is a prospective hospital-based screening study of 80 patients aged >12 years with clinical diagnosis of AP who underwent contrast-enhanced CT study of the abdomen. Comparative analysis between MCSI and CSI with Ranson’s criteria and clinical outcome parameters was assessed by Chi-Squared test.
Results: The accuracy of CSI and MCSI in predicting the requirement of critical care, superadded infection, multiple organ dysfunction syndrome (MODS) and requirement of intervention were 73.0%, 64.5%, 69.8% 60.9% and 77.2%, 76.0%, 74.4% & 56.6% respectively. Area under the curve for MCSI score was significantly higher (AUC: 0.861; 95% CI: 0.736-0.986) than CSI score (AUC:0.815;95% CI:0.749-0.941). MCSI and CSI showed significant correlation with Ranson’s criteria; however, MCSI correlation was better (r:0.53; p<0.01) than CSI (r:0.35;p:0.04).
Conclusion: CSI and MCSI are better predictors of severity, clinical outcome and mortality compared with Ranson’s criteria, with MCSI being more accurate and better predictor than CSI. The accuracy of MCSI is better than CSI for prediction of requirement of critical care, development of superadded infection and development of MODS in AP. However, CSI and MCSI have low accuracy in predicting intervention in AP.
Acute Pancreatitis, CT severity index, Modified CT severity index, clinical outcome parameters, Ranson’s criteria, hospital stay, multisystem organ dysfunction syndrome, sepsis.
Version 2 includes significant changes, updates, and improvements based on the suggestions from the reviewers. We have changed the first line in the introduction. We have also added the limitations of CSI, why we chose Ranson’s criteria with CSI and MCSI & added the pros and cons of the Atlanta classification in the introduction. In the methodology, we have included the institutional protocol for diagnosing and managing pancreatitis and the institute’s protocol for admitting patients for critical care. We have included the uniqueness and limitations of the study in the discussion. We have put the MSCI and CSI results compared to the same parameter together in one figure but not separately in Figures 1-5, 7. Ranson's score result is added to the area under the curve analysis (figure 9) with a resultant change in Table 6 (area under curve analysis) to compare CSI and MCSI with Ranson’s score in outcome prediction. Reanalysis of statistics of the same data provided a mild change in conclusion that Ranson’s score is a better & near perfect predictor of mortality than CSI, which is better than MCSI for the same. Similar changes are reflected in the abstract. I have also acknowledged the people who helped us with this manuscript's statistical analysis and clinical aspects.
See the authors' detailed response to the review by Saurabh Dawra
See the authors' detailed response to the review by Juan Xiao
See the authors' detailed response to the review by Jan Trna
Acute pancreatitis (AP) is one of the most common causes of acute abdomen. Based on the severity, 80% of cases are mild, and 20% of cases are severe, which morphologically correlate with oedematous and necrotizing forms of AP, respectively. The mild form is self-limiting without causing major physiological insult. The severe form is life-threatening and can lead to early or late multiple organ dysfunction syndrome (MODS) and superadded infection.1–3
Contrast-enhanced computed tomography (CT) of the abdomen is the gold standard4 in identifying necrosis and fluid collections in AP. This can aid in predicting disease severity and the patient’s prognosis, thus guiding the management.
The original Ranson criteria were developed in the 1970s, and while they have been validated over time, there are calls for updates to reflect modern clinical practices. The Ranson score is often considered better for early assessment of acute pancreatitis severity upon admission and within the first 48 hours. This is because it incorporates both clinical and laboratory parameters that can be evaluated upon hospital admission, providing an early prognostic indicator. The Ranson score’s criteria can be calculated without the need for advanced imaging, making it accessible in settings where CT scans may not be immediately available. This simplicity and the ability to rapidly assess the severity can be crucial in acute settings. Studies have shown that the Ranson score has a good predictive value for mortality and morbidity in AP, especially when assessed within the first 24 hours. It allows for the stratification of patients into risk categories, which can guide initial treatment decisions.5 There are a few limitations to Ranson’s score. Few meta-analyses have shown that other scoring systems have better sensitivity & specificity than Ranson’s score. The exact score and severity cannot be determined until 48 hours have passed, which becomes a problem in emergency situations before 48 hours to assess severity. Ranson’s score has 11 parameters that make it relatively complex to assess. It cannot be used in pediatric patients & patients at high altitudes.6
The Atlanta classification is based on a morphological assessment of AP severity on CT, which usually takes place after at least 48-72 hours based on which CSI & MCSI scores are calculated.7 Various studies suggest evidence that severity can be better assessed by CT than the clinical grading systems due to direct visualization of necrosis and complications of AP on CT.8,9 Although the CT severity index (CSI) shows a good correlation with the severity of AP, few studies suggested few limitations. One of the main challenges is the subjectivity in interpreting CT findings, as different radiologists may have varying interpretations. This can lead to inter-observer variability and affect the accuracy of the CSI score. Also, CSI is less immediately accessible due to the need for contrast-enhanced CT scans, which are usually performed after at least 48-72 hours.7 Moreover, the CSI does not consider certain important factors, such as the patient’s clinical condition, laboratory parameters. vascular and extrapancreatic complications. Few studies suggest that CSI dint show a good correlation with clinical outcome, mortality & need for surgical or percutaneous interventional procedures, MODS, and superadded infection.3 These shortcomings led to the modification and simplification of CSI by Mortele et al.3 leading to the formation of a modified CT severity index (MCSI) which took into account of vascular & extrapancreatic complications which theoretically should better correlate with clinical outcome.7 The Atlanta classification’s strength lies in its comprehensive approach to defining the disease and its complications such as necrosis, abscess, and pseudocyst formation, but it is less directly predictive of mortality.5 By identifying specific complications, the Atlanta classification helps plan the timing of potential surgical interventions. The present study is performed to assess the predictability of the two CT severity indices, namely CSI & MCSI in comparison with Ranson’s score in AP with respect to severity, mortality & clinical outcome parameters.
This is a prospective hospital-based screening study performed in the department of Radiodiagnosis affiliated to Kasturba Medical College (KMC), Mangalore (MLR), Manipal Academy of Higher Education (MAHE), Manipal, India on 80 patients with the clinical diagnosis of AP who underwent contrast-enhanced CT abdomen over a period of 2 years from September 2019 to September 2021. The study was performed after the approval from the Institutional Ethics Committee (IEC), KMC, MLR, MAHE with approval number of IEC KMC MLR 09-19/411. The diagnosis of acute pancreatitis was made based on a combination of one or more clinical features like relevant history of alcohol consumption, presence of gall stones, abdominal pain, vomiting, features of ileus, fever, tachycardia, hypotension, and other organ dysfunctions. The clinician then correlates with elevated lipase and amylase laboratory parameters, about threefold above the laboratory upper limit. Finally, the clinician confirms the diagnosis based on imaging findings, such as CECT abdomen or ultrasound of the pancreas. The diagnostic decision rests with the treating clinician.
Acute Pancreatitis cases at our institute are managed by the Department of Surgery. Patients with clinical features of acute pancreatitis or those being evaluated for abdominal pain in the emergency department are thoroughly evaluated and admitted based on the discretion of the attending surgeon. The patients are managed conservatively with bowel rest, fluid resuscitation, intravenous (i.v) antibiotics and analgesics, and other supportive measures as and when indicated, including supplemental oxygen, inotropic or ventilatory support, and transfusions if indicated for Disseminated intravascular coagulation (DIC). Patients with mild cases and stable vital parameters were admitted to the wards, and those with one or more organ dysfunction or unstable vitals were admitted to the intensive care unit (ICU). The investigations for Ranson’s score were not performed for all cases but based on the clinical condition at the discretion of the attending surgeon. Complete blood counts, glucose, calcium, liver and renal function tests, and blood gas were performed at baseline for all cases, whereas the post-48-hour investigations were done selectively based on the clinical condition of the patient.
Patients were followed up with contrast-enhanced CT (CECT) abdomen based on Ranson’s criteria and suspected complications and treated accordingly. Mild cases and those showing an improving trend were not subjected to further imaging.
Usually, CT is deferred for at least 48-72 hours post-onset of illness to allow the acute process to subside. Patients in the ICU are imaged only after the stabilization of their vital status. There is a Surgical intensive care unit in the hospital with ventilators and routine intensive care support systems. Unfortunately, no step-up or high-dependency unit (HDU) facility is available in that hospital. Any patient who is found to have any organ dysfunction or features of shock is admitted to the ICU for monitoring and treatment and started on appropriate treatment. They are provided with supplemental oxygen, Non-invasive ventilation (NIV) or endotracheal intubation with ventilation as and when required, hemodynamic support with transfusions or inotropic and pressor supports, and routine conservative management. Paediatric patients <12 years of age were excluded from the study, as Ranson’s criteria scoring is not done in this group of patients in our hospital. Patients with poor imaging results due to poor compliance or motion artifacts were excluded. Patients without intravenous (i.v.) contrast administration were also excluded. Patients with a diagnosis of acute-on-chronic, recurrent, and calcific pancreatitis and those who got discharged against medical advice or were lost to follow-up were also excluded from the study. Patients with cardiac, renal & respiratory comorbidities were excluded from the study. Since informed consent is routinely taken prior to every CT study and research data are obtained from the CT machine computer and patient case files with no direct interaction with the study participants, IEC, KMC, MLR waived off additional informed consent from the study participants for this research.
16-slice and 32-slice CT scanner machines were used to acquire 5-mm plain CT axial sections followed by the administration of 1.5–2.0 mL/kg body weight (80–100 mL) of non-ionic i.v. contrast through the automated injector. This was followed by around 1 mL/kg body weight (40–50 mL) of normal saline. The rate of injection for both contrast and saline administration was ~4 mL/s which was altered in accordance with haemodynamic status, body weight and size of the i.v. cannula. The images were acquired in the arterial and porto-venous phases at 6–8 and 35–45 seconds respectively in all cases by bolus tracking method which is described as follows. A locator was placed on the aorta at D12–L1 level and the contrast injection got automatically triggered via the automated injector once the aorta at this level showed optimum contrast opacification. Axial sections of 5 mm slice thickness were then reformatted to thin 0.6 mm axial, sagittal and coronal sections. The clinical and laboratory details of the patient were obtained from the CT requisition form and patient case file. This was followed by assessment of severity of acute pancreatitis using both CSI (Tables 1a, 1b and 1c) and MCSI (Tables 2a, 2b and 2c). Accordingly, severity of AP was graded as mild, moderate and severe based on the scores.
| Percentage of necrosis (%) | Points |
|---|---|
| Absent | 0 |
| <30 | 2 |
| 30–50 | 4 |
| >50 | 6 |
| Percentage of necrosis (%) | Points |
|---|---|
| Absent | 0 |
| <30 | 2 |
| >30 | 4 |
| Extra-pancreatic complications (one or more of pleural effusion, ascites, vascular complications or gastrointestinal tract involvement) | 2 |
Wherever available Ranson’s criteria score was noted down from patient case file and the correspondence of both the CT indices were studied with respect to the Ranson’s criteria. Ranson’s criteria score consists of 11 prognostic parameters, out of which five parameters are assessed at the admission and six parameters are assessed during initial 48 hours of hospital stay (Tables 3a and 3b).
Ranson’s score of 0 or 1 suggests complications will not develop in AP and mortality is negligible. On the other hand, Ranson’s score of 3 or more predicts severe AP with possible mortality.6 The mortality in AP is directly proportional to Ranson’s criteria score (Table 3c).
| Ranson’s criteria score | Mortality (%) |
|---|---|
| 0–2 | 0–3 |
| 3–4 | 15 |
| 5–6 | 40 |
| 7–11 | 100 |
The clinical outcome parameters9,10 were noted down from all the patient case files and its association with CT severity indices were studied and are as follows:
1. The extent of hospital or intensive care unit (ICU) stay (greater than or equal to 15 days);
2. Requirement of critical care, (Arterial oxygen tension (PO2) <60 mmHg or requirement of ventilation, systolic blood pressure (BP) <90 mmHg);
3. Requirement for (surgical/percutaneous) intervention (like drainage and aspiration);
4. Evidence of infection, (combination of a fever more than 100°F and elevated WBC count greater than 15,000 cells/mm);
5. Existence of organ failure (Arterial PO2 <60 mmHg or requirement of ventilation, serum creatinine of >3 mg/dL or urine output of <500 mL per 24 h and systolic BP of <90 mmHg); and
6. Death.
Outcome Variables that were studied are as follows:
• Sensitivity, specificity, positive predictive value, negative predictive value and accuracy of CSI and MCSI with respect to clinical outcome parameters like mean hospital stay, requirement of critical care, superadded infection, MODS, requirement of intervention & mortality.
• Concordance of CSI and MCSI with the score of Ranson’s criteria.
The data was collected on a pre-designed study proforma. Qualitative data was expressed as percentage and frequency. Chi-Squared test was used to assess the association among the qualitative variables. The level of significance was represented by p-value of less than 0.05. Screening efficacy was computed using standard formulae. Wherever necessary, the results were graphically represented. Pearson correlation was used to assess the magnitude and direction of association between CSI and MCSI with Ranson’s score. Receiver operating characteristics (ROC) curves were used to compare the role of CSI and MCSI in predicting the mortality in AP with r value from +1 to −1. The r value of +0.1 to +1, 0 and −0.1 to −1 was suggestive of positive, zero and negative correlation respectively. Area under the curve (AUC) between CSI and MCSI as predictor of mortality was analyzed. Statistical package for social sciences (SPSS) version 21.0 (RRID:SCR_002865) and Microsoft Excel 2010 (RRID:SCR_016137) were used for most of the analysis and graphical representation respectively.
The patients with AP in this study were more or less equally distributed across all the decades from the 2nd to 6th decade with a mean age of 44.41 years. There was clear male predominance of 77.5% with 22.5% female patients with a male-to-female ratio of 3.5:1. The most common cause for acute pancreatitis was alcoholism (56.3%) followed by gallstones (28.8%).
As per MCSI, more than half of patients (56%) with AP had mild disease, about one-third of them (36.3%) had moderate disease, and a small percentage (7.5%) had severe disease (Figure 1). As per CSI, about half of patients (52.5%) with AP had moderate disease, about one-fourth of them (26.3%) had severe mild disease, and 21.3% had mild severe disease (Figure 1).
1) Requirement of the critical care
Based on the MCSI score, all the patients with severe AP (100.0%) required critical care, 82.8% of moderate disease needed critical care, and only third one-third of patients with mild disease (31.1%) needed intensive care with (p<0.01) (Figure 2). The overall sensitivity and specificity for the prediction of critical care requirement was 85.7% and 68.9% respectively with an accuracy of 77.2%. As per the CSI score, most (95.2%) of severe disease, about half (52.4%) of moderate disease, and a small percentage (11.8%) of mild disease required critical care (p<0.01) (Figure 2). The overall sensitivity and specificity for the prediction of critical care requirement were 66.7% and 88.2% respectively with an accuracy of 73%.
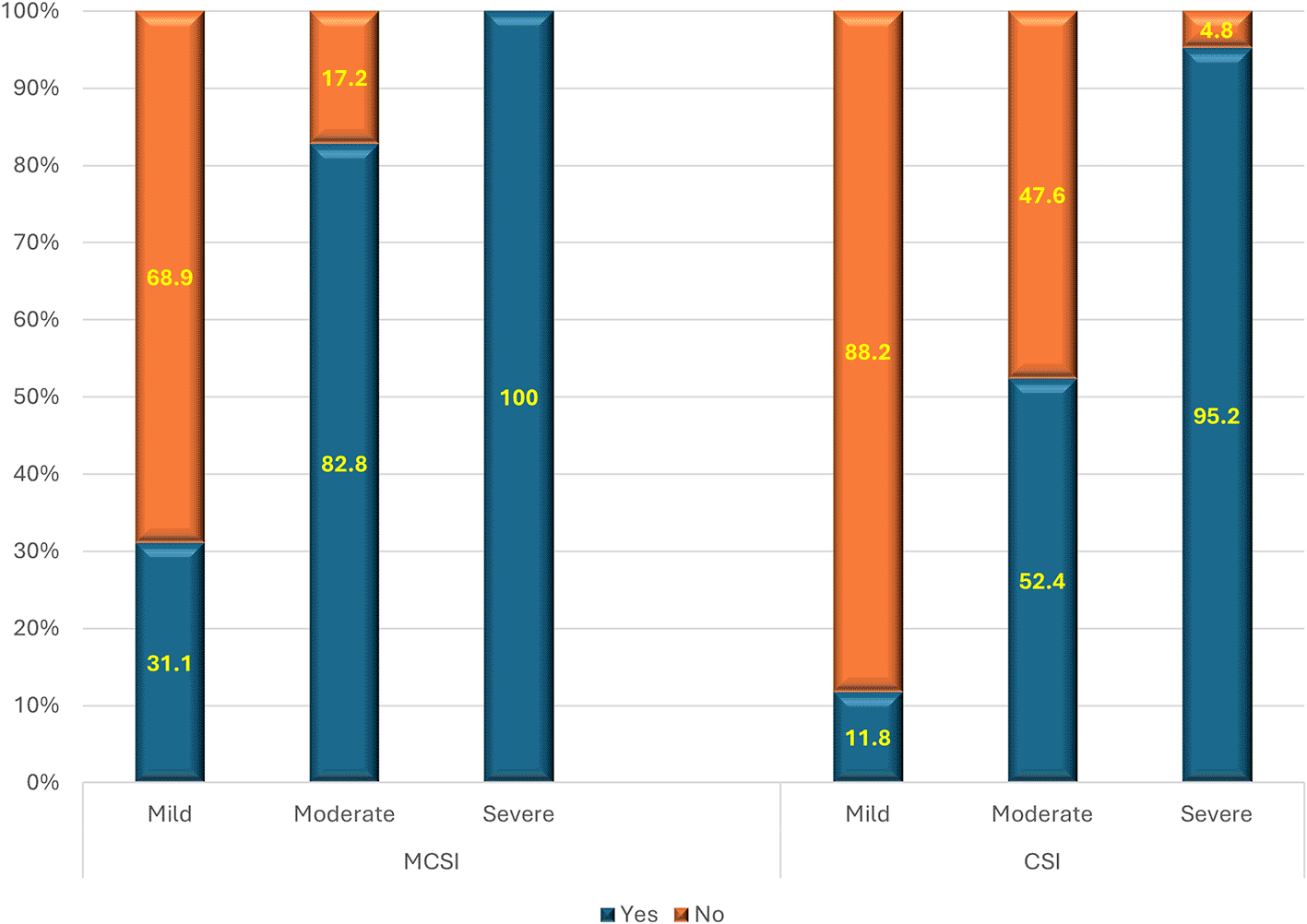
2) Development of superadded infection
As per MCSI, the superadded infection was seen in 83%, 41% and 4% of severe, moderate and mild disease of AP respectively (p value<0.01) (Figure 3). The overall sensitivity and specificity were 49% & 96% respectively with an accuracy of 76% in predicting superadded infection in AP patients. Based on the CSI score, there was no (0.0%) superadded infection in mild disease, while it was present in slightly less than half (47.6%) of severe disease and about 21.4% in moderate disease (p<0.01) (Figure 3). Hence the overall specificity & sensitivity for prediction of the presence of superadded infections were 100.0% and 30.2% respectively with an accuracy of 64.5%.
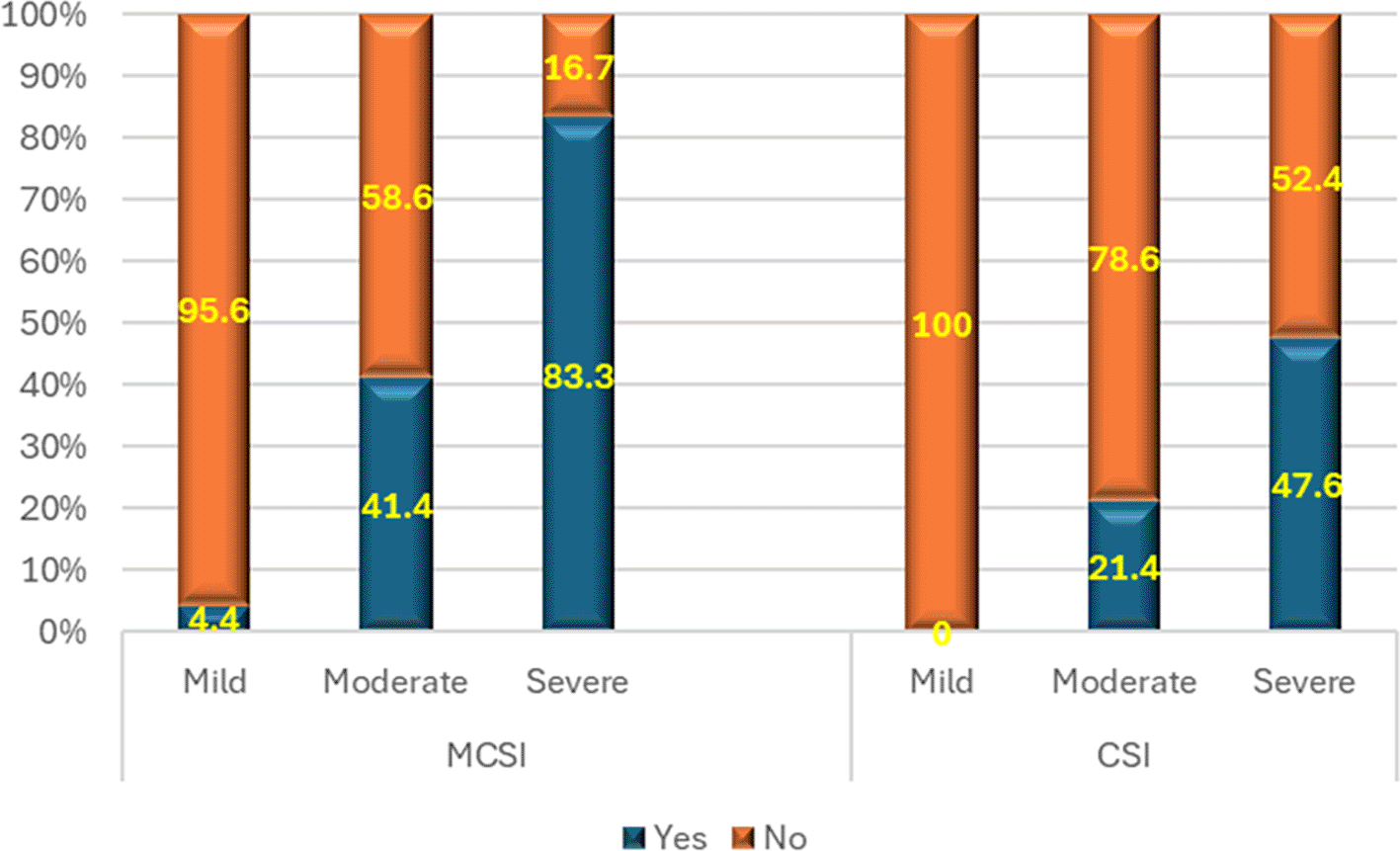
3) Development of multiple organ dysfunction syndrome (MODS)
As per MCSI, MODS developed in 15.6% of mild, 58.6% of moderate and 83.3% of severe AP (p<0.01) (Figure 4). Overall sensitivity & specificity for prediction of development of MODS were 62.9% and 84.4% respectively with an accuracy of 74.4% respectively. As per the CSI score, there was no (0.0%) development of MODS in mild disease. On the contrary, most (95.2%) of severe disease and 21.4% percentage of moderate disease developed MODS (Figure 4). The overall specificity & sensitivity for the prediction of the development of MODS was 100.0% and 40.0%, respectively, with an accuracy of ~69.8%.
4) Requirement of intervention
As per MCSI, intervention was performed in 55.6% of mild, 65.5% of moderate & 83.3% of severe cases of AP (p<0.01) (Figure 5). The overall sensitivity & specificity for prediction of the requirement of intervention was 68.6% and 44.4% respectively with an accuracy of 56.6%. As per CSI score, approximately three-quarters (76.2%) of patients with severe AP required intervention, 61.9% of patients with moderate disease and 41.2% of patients with mild disease required intervention (p<0.01) (Figure 5). The overall sensitivity and specificity for prediction of the requirement of intervention by CSI was ~66.7% and ~58.8%, respectively, with an accuracy of ~60.9%.
The MCSI score showed good sensitivity and specificity for the development of MODS, good sensitivity for predicting the requirement for critical care and intervention, and good specificity for the development of superadded infection (Figure 6a).
CSI score showed high specificity for the development of MODS and superadded infection. The overall accuracy is better with the MCSI score than the CSI score for the prediction of the requirement of critical care, development of superadded infection & development of MODS. Both MCSI and CSI scores had low accuracy in predicting the requirement of intervention (Figure 6b).
As per the MCSI score, the mortality rate was 67% in severe AP, 24.1% in moderate disease and 2.2% in mild disease (Figure 7). The overall specificity and sensitivity for the mortality prediction were 91% and 33%, respectively, with an accuracy of 87.5%. As per the CSI score, the mortality rate was highest for severe AP (43%) followed by moderate AP (7%) and nil (0%) in mild AP (Figure 7). The overall sensitivity & specificity for prediction of mortality was 75% and 82.4%, respectively, with an accuracy of 73.8%.
The mean hospital stay by MCSI was highest in the moderate grade of AP with ~22 days as compared to approximately 12 & 13 days in mild & severe disease respectively (p<0.01) (Table 4a).
| MCSI Score | N | Mean hospital stay (days) |
|---|---|---|
| Mild | 45 | 12.27 |
| Moderate | 29 | 22.14 |
| Severe | 6 | 13.33 |
| Total | 80 | 15.93 |
The mean hospital stay as per CSI score was significantly higher in moderate and severe grade of acute pancreatitis, corresponding to approximately 18 and 19 days, respectively, as opposed to approximately 8 days in mild disease (p<0.01) (Table 4b).
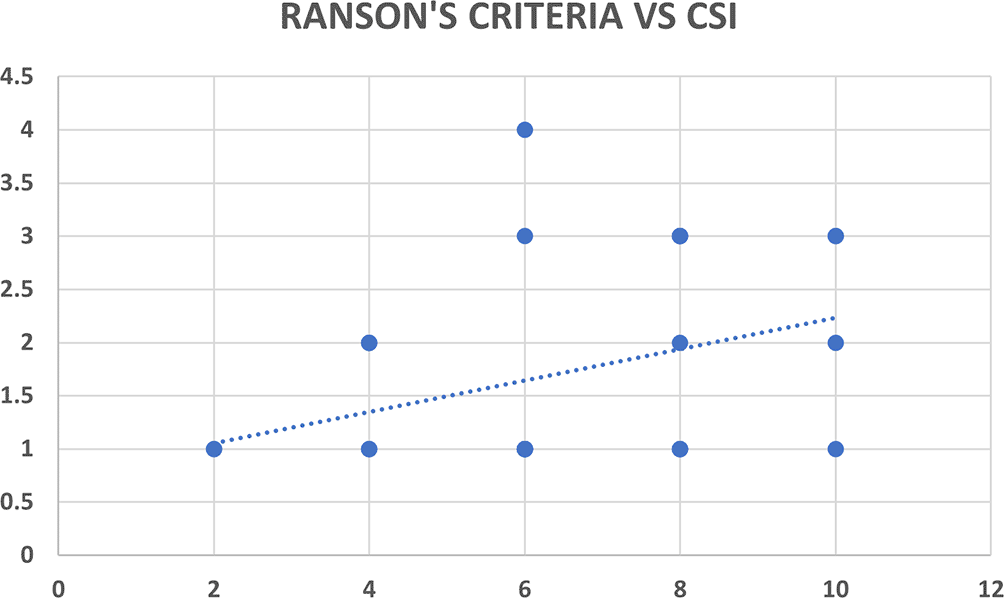
Ranson’s criteria score was available with 31 out of 80 patients (38.8%). There was significant correlation between Ranson’s criteria and both CT severity indices (CSI and MCSI) but the correlation was highly statistically significant and better with the MCSI score (r=0.53; p<0.01) as compared to the CSI score (r=0.35; p=0.04) (Table 5, Figures 8a & 8b).
| Pearson co-relation | ||
|---|---|---|
| Ranson’s criteria | r-value | p-value |
| CSI Score | 0.35 | 0.040 |
| MCSI Score | 0.53 | <0.01 |
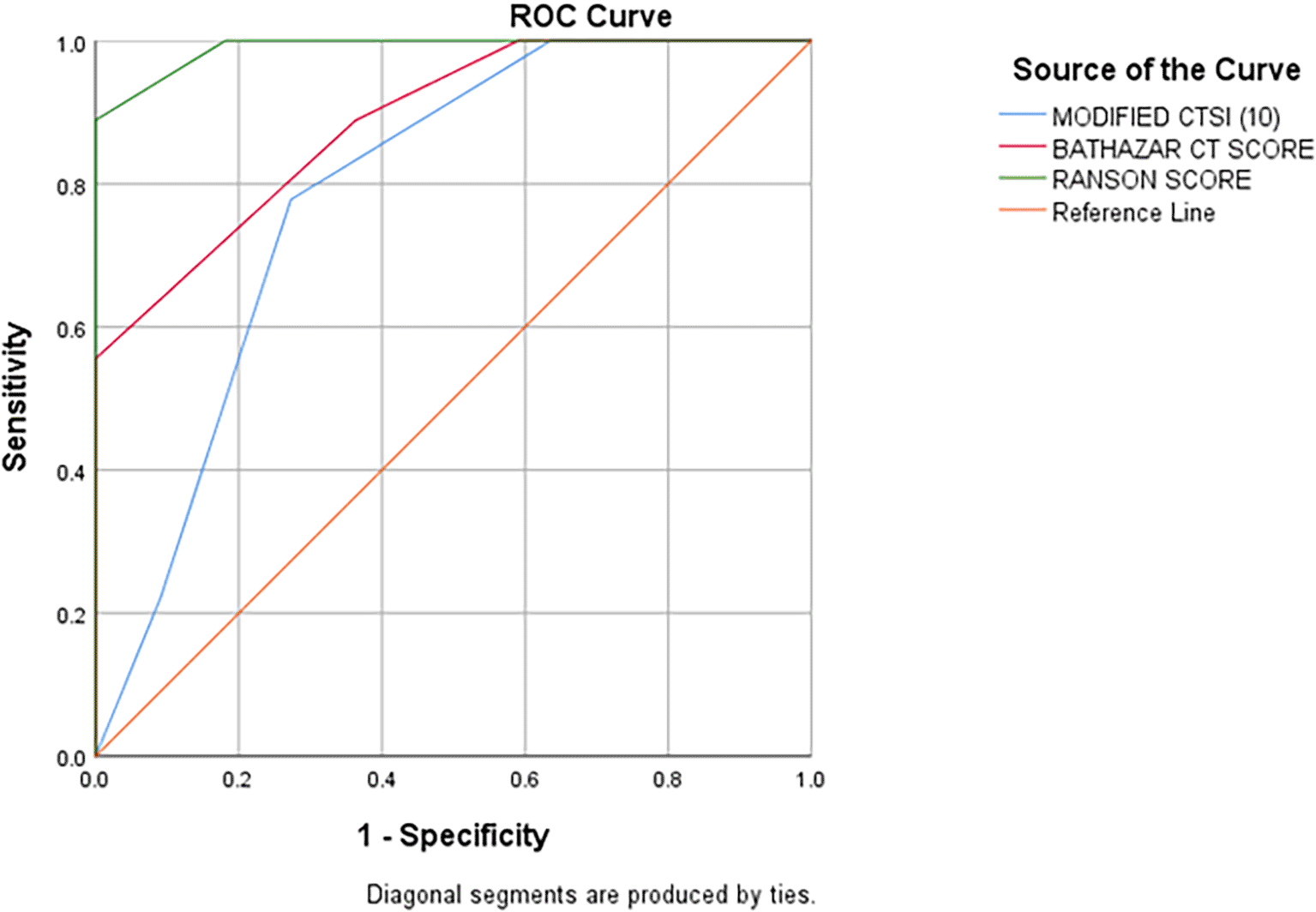
According to ROC Curve analysis, CSI, MCSI & Ranson’s scores were significant predictors of the development of mortality in AP. However, the area under curve (AUC) was near perfect and the best predictor of the mortality (AUC 0.990; 95% CI: 0.962-1.017) of AP followed by CSI score (AUC: 0.886; 95% CI: 0.759-1.014) which is better predictor of mortality than the MCSI score (AUC: 0.788; 95% CI: 0.627-0.949) (Table 6 & Figure 9).
Contrast-enhanced computed tomography of the abdomen is the imaging modality of choice and is the gold standard in diagnosing AP. The necrotizing form of AP, though less common, if present, is associated with a myriad of life-threatening complications. Among all the diagnostic tests available, CT has the highest diagnostic accuracy in detecting pancreatic necrosis.13
Steinberg et al.14 in their study suggested the evidence that 80 to 90% of AP was due to cholelithiasis & chronic alcoholism. Our study suggests evidence of alcoholism as the most common etiological factor for AP (56.3%) followed by gallstones (28.8%). Similar evidence was suggested by Wongnai et al.15 in their study on 90 patients of AP, where alcoholism and pancreaticobiliary ductal calculi were reported as an aetiological factor in 60% and 18% of patients respectively. In India, alcohol consumption is predominantly seen in males (male-to-female ratio of 24.3:1).16 The suggestive evidence of alcohol abuse as the commonest aetiological factor of AP combined with the male predominance of alcohol consumption in India explains the male to female preponderance (3.5:1) in this study. Similar evidence was suggested by Dugernier T L et al.17 and Balthazar EJ et al.18
On the contrary, Raghuwanshi S et al.19 suggested the evidence of most common aetiology for AP as cholecystolithiasis (42%) followed by alcoholism (38%) with remaining 20% aetiology for AP belonged to rest category which included idiopathic, trauma and drug induced cases (24%, 2% and 2% respectively). Casas et al.20 in their study on 148 patients suggested cholelithiasis (57%) as the most common aetiological factor for AP followed by alcoholism (21%) with both together contributing to another 5% of AP patients. Bollen TL et al.21 and Jauregui et al.22 also suggested the evidence of cholelithiasis as the predominant aetiological factor for AP.
This study is comparative analysis between MCSI and CSI grading systems in assessing severity and clinical outcome. Majority of the patients with AP belonged to mild category as per MCSI and moderate category as per CSI. This resulted in a small group of patients who had different categories of severity by CSI and MCSI. The present study suggests MCSI to be more accurate predictor of severity than CSI as it predicted clinical outcome more accurately in those patients who were differently categorized in severity by CSI. This better prediction of severity and clinical outcome by MCSI in AP may be attributable to inclusion of extra-pancreatic complications of AP like ascites, pleural effusion, vascular complications and gastrointestinal complications in the assessment of MCSI which are not included in CSI. Kondekar S et al.10 and Banday et al.23 suggested partially opposing evidence from our study where the majority of the patients by MCSI belonged to the mild category as per our study and the majority of the patients belonged to the severe category as per CSI unlike our study.
Banday et al.23 in their study suggested evidence of increasing mean duration of hospital stay with increasing severity by MCSI score and concluded that the duration of mean hospital stay is directly proportional to severity grading by MCSI system in acute pancreatitis.
Our study suggests mean hospital stay in AP by CSI score is significantly longer in moderate and severe disease as compared to mild disease (p<0.01) whereas the mean hospital stay by MCSI is significantly longer in moderate disease as compared to mild and severe disease (p<0.01). This can be attributed to the fact that mild cases were discharged relatively early from the hospital in contrast to moderate category cases, and very severe cases had higher mortality with fewer hospital stays.
Overall in the present study, MCSI score showed good sensitivity for prediction of requirement of critical care, development of MODS and requirement of intervention. MCSI showed good specificity for MODS and development of superadded infection. CSI showed high specificity for MODS and development of superadded infection. Overall accuracy of MCSI was better than CSI for prediction of requirement of critical care, development of superadded infection and development of MODS. Both scores showed lower accuracy with regard to requirement of intervention.
The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of MCSI in predicting severity according to the study by Bollen TL et al.21 were 71%, 93%, 69% and 94%, respectively. This study suggested evidence of an accurate correlation of clinical scoring systems with systemic complications & mortality in AP. The study also suggested evidence that the radiological scoring system was more accurate in predicting the severity of acute pancreatitis, superadded infection and need for intervention than the clinical scoring system. Among the two radiological scoring systems, the study suggested no evidence of significant differences between CSI and MCSI in predicting the severity of acute pancreatitis.
Bollen et al.24 suggested CSI showed better sensitivity, specificity, PPV and NPV than MCSI. Whereas Jauregui-Arrieta LK et al.21 suggested different evidence where MCSI showed better sensitivity, specificity and PPV than CSI in severe AP and concluded that MCSI is a better screening test than CSI in severe AP. Sharma et al.25 suggested sensitivity and NPV is better with MCSI (98.6% and 90%, respectively) than CSI (87.3% and 57.1%, respectively) with similar PPV for both (~74%) and low specificity of 26.5% and 35.3% for MCSI and CSI, respectively.
The present study shows a significant correlation between Ranson’s criteria and both severity indices on CT (CSI and MCSI), but the correlation of MCSI with Ransons’ criteria is highly statistically significant, which suggests that MCSI is a better predictor of severity and clinical outcome than CSI.
On receiver operating characteristic (ROC) curve analysis, the present study suggests evidence that both CSI and MCSI are significant predictors of development of mortality in AP. However, the area under curve was significantly higher for CSI score (AUC: 0.886; 95% CI: 0.759-1.014) as compared to MCSI (AUC: 0.788; 95% CI: 0.627-0.949) which suggests CSI as better predictor of mortality in AP than MCSI. The AUC for Ranson’s score was significantly highest with near complete to 1 (AUC: 0.990; 95% CI: 0.962-1.017) indicating that it is the best predictor of mortality in AP than both the CT severity indices.
Mangalanandan S et al.26 suggested evidence of strong correlation between Ranson’s criteria and MCSI with mild and severe forms of AP showing 100% agreement with each other. But moderate category in MCSI score had disagreeing results because Ranson’s criteria has only mild and severe categories due to which moderate category patients could not be studied. Their study suggested that MCSI (sensitivity of 93.33% and specificity of 54.17%) is more sensitive but less specific than Ranson’s criteria (sensitivity of 80% and specificity of 83.3%) in predicting actual outcome of AP. Although Chand P et al.27 suggested evidence of lack of statistical significant difference between Ranson’s criteria and MCSI in evaluation of the outcome of AP with respect to the systemic complications,7 there was statistically significant difference between MCSI and Ranson’s criteria with respect to local complications with increased incidence of local complications with higher Ranson’s criteria. The uniqueness of this study is that there was no other study in the literature comparing both radiological severity indicators CSI & MCSI with Ranson’s score alone in this combination at the time of conceptualization of this study. There were studies comparing CSI alone or MCSI alone with Ranson score or correlating radiological scores (CSI/MCSI) with multiple clinical scoring systems in various combinations. One of the drawbacks of the study was pediatric patients below the age of 12 years were not included as Ranson’s criteria is not done for them in our hospital. Also, a smaller sample size which may increase the margin of error when compared to a study with a relatively larger sample size. Third drawback is the lack of availability of Ranson’s criteria score in 61.2% of the patients in the study due to the usage of various other alternative clinical grading systems like Revised Atlanta Classification, Acute Physiology and chronic health evaluation (APACHE) II & Bedside index of severity in acute pancreatitis (BISAP) by the treating clinician. These alternative clinical grading systems are affordable, quick & require less effort in assessing the severity of AP than Ranson’s criteria. Since no single scoring system can universally predict the patient outcome perfectly, it is a limitation of the study. There is a delay of at least 48 hours to obtain the complete Ranson’s score, and CSI & MCSI can only be obtained after at least 48-72 hours of admission. This delay of 48-72 hours in obtaining scores leads to lesser sensitivity in the very early stage of the disease.
Both CSI and MCSI are better predictors of severity, clinical outcome, and mortality, with Ranson’s criteria being the best predictor of mortality & CSI being a better predictor of mortality than MCSI in patients with AP. The accuracy of MCSI is better than CSI for the prediction of the requirement of critical care, development of superadded infection, and development of MODS. Both CSI and MCSI scores have low accuracy in predicting the intervention requirement in AP patients.
Mendeley: Underlying data for ‘Comparative analysis of computed tomography severity indices in predicting the severity and clinical outcome in patients with acute pancreatitis’, https://doi.org/10.17632/htkkzr9zbr.2.28
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to acknowledge Dr. Ashvini Kumar, Former Head of Department and former Professor of Radiodiagnosis at Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India, who provided insight and expertise that greatly assisted this research. We acknowledge Dr. Sushrit A Neelopant, Assistant Professor, Department of Community Medicine, Raichur Institute of Medical Sciences, Raichur for statistical analysis of this study. We also acknowledge Dr. Rajat Choudhary, a former postgraduate resident in the Department of General Surgery at Kasturba Medical College, Mangalore, Manipal Academy of Higher Education, Manipal, India for providing surgical insight to this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pancreas, Liver
Competing Interests: No competing interests were disclosed.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biochemistry, baic medicine,acute pancreatitis
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: gastroenterology, endoscopy
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pancreas, Liver
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 18 Jul 24 |
read | read | |
|
Version 1 08 Nov 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)