Keywords
Eutectic, Phase change materials, Non-paraffins, Thermal energy storage, Solar dryer
This article is included in the Energy gateway.
This article is included in the Uttaranchal University gateway.
This article is included in the International Conference on Clean Energy Systems and Technologies collection.
Eutectic, Phase change materials, Non-paraffins, Thermal energy storage, Solar dryer
Update to the data availability statement.
See the authors' detailed response to the review by Deepika P. Joshi
See the authors' detailed response to the review by Abuelnuor Abdeen Ali Abuelnuor
Modern civilization has grown and developed mostly as a result of energy, which has always been important from the perspective of the development of the global economy. However, along with increased efficiency in the energy sector, significant barriers must also be overcome, including the creation of conventional energy sources, reducing the use of fossil-based fuels, as well as reducing greenhouse gas emissions i.e., CO2 (carbon dioxide), SOX (oxides of sulfur), CH4 (methane), NOX (nitrogen oxides).1 Researchers around the world have been looking for new technologies in the last 30–40 years that will reduce the use of fossil fuels and, lessen the detrimental effects that energy production has on the climate and environment. A practical way of using alternative energy is through energy storage, in addition to conserving energy, energy storage also improves the stability and quality of the energy supply and reduces the variation between supply and demand. One of the popular techniques of energy storage is thermal energy storage (TES), which can be classified as2: i) Latent heat storage and ii) sensible heat storage.
Latent heat storage is reliant on heat absorption or release phenomenon, during the phase transition of material from solid to liquid or liquid to gas or vice versa. These materials involved in phase transition are known as phase change materials (PCMs). Whereas in sensible heat storage, energy is stored by increasing the temperature of liquid or solid. In the charging and discharging process, a sensible heat storage system makes use of the material's heat capacity and temperature variation. Stored heat in materials depends on the amount of storage material, the specific heat of the medium, and the temperature change.3
Due to applied thermal energy, some materials change their state by storing some amount of latent heat during the state/phase transition, such materials are known as PCMs. During the transition from solid to liquid or from liquid to solid, thermal energy is transferred. These solid-liquid PCMs function initially like traditional storage materials, as they absorb heat, their temperature increases, but release heat at a practically constant temperature, in contrast to conventional (sensible) storage materials.4 PCMs use chemical bonds for energy storage and release. Although in a sense every material is a PCM, the materials are only categorized as PCM if they have some characteristics of energy storage. High thermal conductivity and significant latent heat should be present in phase transition materials used for energy storage. Additionally, the materials melting points should be within a practical application range; materials should melt consistently with the least amount of supercooling and should be chemically stable. The materials should not be toxic, or chemically corrosive, and should be economical for practical applications.5 PCMs are often divided into three groups i.e., organic, inorganic, and eutectics, which is the combination of two or more materials.
This group of PCMs is divided into salt hydrates and metallics. Due to their low cost, better thermal conductivity, cost-effectiveness, and minor volumetric changes for storage, salt hydrates are very appealing materials for phase change energy storage. Salt hydrates are a typical crystalline solid that are a mixture of water (H2O) and inorganic salts and is written as AB.nH2O. Salt hydrates can change from a solid to a liquid state by dehydrating or hydrating the salt, even though this process thermodynamically mimics melting or freezing. Generally, a salt hydrate melts into water, and salt hydrate6 i.e.,
Most salt hydrates have the problem of incongruent melting. The hydrate crystals disintegrate into anhydrous salt and water, or a lower hydrate and water, at the melting point. The fact that the water released during crystallization is insufficient to completely dissolve all of the solid phases present causes incongruent melting, which is one issue with the majority of salt hydrates. The lower hydrate (or anhydrous salt), due to the density difference, descends to the bottom of the container. Many salt hydrates also have weak nucleating capabilities, which causes the liquid to supercool before crystallization starts. The addition of a nucleating agent, which supplies the nuclei on which crystal formation is started, is one approach to solving this issue. Another option is to keep some crystals in a small, cold area so they can act as nuclei. Some of the salt hydrates with their latent heat of fusion and melting point are listed in Table 1.
Most of the metal eutectics and low melting metals come under the inorganic metallic PCM category, but due to their heavy weight, metallics have not been given substantial consideration for PCMs. They are reasonable candidates when the volume is taken into account due to the high latent heat of fusion output per unit volume. The employment of metallics brings forth a variety of peculiar technical issues. The strong heat conductivity of the metallics distinguishes them significantly from other PCMs.8
Organic PCMs are divided into the paraffin and non-paraffin subgroups. Without any loss in their latent heat of fusion and phase segregation, these materials have the property of congruent melting i.e., repeatedly melting and freezing. It also exhibits the property of non-corrosiveness and self-nucleation.
Paraffin is mostly made up of an alkanes chain (CH3–CH2–CH3…), and the crystallization of these chains generates a significant amount of latent heat. In general, paraffin is stable below 500°C, and there are no significant changes in the volume on their melting; also they have low vapor pressure while melting.9 The melt-freeze cycle of paraffin is often relatively long. With more carbon atoms present, alkane has a higher melting point. The fact that paraffin is accessible in a wide range of temperatures is the primary factor in its qualification as an energy storage material. Along with other beneficial traits like consistent melting and good nucleating qualities, paraffin has several other advantages.10 They have a few unfavorable characteristics, including low thermal conductivity, incompatibility with plastic containers, and considerable flammability. By slightly modifying the wax and the storage unit, all these negative effects can be somewhat removed. Some of the most desirable and moderate desirable paraffin are shown in Table 2.
Of all phase transition materials, non-paraffin organics are the most prevalent and have the widest range of features.11 Unlike paraffin, which has extremely comparable properties, each of these materials will have unique characteristics. This is the broadest group of potential PCMs. After conducting a thorough analysis of organic materials, Buddhi and Sawhney found several esters, fatty acids, alcohols, and glycols that might be useful as energy storage materials.12 Fatty acids and other non-paraffin organic compounds are other subgroups of these organic molecules. Due to their flammability, fatty acids and non-paraffin organic materials cannot be subjected to extreme heat, flames, or oxidizing agents. Compared to paraffin, fatty acids have high heat of fusion and have repeatable behavior in their melting and freezing. Fatty acids also freeze without supercooling. All fatty acids are described by the chemical formula CH3(CH2)2n.COOH. Their main disadvantage is that they are 2–2.5 times more expensive than technical-grade paraffin. They are also barely corrosive. Some of the non-paraffin compounds are listed in Table 3 with their melting point and latent heat of fusion.
A minimal-melting composition of at least two or more materials is known as a eutectic, and during crystallization, each of these materials melts and freezes concurrently to form a mixture of the material crystals.13 Because they freeze to a close-knit combination of crystals, eutectic materials rarely melt or freeze without the components segregating. Both components simultaneously liquefy when heated, making separation unlikely. Since they are minimum melting, some segregated PCM compositions have been wrongly referred to as eutectics. But it would be more accurate to refer to them as peritectic as they undergo a peritectic reaction during phase change. Some of the compositions of the eutectics are shown in Table 4.11
Now, if there is a discussion on the merits and demerits of the three types of PCMs mentioned above (inorganic, organic, and eutectic), there are many discrepancies, some of which are listed in Table 5.
PCMs, Phase change materials.
| PCMs | Merit | Demerit |
|---|---|---|
| Inorganic | ||
| Organic | ||
| Eutectics | - |
Other than the listed eutectic PCMs, many more eutectic mixtures were prepared with the good latent heat of fusion and melt-freeze cycle. In this series, beeswax-Stearic (BWSA), beeswax-Palmitic (BWPA), beeswax-Myristic (BWMA), and beeswax-Lauric (BWLA) are prepared and tested. These four prepared eutectic mixtures are a composition of organic non-paraffin PCMs.
Organic non-paraffin compound beeswax (BW) in different compositions mixed with another organic non-paraffin compound, Palmitic (PA), Myristic (MA), and Lauric (LA) acid at a temperature range of 50–60°C with the help of a magnetic stirrer at 200 rpm for 3–4 hrs followed by sonication for 15 minutes using Sonics Vibracell probe sonicator and get eutectic PCMs. Organic non-paraffin compounds are mixed in different compositions to get desired eutectics. A total of 20 wt % of BW mixed was with 80 wt % of SA to prepare BWSA28. Similarly, 40, 10, and 10 wt % of BW were mixed with 60, 90, and 90 wt % of PA, MA, and LA, respectively, to form BWPA46, BWMA19, and BWLA19 eutectic PCMs. Each sample is prepared in a quantity of 10 gm and acids SA, PA, MA, and LA used here were sourced from MOLYCHEM with product codes 19060, 16705, 16392, and 12520, respectively. Whereas BW was sourced from the center of Excellence on Honey Bees (Nalanda College of Horticulture, Nalanda, Bihar).
The PerkinElmer DSC 4000 equipment was used for the differential scanning calorimetry (DSC) study. In order to measure this little amount (mg) of the samples, an analytical digital weighing machine with a precision of 0.00001 g was used. The weighted sample between 10 to 15 mg was filled into an aluminum pan, and the DSC procedure was carried out in a nitrogen environment at a flux of 20 ml/min at a heating rate of 2°C/min. The accuracy of the DSC device was ±2% for enthalpy measurement and ± 0.1°C for temperature measurement. The reference pan and the sample pan are heated at the same rate during the DSC analysis. The latent heat of fusion, peak melting temperature, and other thermophysical parameters was measured. The top point of the curve offers the peak melting temperature, while the area that comes under the curve explicated latent heat of fusion and crystallization, and the tangent of the highest slope explicated the onset melting point.
Eutectic PCM molecules start to oscillate when heated, this oscillation causes the atoms to move farther apart, occupying more space and resulting in an increase in volume. Further raising the temperature caused molecules to move more quickly due to an increase in kinetic energy, quick movement of molecules disrupting the supramolecular link between the individual atoms. The ordered crystal structure transforms into a randomly oriented liquid state as the temperature rises to a specific critical point, DSC is used to measure this critical point of temperature known as phase transition temperature. Therefore, using DSC analysis, the thermal energy storage properties of BWSA28, BWPA46, BWMA19, and BWLA19 were determined. The DSC curves for BWSA28, BWPA46, BWMA19, and BWLA19 are shown in Figure 1. The DSC curves for BWSA28, BWPA46, BWMA19, and BWLA19 displayed comparable patterns and nearly equal forms. For BWSA28, BWPA46, BWMA19, and BWLA19, the onset melting points were 49.59°C, 48.85°C, 50.91°C, and 41.02°C, respectively, which are listed in Table 6. Similarly, the peak melting temperatures for these materials were 55.17°C, 56.85°C, 55.22°C, and 44.96°C, respectively. Onset and freezing temperatures obtained from BWSA28, BWPA46, BWMA19, and BWLA19 were 50.79°C, 50.22°C, 45.44°C, 36.77°C, and 48.93°C, 49.59°C, 45.34°C, 36.08°C, respectively. In the DSC plot, the area under the curve provides latent heat of fusion and latent heat of crystallization as shown in Figure 1 and marked with the arrow. So, the obtained latent heat of fusion and latent heat of crystallization for BWSA28, BWPA46, BWMA19, and BWLA19 were 174.52 kJ/kg, 166.03 kJ/kg, 192.85 kJ/kg, 195.73 kJ/kg, and 177.94 kJ/kg, 155.55 kJ/kg, 211.52 kJ/kg, and 201.04 kJ/kg, respectively.
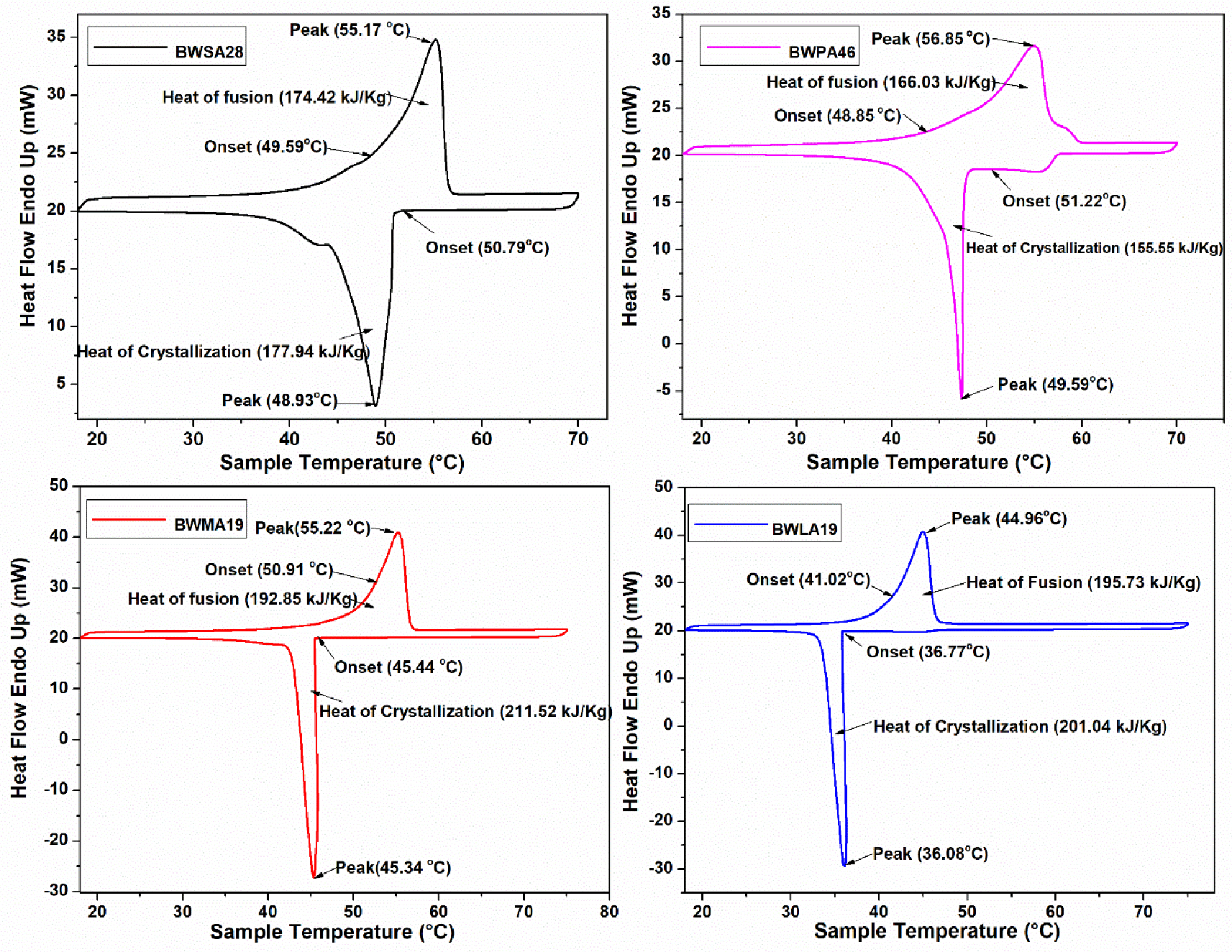
DSC, differential scanning calorimetry; BWSA, Beeswax-Stearic; BWPA, Beeswax-Palmitic; BWMA, Beeswax-Myristic; BWLA, Beeswax-Lauric.
PCMs, Phase change materials; BWSA, Beeswax-Stearic; BWPA, Beeswax-Palmitic; BWMA, Beeswax-Myristic; BWLA, Beeswax-Lauric.
Foods and agricultural products can be stored using drying techniques, which is a better technique for reducing the moisture content of products that will also not reduce their quality. Fruits and vegetables are dried at an operating temperature of 40–60°C.15 Where humidity level and operating temperature can be used to regulate the moisture content and product quality (such as nutritional characteristics).16 The food and agricultural industries have focused their attention on the solar drying technique as it is an inexpensive way to preserve food and agricultural products. Additionally, it has significant ecological advantages.17 The prepared eutectic PCMs had melting and freezing temperatures in the range of 36°C to 56°C, which is very much suitable for the solar drying application.
Due to its capability to enhance system performance, energy storage is particularly alluring to a wide range of parties. Technology development is more efficient and practical when excess energy is stored for later use rather than being replaced by new power plants. The latent heat of the phase change is associated with prepared eutectic PCMs and has a crucial influence on their ability to store greater amounts of energy. A target-oriented settling temperature is also supported by PCMs due to the fixed phase change temperature. This paper deals with the development of eutectics PCM. The foremost advantages of these prepared eutectic PCMs were their low cost and eco-friendly nature. DSC analysis of this prepared eutectics BWSA28, BWPA46, BWMA19, and BWLA19 showed good thermal energy storage capacity, and it lies between 155–211 kJ/Kg. These eutectics have a temperature range between 36–56°C.
Figshare: Dataset, https://doi.org/10.6084/m9.figshare.23496929.v1. 18
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phase Change Materials, Solar Drying
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: composite, core-shell nanomaterials , thermal energy storage
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 06 Aug 25 |
read | read | |
|
Version 2 (revision) 20 Sep 23 |
read | read | |
|
Version 1 09 Nov 22 |
|||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)