Keywords
Chronodisruption, circadian misalignment, coupling, inter-tissue desynchrony, intra-tissue desynchrony, phase incoherence, shiftwork
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Circadian Clocks in Health and Disease collection.
Chronodisruption, circadian misalignment, coupling, inter-tissue desynchrony, intra-tissue desynchrony, phase incoherence, shiftwork
In the revised version of the manuscript, we have addressed all comments. Specifically we attempted to
(1) indicate specific model and tissue used of cited studies as this influences described circadian parameters,
(2) elaborate on possible approaches to clarify knowledge gaps,
(3) discuss other risk factors for development of mentioned diseases other than circadian disruption,
(4) elaborate on effects of diet composition and time of food intake on circadian rhythms and physiology and discuss time-restricted food intake as potential therapeutic approach,
(5) discuss behavioral changes and possible coping strategies in shift worker and outline the mixed literature on associations between shift work and numerous diseases,
(6) elaborate on the relationship of aging and circadian (de)synchrony as well as on effects of social jetlag as a population-wide chronic desynchronised state,
(7) change figure 2 to clarify the term inter-tissue desynchrony and show the different possibilities of misaligned zeitgebers.
We hope all these changes and edits will contribute to the clarity and readability of the manuscript.
See the authors' detailed response to the review by Frank A.J.L. Scheer
See the authors' detailed response to the review by Andrew N. Coogan
Mammals possess a ubiquitously expressed circadian clock system with a master pacemaker located in suprachiasmatic nucleus (SCN), in the hypothalamus, driving physiological and behavioral rhythms. Such rhythms can be observed in, e.g., hormonal release, eating patterns, sleep behavior and body temperature (Moore and Eichler, 1972; Ralph et al., 1990; Sawaki et al., 1984; Stephan and Zucker, 1972). Functional clocks have been found in the SCN but also numerous other tissues including liver, kidney, and adipose tissues (Aschoff, 1965; Aschoff et al., 1967; Balsalobre et al., 1998; Lamia et al., 2008; Yamazaki et al., 2000; Yoo et al., 2004). All circadian clocks share three common properties. First, circadian oscillators are self-sustained, i.e., they are capable of driving ~24-hour circadian rhythms in transcription and translation. Second, they preserve the same kinetics over a broad range of temperatures (Barrett and Takahashi, 1995; Menaker and Wisner, 1983; Pittendrigh, 1954; Reyes et al., 2008). Third, circadian oscillators can be synchronized (or entrained) by environmental cues, so called zeitgebers (German for ‘time giver’) such as light and food (Balsalobre et al., 1998; Yoo et al., 2004). Photic zeitgeber input synchronizes the SCN that in turn aligns the central nervous system (CNS) and peripheral-tissue clocks with each other and with external time via humoral and neural signals. Together, these give rise to rhythmic circadian output (Figure 1). Single circadian oscillators have distinct period (cycle length), and it is thought that they must be synchronized to the 24-hour light/dark (LD) cycle to provide coherent rhythmic control over physiological processes such as maintaining temporal separation of chemically incompatible processes (Honma et al., 1998; Liu et al., 2007; Nagoshi et al., 2004; Welsh et al., 1995). Modern lifestyles are often characterized by deregulated food intake rhythms, lack of exercise, disrupted sleep/wake patterns, and nocturnal light exposure. Such mismatch of zeitgeber signals is believed to lead to disruptions in the phase coherence between internal and external time and between different tissue clocks in a state termed chronodisruption (Erren and Reiter, 2009, 2013; Kiehn et al., 2017).
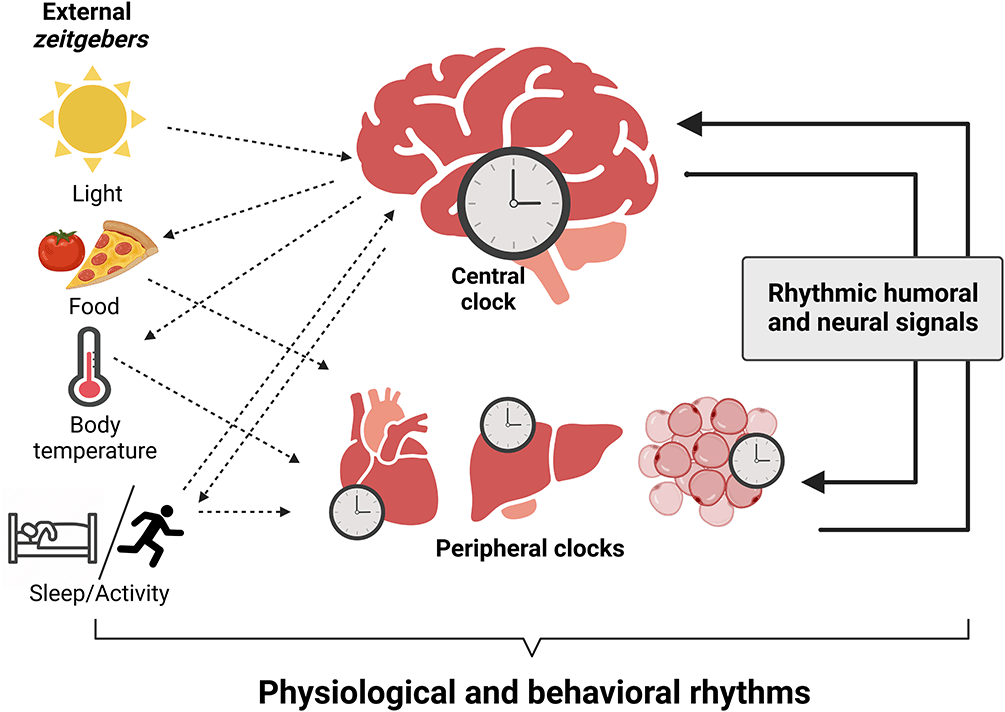
The primary zeitgeber, light, entrains the master pacemaker, the suprachiasmatic nucleus (SCN), with geophysical time. The SCN synchronizes subordinated central nervous system (CNS) and peripheral tissue clocks by humoral and neural signals and the temporal coordination of food intake, body temperature, and rest/activity cycles. Outputs of peripheral tissues feedback to clocks in the brain and stabilize circadian synchrony. Integration of external signals, collective output of tissue clocks, and rhythmic humoral and neural signals generates physiological and behavioral circadian rhythms. Figure created with BioRender.com
The circadian clock system regulates energy homeostasis, and dysregulation of circadian clock-metabolism crosstalk can seriously impact overall metabolic health (Reinke and Asher, 2019). For example, eating at a biologically inappropriate phase results in impaired glucose tolerance and transient insulin resistance in a laboratory study (Scheer et al., 2009). Nighttime eaters gain more weight compared to non-nighttime eaters (Gluck et al., 2008). Increased light at night (LAN) exposure is associated with a higher incidence of obesity and metabolic syndrome (McFadden et al., 2014). Possibly, this may be provoked by light-mediated resetting of the SCN circadian clock and inhibition of melatonin release (Boivin et al., 1996; Zeitzer et al., 2000). Mice exposed to dim light at night have blunted rhythms of essential clock genes at both mRNA and protein levels in the SCN. Changes are also observed in feeding behavior in those mice (Fonken et al., 2013; Shuboni & Yan, 2010). Melatonin is crucial for insulin biosynthesis, secretion, and action, as well as for regulating the rhythm of leptin. Hence, the disruption of its nocturnal production as observed due to exposure to illumination at night may underlie obesity and metabolic dysfunction (Chakir et al., 2015; Cipolla-Neto et al., 2014). In modern societies roughly 21% of employees work outside the regular working hours or in shifts, which has been associated with various adverse health outcomes (Karatsoreos et al., 2011; Parent-Thirion et al., 2016; Streng et al., 2022). Shift work is believed to affect the alignment of an individual’s behavioral cycle with both external and endogenous rhythms resulting in a loss of phase coherence of circadian and diurnal rhythms or between two or more circadian rhythms (Boudreau et al., 2013; Skene et al., 2018). Numerous epidemiological studies have demonstrated a higher incidence of various disorders in shift workers – from impaired mental health to metabolic syndrome, obesity, cardiovascular disease, autoimmune disorders, and cancer (Chellappa et al., 2020; Davis et al., 2001; de Bacquer et al., 2009; di Lorenzo et al., 2003; Ellingsen et al., 2007; Karlsson et al., 2001; Kawachi et al., 1995; Li et al., 2022; Magrini et al., 2006; Schernhammer et al., 2003). It is worth mentioning that shift work can affect sleep quality, leading to shorter sleep duration, sleep disturbances, and daytime dysfunction. Some individuals may cope with sleep-related issues by increasing their alcohol consumption and tobacco use which in turn may further contribute to the development of various diseases (Bae et al., 2017; Jung et al., 2022; Lim et al., 2020; Thach et al., 2020). Of note, some published studies have not found an association between shift work and the likelihood of developing cardiovascular disease, cancer, type 2 diabetes mellitus or depression (Barul et al., 2019; Behrens et al., 2021; Bøggild et al., 1999; Vetter et al., 2018). Circadian misalignment can also be caused by travelling across several time zones leaving us with sleep problems, fatigue, cognitive impairments, and gastrointestinal issues summarized as jetlag disorder. During jetlag, the body’s internal time is misaligned to local geophysical time, and the circadian clock network needs some time to entrain to the new time zone (Diekman and Bose, 2018; Kiessling et al., 2010; McGuckin et al., 2014; Roach and Sargent, 2019; Wright et al., 1983). Another form of chronic circadian misalignment is known as social jetlag, which is characterized by an ongoing discrepancy of an individual's sleep/wake cycle between workdays and free days. Importantly, several health consequences, including mood disorders, cardiac disease, obesity, metabolic syndrome, and type 2 diabetes mellitus, have been strongly linked to social jetlag (Gamboa Madeira et al., 2021; Islam et al., 2020; Koopman et al., 2017; Roenneberg et al., 2012).
The SCN constitutes a network of interconnected neural clocks with distinct periods in vitro (Honma et al., 2004; Welsh et al., 1995). However, a robust circadian output is observed probably arising from strong inter-cellular coupling within the SCN. Non-SCN tissue clocks show fast dampening of circadian oscillations in vitro likely due to weak inter-cellular coupling and the high dependence on systemic signals to keep synchrony (Yoo et al., 2004). Coupling can be observed on different levels such as systemic (inter-tissue) and intra-tissue which can be further subdivided into inter-cellular and molecular coupling. All contribute to the generation or maintenance of coherent rhythms in behavior and physiology.
The SCN synchronizes the clock network via numerous signals. Vice versa, rhythmic signals from the periphery feedback to the SCN to stabilize and fine-tune the clock system. This type of crosstalk occurring between different tissue clocks is known as systemic coupling (Pilorz et al., 2020). The autonomic nervous system and the endocrine system are important routes utilized by SCN to deliver humoral and neural signals to peripheral tissues. Thereby, the SCN regulates changes in the blood supply, kidney filtration rates, hormone secretion, sensitivity for hormones, and insulin sensitivity in a time-dependent manner (Buijs et al., 1999, 1993; Cailotto et al., 2005; Cui et al., 2001; Dai et al., 1997; Deering and Coote, 2000; Kalsbeek et al., 1993; Kalsbeek and Strubbe, 1998; Vujovic et al., 2015). The best studied circadian hormones are glucocorticoids and melatonin, and both act as systemic synchronizing signals (Cheifetz, 1971; de Kloet and Sarabdjitsingh, 2008; Klemcke et al., 1989; Lincoln et al., 1982; Oster et al., 2006; Perlow et al., 1981; Redman et al., 1983). On the other hand, systemic signals derived from peripheral tissues reach the SCN for a proper synchronization of physiological rhythms (Balsalobre et al., 2000; Buijs et al., 1999; Gerber et al., 2013; Guo et al., 2005; Oster et al., 2006; Redman et al., 1983). For this reason, various hormone receptors are present in the SCN modulating and fine-tuning internal circadian timing. Intriguingly, the hepatokine fibroblast growth factor 21 (FGF21), a starvation signal secreted from the liver into the blood stream, can affect the SCN, thereby modulating several physiological functions (Bookout et al., 2013). Another example, leptin, an adipocyte derived peptide, conveys the metabolic state of peripheral tissues to the SCN. Interestingly, leptin receptor deficient rats show disrupted circadian rhythms of food intake (Li et al., 2012). The stomach-derived peptide hormone ghrelin can modulate food anticipatory activity through its action on the mediobasal hypothalamus (MBH) (Merkestein et al., 2014; Wang et al., 2018). Thus, systemic coupling mechanisms are crucial to efficiently synchronize circadian rhythms across the entire body.
On tissue level, one can discriminate between inter-cellular and molecular coupling. For inter-cellular coupling, single cells within a tissue communicate with each other using various signaling mechanisms, thereby, creating internal synchrony across the organ. Coupling between different cells of the same tissue is best described for the SCN. Here, gap junctions, paracrine signals, chemical synapses, and electrical signaling are used to keep SCN cell rhythms synchronized and aligned with each other (Maywood et al., 2011; Rash et al., 2007; Yamaguchi et al., 2003). Tetrodotoxin-mediated inhibition of neurotransmission results in reduced amplitudes of circadian clock gene expression (Yamaguchi et al., 2003). Strong inter-cellular coupling in the SCN results in robust rhythms which can also compensate genetic clock disruption for which peripheral tissues are much more sensitive (Liu et al., 2007). In line with this, SCN explants show circadian oscillations for weeks whereas circadian oscillations diminish in peripheral tissue explants after some days suggesting much weaker inter-cellular coupling (Yoo et al., 2004). Hepatocyte clocks in SCN ablated mice show fast and stable entrainment to daytime feeding highlighting how the feeding rhythm synchronizes peripheral tissue clocks, including those in the liver (Saini et al., 2013). Despite this, it remains elusive whether the synchronized circadian oscillations in liver arise through the integration of systemic signals or by coupling on the tissue level. Hepatocyte cell culture experiments suggest that there is weak coupling between the cells since desynchronization over time is slower than in a simulation of a model where no coupling is assumed. Coupling in the periphery is weak and locally more restricted compared to what is shown for the SCN (Guenthner et al., 2014), and the underlying mechanisms are largely unknown.
Molecular coupling within a rhythmic cell is established through interlocked transcriptional- translational feedback loops (TTFLs) regulating rhythmic transcription and post-translational modifications controlling the stability of clock proteins (Buhr and Takahashi, 2013). Within each rhythmic cell, the components of the molecular clock have a normal phase distribution. In the SCN of mice, the new transcriptional cycle of the molecular clock starts once brain and muscle ARNT like protein 1 (BMAL1): circadian locomotor output cycles kaput (CLOCK) heterodimers bind to E-box promotor elements in period1–3 (Per1–3) and cryptochrome1/2 (Cry1/2) genes inducing their transcription in the morning (Kume et al., 1999; Shearman et al., 1997). Later, towards the night, PER and CRY heterodimers are translocated to the nucleus where they repress their own transcription by inhibiting binding of BMAL1:CLOCK to E-boxes (King et al., 1997; Konopka and Benzer, 1971; Reddy et al., 1984; van der Horst et al., 1999; Zheng et al., 1999). Beside the E-box elements, other response elements such as D-boxes and retinoic acid receptor-related orphan receptors elements (ROREs) are modulating and stabilizing circadian oscillations (Akashi and Takumi, 2005; Mitsui et al., 2001; Nakajima et al., 2004; Ohno et al., 2007; Preitner et al., 2002; Sato et al., 2004; Ueda et al., 2002; Yamaguchi et al., 2000). D-boxes are bound among others by the transcriptional repressor nuclear factor interleukin-3-regulated protein (NFIL3, also known as E4BP4) and the transcriptional activator D-box binding protein (DBP) modulating the expression of, e.g., Per1–3, repressive reverse-erythroblastosis virus α/β (Rev-Erbα/β), and activating retinoic acid receptor-related orphan receptors (Rorα/β/γ) (Mitsui et al., 2001; Ohno et al., 2007; Ripperger and Schibler, 2006; Yamaguchi et al., 2000). Repressing REV-ERB α/β and activating RORα/β/γ proteins compete for binding to ROREs modulating the expression of Bmal1, Clock and Cry1 (Guillaumond et al., 2005; Nakajima et al., 2004; Preitner et al., 2002; Sato et al., 2004; Ueda et al., 2002). The interlocked feedback-loops result in ~24- hour oscillation of gene expression and repression. Importantly, such oscillations are further stabilized and fine-tuned by chromatin remodeling, post-transcriptional, and post-translational modifications impacting the stability of distinct clock mRNAs/proteins (Doi et al., 2006; Duong and Weitz, 2014; Gallego and Virshup, 2007; Grimaldi et al., 2007; Nakahata et al., 2008; Grimaldi et al., 2009; Kojima et al., 2011; Preußner et al., 2014). Together, these lead to a stable phase relationship/coherence between the different genes. In general, expression of Bmal1 is almost anti-phasic to the expression of Per2, Nr1d2 and Cry2 (10–12 h), whereas expression of Nr1d1 is only phase-delayed by 6–10 h. Interestingly, the peak expression of Bmal1 and Cry1 differs only between 2–4 h (Mure et al., 2018). Of note, the phase relationship of the clock genes to each other is tissue- and species-specific (Harbour et al., 2014; Korenčič et al., 2014; Mure et al., 2018; Pett et al., 2018; Yeung et al., 2018). Moreover, there is a stable phase relationship of single-tissue clocks to each other. In general, non-SCN tissues are phase-delayed compared to the SCN. Adrenal gland clocks show a small phase delay compared to those in the liver, whereas liver, adipose tissue, and muscle are similarly phased (Korenčič et al., 2014; Mure et al., 2018; Yang et al., 2006). Using the human genotype-tissue expression (GTEx) data, most recent studies suggest differences in the phase relationship over the circadian and seasonal cycle as well as a sex-and age-dependency between tissues (Talamanca et al., 2023; Wucher et al., 2023). The GTEx data set is currently the best multiple tissue-spanning data set available. However, samples in these studies were collected from hospitalised patients after the death which strongly suggests that the patients lacked the circadian synchronous (healthy) state due to the diseased conditions and imposed clinical environment.
Since all tissue clocks contribute to generate coherent circadian rhythms, the phase coherence within the cell, between the cells and among the tissues is potentially important to maintain homeostasis. Genetic mutations or knockouts (KO) of circadian clock genes lead to the development of various diseases ranging from sleep disorders to cardiovascular, mental, and metabolic deteriorations (Dibner and Schibler, 2015; Kiehn et al., 2017; Valenzuela et al., 2016). In recent years, evidence has been accumulated on the physiological relevance of distinct tissue clocks. Liver-specific Bmal1 KO results in abolished rhythms in glucose regulatory genes creating problems in the maintenance of blood glucose levels especially during fasting periods (Lamia et al., 2008). Elevated levels of blood lipids were observed in mice with hepatocyte-specific ablation of REV-ERBα/β (Bugge et al., 2012; Cho et al., 2012). Skeletal muscle clock regulates glucose metabolism. Mice lacking a functional muscle clock show defective insulin-stimulated glucose uptake attributed to impaired glucose transporter 4 (GLUT4) translocation to plasma membrane (Dyar et al., 2014; Harfmann et al., 2016). In adipose tissue, circadian clocks regulate several metabolic processes in a time-of-day dependent manner. Adipocyte-specific Bmal1 KO results in obesity and increased rest-phase food intake probably related to altered fatty acid signaling to the hypothalamus (Paschos et al., 2012). In heart, genetic ablation of Bmal1 results in perturbed systolic function and abnormal glucose utilization (Young et al., 2014). Lastly, adrenal cortex-specific Bmal1 KO mice display dampened glucocorticoid and locomotor activity rhythms (Dumbell et al., 2016; Son et al., 2008). Of note, local tissue clocks are not sufficient to drive the local transcriptome independently (Koronowski et al., 2019). Altogether, these studies emphasize the importance of specific tissue clocks, but they also show that coherence among different tissue rhythms is important to maintain whole body homeostasis.
Circadian clocks throughout our body integrate external and internal rhythmic signals to drive coordinated rhythmic physiological and behavioral outputs. When these inputs are appropriately aligned, the body is in a resonant or synchronous state which enhances resilience to pathogenic insults and thus, stabilize health and wellbeing. However, when zeitgeber input is perturbed, e.g., the occurrence of temporal stimuli is temporally shifted or the duration is altered, chronodisruption may emerge. With regard to different zeitgebers, the LD cycle is considered as the most potent zeitgeber for entraining circadian and seasonal rhythms in most species. In real life, a misalignment of geophysical time and circadian rhythms is observed after rapidly crossing different time zones. This provokes jetlag disorder with disrupted sleep/wake cycles, fatigue and cognitive impairments until the internal clock system is entrained to the new geophysical time. While for the general population jetlag occurs only once in a while, which has little persisting health effects, long-distance flight personnel experiences repeated jetlag which may cause long-term impairments. Most people in modern societies, on the other hand, are exposed to artificial light during natural dark periods. Increasingly more people are experiencing LAN during work in shifts (reviewed in detailed by Touitou et al., 2017). Shift workers commonly have inefficient and poor quality sleep causing fatigue (Paech et al., 2010). Moreover, forced activity and sleep deprivation in the normal rest phase strongly impacts the immune system (Irwin et al., 2006), and shift workers are more likely to develop chronic medical conditions like cardiovascular diseases (Ha and Park, 2005), metabolic syndrome (Cheng et al., 2021), psychological disorders (reviewed by Cheng & Drake, 2018) and others. Simulations of shift work and abnormal LD cycles in laboratory settings are used to investigate the mechanisms of chronodisruption and will be described in detail in the next sections. Usually feeding and social behavior are also almost exclusively scheduled during active phases of the circadian cycle. They serve as potent entraining stimuli to some CNS and peripheral tissue clocks. Availability of food round-the clock and energy requirement during shift work leads to mistimed food intake in humans that manifests into various chronic metabolic diseases including obesity and type-2 diabetes. Tissues clocks that are strongly influenced by feeding signals, e.g. liver or kidney, can be uncoupled from the SCN inducing disturbance in inter-tissue synchrony (Damiola et al., 2000; Hara et al., 2001; Stokkan et al., 2001).
All of the above described real-life situations result in chronodisruption which promotes metabolic impairments but also increases the prevalence for major depression, cardiovascular disease, autoimmune disorders, and certain cancers (Davis et al., 2001; de Bacquer et al., 2009; di Lorenzo et al., 2003; Ellingsen et al., 2007; Erren and Reiter, 2009, 2013; Karlsson et al., 2001; Kawachi et al., 1995; Li et al., 2022; Magrini et al., 2006; Schernhammer et al., 2003). It is important to note that other factors like gender, daily lifestyle, socio-economic status, levels of stress, environmental conditions or daily exposure to certain pollutants and many others probably contribute to the development of many of the above mentioned diseases (Almetwally et al., 2020; Kautzky-Willer et al., 2016; Loef & Walach, 2012; O’Connor et al., 2021; Radkiewicz et al., 2017; Regitz-Zagrosek et al., 2006; Wang & Geng, 2019). Furthermore, a bi-directional relationship between ageing and a circadian-resonant state is known. Ageing leads to downhill changes in physiology and body homeostasis gradually weakening the circadian system. And in turn a weakened circadian system promotes the age-related morbidities (reviewed in Duffy et al., 2015; Verma et al., 2023; Xu & Li, 2022). Studies are therefore, in demand to test and state the cause-and-effect relationship between the disease and the risk factors. Laboratory studies are usually well controlled, and results can refine our understanding of the cause-and-effect relationship. Fortunately, in the past decade an increasing number of studies investigate the effects in women. However, the participants are young (<50 years old) and healthy in most laboratory studies. More studies including all kind of humankind are needed to disentangle the relevance and cause-and-consequence relationship of circadian disruption are needed.
Although the concept of chronodisruption as a risk factor is established, its pathogenic mechanism remains largely elusive. Chronodisruption can occur at different levels, i.e., (1) a mismatch of external time and internal circadian rhythms (external-internal desynchrony), (2) phase incoherence between different tissue clocks (inter-tissue desynchrony), (3) phase incoherence within a tissue clock (intra-tissue desynchrony) which can further be divided into (3a) phase incoherence among different cells of the same tissue (inter-cell desynchrony) and, (3b) phase incoherence at the molecular level (molecular desynchrony) (Figure 2). In the following sections we will describe these different levels of desynchrony and outline what is known about their physiological consequences. Experimental studies referred to in this review are conducted in adult subjects or animals, unless otherwise specifically stated.
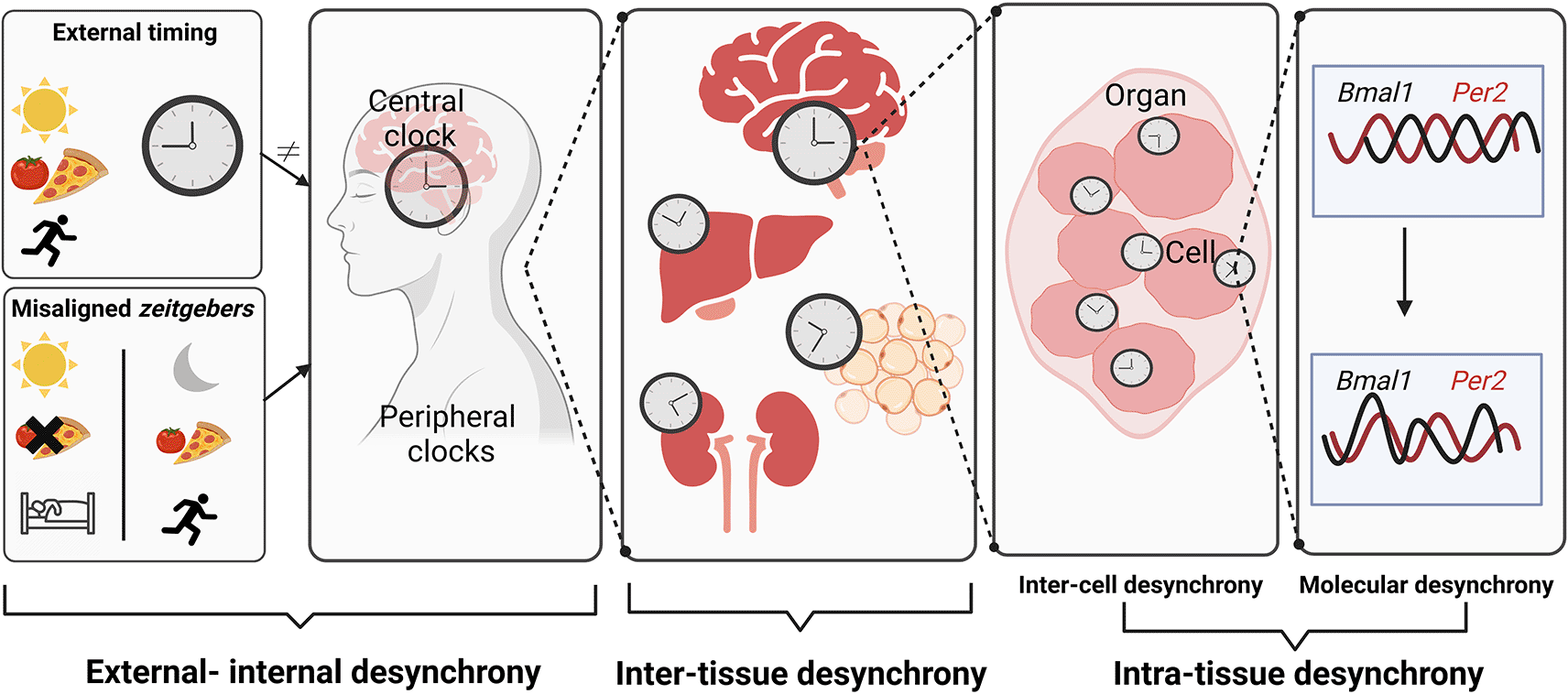
Misaligned zeitgebers like artificial lighting conditions, mistimed food intake, or activity in the rest phase create dissonance between the internal circadian timing system and geophysical time – a state somewhat vaguely termed as chronodisruption. Desynchrony of circadian rhythms can occur at various levels – from misalignment of internal and external time (as during jetlag) to alterations in the coordination of different clock gene expression rhythms at the cellular level. It is still poorly understood how the different levels of circadian desynchrony contribute to the adverse health effects of chronodisruption. Figure created with BioRender.com
Misalignment between zeitgebers and internal circadian rhythms evokes chronodisruption (Figure 2). This state is often caused or enhanced by perturbed zeitgebers such as phase-shifted LD cycles, activity in the inactive phase (shift work), or mistimed food intake, all of which have been suggested to promote metabolic and psychological ailments.
Mismatch between geophysical time and internal biological timing, as observed in jetlag conditions, causes physiological behaviors like sleep, hunger and defecation to occur according to internal timing at non-regular times of the day. However, people in aviation industries who are exposed to repeated jetlag report chronic sleep-inefficiency associated physiological (reviewed in Arendt and Marks, 1982) and cognitive health problems (Cho et al., 2000). Simulated chronic jetlag conditions induce obesity in mice (Oike et al., 2015). However, body weight gain is observed only in advanced but not in delayed LD cycles (Casiraghi et al., 2016). Chronic jetlag retains oscillations but induces phase shifts in clock gene expression rhythms in SCN and liver of mice (Iwamoto et al., 2014). In laboratory settings, rodents are exposed to non-24-hour LD cycles (T-cycles) to investigate the mechanisms of entrainment. Chronic exposure to short T-cycles (4 h light: 4 h darkness (4:4 LD)) — but not long T-cycles (18:18 LD) — decreases life span. Interestingly, clock deficient mice show no differences in mortality rates under long and short T-cycles. The authors suggest that the combination of specific LD cycles and perturbations of the circadian clock network results in impaired homeostatic regulation impacting longevity (Park et al., 2012). In mice, T-cycles, both 11.25:11.25 LD and 13.5:13.5 LD, reduce energy efficiency evidenced by higher food intake despite no body weight gain. Animals increase their food consumption during the light phase. Thereby, mice predominantly utilize carbohydrates to fuel the body which hints to an impaired metabolic homeostasis (West et al., 2017). Under 11:11 LD cycles, rats show dyslipidemia and lowered expression levels of proteins critical for insulin signaling as well as increased levels of enzymes involved in gluconeogenesis in liver indicating metabolic disruptions induced by forced desynchrony (de Oliveira et al., 2019). Non-24-hour LD cycles result in abolished rhythmicity in heart rate and blood pressure (Molcan and Zeman, 2017; West et al., 2017). In humans, circadian misalignment evokes an overall increase in blood pressure (Morris et al., 2016; Scheer et al., 2009). Of note, the loss of rhythmic LD cycles as experienced during LAN or constant light (LL) conditions also impacts circadian outputs such as food intake patterns, energy expenditure and corticosterone rhythms promoting elevated body weight, metabolic impairments and depressive-like behavior (Coomans et al., 2013; Fonken et al., 2012, 2010; Tapia-Osorio et al., 2013).
Shift workers are forced to be active during their normal rest phase. Such work regimes are often accompanied by food consumption at inappropriate times. Thus, shift workers are simultaneously exposed to various chronodisrupters such as LAN, mistimed food intake and mistimed behavioral rhythms. Such employees show dampened cortisol and testosterone rhythms (Touitou et al., 1990). Furthermore, shift workers exhibit higher triglyceride and lower HDL (high density lipoprotein) cholesterol levels and higher abdominal obesity than regular day-time workers (Karlsson et al., 2003). Night shift work can be simulated in rodents by forced activity in the normal resting phase. This shifts activity and food intake into the resting phase. Consequently, daily blood glucose rhythms are abolished, triglyceride rhythms are reversed, and corticosterone levels are transiently increased (Salgado-Delgado et al., 2008). In laboratory studies, shift work can be simulated by imposed 28-hour days in humans. In such conditions, sleep and food intake patterns are 12 h out of phase from the habitual times within a few days. On these (misaligned) days, participants exhibit completely reversed cortisol rhythms and increased arterial pressure. In addition, they show impaired metabolic homeostasis with increased blood glucose despite increased insulin levels compared to the circadian aligned baseline day (Scheer et al., 2009). Interestingly, daytime-restricted food consumption prevents the dissociation of body temperature rhythms, a measure of the central clock, and peripheral glucose rhythms. In turn, this prevents detrimental outcomes in glucose tolerance especially in the biological morning in the 28-hour shift work protocol (Chellappa et al., 2021). The studies show that already short-term chronodisruption generates adverse metabolic and cardiovascular effects. In addition to metabolic and psychological repercussions, far more detrimental effects of chronodisruption induced by shift work are carcinogenesis and malignancy. Numerous studies have shown cancer as a consequence of such a work regime (Davis et al., 2001; Mazzoccoli et al., 2011; Schernhammer et al., 2003; Viswanathan et al., 2007). However, a recent meta-analysis shows overall no significant association between the two (Dun et al., 2020). The meta-analysis considers factors like geophysical region, time and gender differences to compare data between different cohorts; however, factors like type of shift work, food intake habits and general health condition of the individual may also play a role in the cancerogenic effects of shift work. Not only shift workers, but even larger parts of the working force face discrepancy in their behavioral cycles between workdays and free days imposing social jetlag. This regular shift in sleep/wake phasing likely represents a condition of chronic circadian misalignment. Magnitude of social jetlag varies over population in terms of chronotype and geographical location preferences (Roenneberg et al., 2007). The risk of being social jetlagged further increases in places where standard (or local) time largely deviates from solar time (rewieved in Roenneberg et al., 2019). Owing to the present technologically advanced society, social jetlag is highly correlated with people using light emitting smartphones before bedtime (Hena & Garmy, 2020; Šmotek et al., 2020). People who experience social jetlag experience high sleep debt leading to chronic sleep deprivation and poor sleep quality (Juda et al., 2013; Sűdy et al., 2019). This is especially stronger in humans with a late chronotype (Wittmann et al., 2006). This holds true also for young school children. Due to poor sleep quality, social jetlagged people tend to be fatigued more often and have poor alertness with overall poor performance in school or workplace than people with healthy sleep quality (Haraszti et al., 2014; Moon et al., 2017; Yong et al., 2016). Chronic sleep deprivation in social jetlagged people can further impinge into mental health issues (reviewed in (Henderson et al., 2019)). People with strong social jetlag show higher cortisol levels and increased resting heart rates (Rutters et al., 2014). Social jetlag is also associated with increased body mass index and metabolic syndrome (Roenneberg et al., 2012; Parsons et al., 2015). Studies in mid-aged people showed that social jetlag is associated with altered metabolic risk factors such as, high levels of total cholesterol, triglycerides and fasting glucose and low levels of HDL cholesterol (Mota et al., 2017; Wong et al., 2015). Unhealthy obese subjects show elevated levels of the inflammatory marker C-reactive protein and the obesity-related biomarker glycated hemoglobin with increased social jetlag (Parsons et al., 2015). Overall these studies show that circadian misalignment in face of social jetlag is pervasive in every-day life at a population level and has significant mental and metabolic health effects. Food intake, being an important zeitgeber especially for peripheral tissue clocks, disturbs internal resonance of rhythms when occurring at the wrong time-of-day. On the long-term, this may result in obesity and other metabolic disorders. Daytime restricted feeding in nocturnal mice uncouples the liver clock from the SCN (Damiola et al., 2000; Hara et al., 2001; Stokkan et al., 2001). Food intake only during the light phase, in mice, also phase-shifts the acrophase of genes involved in lipid homeostasis and bile acid metabolism which may cause adverse metabolic outcomes (Cui et al., 2022). High-caloric food is shown to disrupt daily food intake patterns and rhythms in clock as well as metabolism related genes. Such disruption can be prevented when the high-caloric diet is only available during the night in nocturnal mice. This intervention also reduces metabolic diseases like hepatic steatosis and hypercholesterolemia observed in ad libitum fed mice (Chaix et al., 2014).
In modern societies, several zeitgebers are misaligned to each other and to the endogenous circadian clock. When mice are subjected to a combined 14:14 LD with a 12:12 fasting/feeding (FF) protocol they become dynamically exposed to aligned and misaligned zeitgeber conditions. Surprisingly, mice transiently show weight gain and impaired glucose tolerance on the day of aligned zeitgeber input (i.e., when feeding coincides with the dark phase) compared to the day of misaligned zeitgeber input (feeding during the light phase). Such zeitgeber misalignment further evokes a change in the phasing of different tissue clocks (Heyde & Oster, 2019). In conclusion, misalignment between internal and external rhythms is associated with alterations in metabolic and psychological states and may promote obesity, diabetes, cardiovascular diseases, depression, anxiety, and cancer.
Desynchrony caused due to external-internal misalignment can impinge further than just the systemic level by inducing phase incoherence between different tissue clocks and circadian tissue outputs. Tissues differ in their susceptibility to external and hormonal signals from each other. Thus, they adapt to changed input rhythms at a different pace inducing misalignment between tissue clocks and rhythms – a state termed as inter-tissue desynchrony.
Circadian homeostasis is maintained by communication and synchronization between and among central and peripheral clocks. Ablation of the SCN eliminates diurnal variation in clock gene expression in peripheral tissues including liver, kidney, heart, skeletal muscle, and spleen. Parabiosis of SCN-lesioned and intact animals re-induces day-night variation in SCN-lesioned mice in liver and kidney but not in heart, skeletal muscle and spleen. This emphasizes the differential dependence on blood-borne signals of distinct tissue clocks (Guo et al., 2005). However, recent studies, in mice, observed that in LD the SCN clock is dispensable for clock network synchronization but becomes necessary under constant darkness (DD) conditions. Animals with an ablated SCN clock show internal desynchrony, i.e., phase incoherence between different tissue clocks, within a few days under DD conditions (Husse et al., 2011; Izumo et al., 2014; Kolbe et al., 2019; van der Vinne et al., 2018). However, effects of internal desynchrony on body weight seem to be dependent on the Cre driver line used (Kolbe et al., 2019; van der Vinne et al., 2018). Of note, internal desynchrony and weight gain are prevented by maintaining the mice on time-restricted feeding schedules under DD conditions highlighting the importance of food intake timing for peripheral clock synchronization (Kolbe et al., 2019).
Many studies use constant light (LL) or repeated jetlag conditions to investigate the effects of abnormal light cycles on different tissue clocks. LL conditions not only weaken the central clock but also disrupt phase alignment between different tissues. Mice subjected to LL show immediate dampening in the amplitude of SCN rhythms (Coomans et al., 2013). LL housing maintains rhythmic PER2 oscillations in liver and kidney but distinctly reduces amplitude and broadly distributes phases of its rhythm. In submandibular gland, this rhythm is lost in a significantly higher number of animals (Hamaguchi et al., 2015). On transcriptional level, the liver and colon of adult male rats, display abolished diurnal expression of clock genes (Polidarová et al., 2011). In addition, to clock outputs, LL conditions eliminate rhythmicity of numerous genes involved in lipid metabolism in liver and white adipose tissue (WAT) in mice. Decreases in amplitude and average levels of gene expression are more pronounced in the liver compared to WAT, indicating higher sensitivity of metabolic and clock gene transcription in the liver to constant light conditions (Yamamuro et al., 2020). In jetlag experiments, distinct tissue clock genes of mice display differences in entrainment speed to the shifted LD schedule. Here, the misalignment is temporary and is resolved when tissue clocks re-align with the new LD cycle. SCN and adrenal rapidly adapt to the changed lighting schedule whereas liver and kidney re-entrain at a slower pace, with the pancreas clock being the slowest (Kiessling et al., 2010). PER1 oscillations in the arcuate nucleus quickly entrain upon a phase-delaying jetlag whereas the paraventricular nucleus (PVN) and pineal gland are faster to re-entrain upon a phase-advancing jetlag in in vitro studies (Abe et al., 2002). These studies indicate that it is likely that jetlag induces internal desynchronization not only between central and peripheral clocks but also between clocks in different parts of the central nervous systems. The sleep/wake cycle is one of the behavioral outputs driven by the SCN. Two weeks of timed-sleep restriction, mimicking human night shift work, have only moderate effects on the levels of clock gene expression in the SCN but markedly change expression levels and phasing in the liver. Moreover, circadian liver transcriptome data show pronounced changes especially in glucose metabolism related genes which results in impaired performance in gluconeogenesis and increased glycogen storage in the beginning of the dark phase. Time-restricted feeding to the normal active phase can prevent the effects of time-restricted sleeping on hepatic clock gene expression and metabolic outcomes (Barclay et al., 2012).
Peripheral clocks show tissue-specific pace in feeding-induced phase resetting. Upon daytime restricted feeding, phases of the clocks in liver, kidney, heart, lungs and pancreas in mice are transiently misaligned to each other. However, all these tissues entrain to the new feeding schedule within a week (Damiola et al., 2000; Hara et al., 2001; Opperhuizen et al., 2016; Stokkan et al., 2001). The dorsomedial hypothalamus of rats also entrains to daytime restricted feeding (Verwey and Amir, 2011). The SCN clock, however, stays aligned to the LD cycle (Damiola et al., 2000; Hara et al., 2001; Opperhuizen et al., 2016; Stokkan et al., 2001). In contrast, clock gene rhythms are abolished in lateral hypothalamus, skeletal muscle and arcuate nucleus in rats fed during the light period (Opperhuizen et al., 2016; Wang et al., 2017). The studies show that feeding time has differential effects on SCN and non-SCN CNS as well as peripheral clocks, thus, weakening inter-tissue phase coherence — which likely interferes with the maintenance of metabolic homeostasis.
In addition to the mistiming of food intake, changes in diet composition can induce internal desynchrony. High-fat diet (HFD) feeding is known to disrupt behavioral and physiological rhythms, but the mechanisms are still not fully understood. It is likely that internal desynchronization of circadian rhythms plays an important role. Long-term HFD feeding in mice alters clock gene expression rhythms in liver and adipose tissue (Kohsaka et al., 2007; Yamamuro et al., 2020). Minimal effects on clock gene rhythms were observed in MBH, a center which controls hunger and satiety, and the medial prefrontal cortex, a region involved in cognitive functions (Kohsaka et al., 2007; Tognini et al., 2020). Of note, HFD reduces the total number of oscillating genes, significantly advances the acrophase and dampens the amplitude of rhythmic genes, but not clock genes, in the SCN demonstrating that the SCN is susceptible to food signals (Tognini et al., 2020). The liver clock is not only susceptible to feeding time but also to food composition. In vitro studies confirm a high susceptibility of the liver clock to food-related signals whereas other tissue clocks such as lung, pituitary and arcuate complex are less receptive (Pendergast et al., 2013). Food related signals which modulate clock resetting include insulin (Chaves et al., 2014; Crosby et al., 2019; Sato et al., 2014; Sun et al., 2015; Tahara et al., 2011), oxyntomodulin (Jorgensen et al., 2007; Landgraf et al., 2015), and many others (reviewed in Reinke and Asher, 2019). Additionally, glucose, amino acids and other metabolites can also phase-shift or entrain circadian clocks (reviewed in Froy, 2007). As such, it can be speculated that re-entrainment of a distinct tissue clock is dependent on its susceptibility to clock modulators. Altogether, the timing of food intake and food composition can rapidly lead to inter-tissue phase incoherences which might be transient or permanent.
Under diverging zeitgeber input (14:14 LD cycle combined with a 12:12 FF cycle) in mice, liver and epididymal WAT clocks align with the FF schedule whereas adrenal and SCN tissue clocks are highly impacted by the extended LD cycle. Thus, animals suffer from internal desynchrony between distinct tissue clocks and circadian output. Interestingly, mice do not gain weight but show circulating body weight changes dependent on the transient state of zeitgeber (mis)alignment (Heyde & Oster, 2019). Such internal desynchrony is already observed after four days of 14:14 LD/12:12 FF conditions and is less pronounced when feeding is restricted to the dark phase under 14:14 LD conditions (Heyde & Oster, 2022).
Taken together, changes in the external environment or (forced) changes in the behavioral cycle result in phase incoherences between different oscillators which may cause disruption of rhythmic output eventually resulting in metabolic impairments. However, more studies investigating the effect of phase incoherences between certain tissues for e.g. studies comparing clock gene expression and protein levels in SCN, extra-SCN CNS regions and peripheral tissues under perturbed zeitgeber inputs and enforced activity are needed to fully elucidate the physiological consequences.
Tissues contain numerous cell types with each cell containing an autonomous molecular clock. Synchronized cells give rise to overt circadian rhythms on the tissue level. Cells integrate several signals to produce coherent rhythms. Phase incoherencies among clocks of different cells within a tissue cause inter-cell desynchrony. Moreover, at the single-cell level the phasing of distinct clock gene rhythms to each other can be disrupted giving rise to molecular desynchrony (Figure 2). Current standard techniques face limitations in distinguishing between cellular and molecular levels of chronodisruption. Thus, in this section we describe both levels together as intra-tissue desynchrony.
With regards to desynchrony caused between different cells the best-studied tissue is the SCN. The SCN consists of two major regions with light responsive neurons in the ventrolateral and light unresponsive ones in the dorsomedial part (Van den Pol, 1980). Abrupt delaying or advancing of LD rhythms rapidly causes incoherence of synchronous rhythms of clock genes between the two SCN regions. In rats, clock genes in the ventrolateral SCN are quick to show large shifts compared to the dorsomedial region which shifts slower and takes a longer time to resynchronize to the new lighting schedule (Nagano et al., 2003). Shifts in lighting conditions also disrupt phase relation, i.e., peak phase differences between dorsal and ventral parts of the SCN. After a phase-delaying shift in the LD cycle, peak phase differences between the regions increase but return to pre-phase shift conditions within three days. In contrast, a phase-advancing shift in the LD cycle leads to reversed phase relations between the regions and the pre-shift state is reached only after six days (Nakamura et al., 2005). A recent study on mice with imposed delays in the LD cycle investigated Per2 expression at a single-cell level throughout the SCN. The ventral part contains about 40% fast phase-shifting and 60% slow phase-shifting neurons while the dorsal SCN almost exclusively comprises slow-shifting neurons (van Beurden et al., 2022).
As mentioned above, phase incoherence can also occur at the level of the molecular clock work itself. In the SCN of mice, Per1 and Per2 rhythms more rapidly react to LD shift advances compared to Cry1 whereas all three genes react rapidly to LD delays (Reddy et al., 2002). Similar transient dissociation is observed between Per1 and Bmal1 rhythms in mice exposed to a single light pulse during DD conditions. Per1 is instantaneously phase-delayed together with activity onset while Bmal1 shifts more gradually in parallel with activity offset (Ono et al., 2017).
Desynchrony within clock gene expression is also observed in peripheral tissue clocks. HFD attenuates amplitude of clock gene profiles in MBH, adipose and liver tissue. Mice studies show that in the MBH, although Per2 and Bmal1 show robust rhythms, Clock RNA expression is completely abolished. In contrast, Clock is diurnally rhythmic in both fat and liver but with altered amplitude. Additionally, Bmal1 is attenuated throughout the 24-hour day, but Per2 shows decreased amplitudes only in the dark phase (Kohsaka et al., 2007). Notably, not only incoherence in phasing of clock genes but also differences in amplitude of their rhythm may contribute to desynchrony. However, it has to be investigated whether changes in amplitude result from impaired clock gene expression on a single-cell level or broader averaging over phase-distributed cells. Amplitude dampening of clock gene rhythms in different tissues may also be an effect of attenuated feeding rhythms (Kohsaka et al., 2007). Notably, mice, fed a HFD restricted to the active phase, show no dampening in daily feeding rhythms, an improved glucose tolerance, decreased adiposity as well as insulin and leptin resistance and lower inflammation than mice fed HFD ad libitum. These mice also show no attenuation in diurnal expression of circadian oscillators (Hatori et al., 2012; Sherman et al., 2012). Unhealthy diet composition, i.e., high-fat or high-carbohydrate, adversely affects metabolism and physiology which can be prevented by nighttime restricted feeding (Chaix et al., 2014). Recent studies show that time of food intake and diet composition impact clock and metabolism-related gene expression in a tissue-specific manner (de Goede et al., 2018; Reznick et al., 2013).
Differential pace of entrainment is observed for distinct clock genes upon jetlag conditions. In the SCN, somatosensory cortex, and adrenals of mice, Per1 and Per2 rapidly entrain to the new LD schedule whereas entrainment of Dbp and Nr1d1 is slower. Interestingly, in the liver, Per1 and Dbp re-entrainment is slower compared to Per2 expression rhythms. Bmal1 shows the slowest pace of entrainment in all tissues. Of note, in the pancreas, Nr1d1 is quickly re-adjusted while Per1 and Per2 are slower (Kiessling et al., 2010). Period genes are rapidly induced by light in the SCN. Per1 is almost induced with the same kinetics as c-fos (within 15–30 min) compared to Per2 (60 min) claiming Per1 as an immediate early gene (Albrecht et al., 1997; Shigeyoshi et al., 1997). Per2 reacts slower and takes longer to stably entrain upon a 6-hour LD cycle phase shift (Reddy et al., 2002). Thus, it cannot be excluded that the gene- and tissue-specific differences in re-entrainment are partly due to the different characteristics of the clock genes. Under dual-zeitgeber intervention conditions, a higher degree of phase incoherence between clock genes is observed under LD-28/FF-28 compared to LD-28/FF-24 conditions in liver, adrenal, and epididymal WAT of mice (Heyde & Oster, 2022). It is plausible that clock genes also vary in speed of resetting upon different cues like feeding and feeding-mediated signals. After 24 hr of fasting, for example, only Per2, Dec1, and Cry1 were expressed within 1-4 hrs of resumed feeding while other clock genes showed no effects until 8 hrs (Oike et al., 2011).
Exploring circadian characteristics at the single-cell level within a tissue is technically challenging and not thoroughly studied yet. In contrast, more evidence on phase incoherence between different clock genes within a tissue is arising. Both levels of intra-tissue desynchrony might impinge on the circadian output of the tissue affecting health, but more studies are needed to elucidate the specific impact.
Circadian clock network coordination is disrupted by alterations in zeitgeber input rhythms. Numerous studies show that such perturbation may result in metabolic, mental, and cardiovascular impairments. It is important to consider that these ailments arise from circadian misalignment which occurs at different levels of organization. However, causality has yet to be proven. Disruption of circadian rhythms usually emerge due to misalignment between external time cues and internal time. Such misalignment promotes internal desynchrony between distinct tissue clocks. Although inter-tissue desynchrony is an established concept, experimental evidence is still sparse and, therefore, the physiological outcomes are not well understood. It can be speculated that desynchrony between cellular clocks of the same tissue contributes to dampening of circadian tissue output, which consequently impacts homeostasis. Again, so far this has not been proven directly. Finally, it remains elusive whether such perturbed circadian output is caused by impaired rhythmic transcription and translation at the cellular level or by phase incoherence between cells within the tissue.
It will be important to understand the mechanisms and effects of internal desynchrony at different levels to develop targeted therapeutic approaches that can prevent chronodisruption-associated diseases. Differentiating the physiological impact of desynchrony on each level may be technically challenging and good experimental paradigms dissecting the different forms of desynchrony are still sparse. As one example, the development of transgenic mice harboring luciferase reporters coupled to a clock gene promotor enabled researchers to investigate effects of zeitgeber intervention in real-time in freely moving animals (Ono et al., 2015; Saini et al., 2013; Yamaguchi et al., 2016). Recently, fluorescent clock-reporters were used to track circadian oscillation in SCN neuronal sub-populations in vivo (Mei et al., 2018). However, so far it is not possible to study changes on single-cell levels in vivo. In vitro, this is overcome by introducing two reporters. Here, one fluorescent clock-reporter gives information on the phase of the clock while the second fluorescent reporter is used to track single cells (Manella et al., 2021). Future developments in this direction might enable us to study the impact of zeitgeber input on single cells in vivo and may help in understanding the physiological effects. Chimeric animals, having fast clocks, i.e. shorter period than 24 h, in almost all tissues but 24-hour clocks in distinct tissues, may be useful to study the consequences of misalignment between tissues (Maywood et al., 2021).
Furthermore, tissue-specific clock KO mice show impairments in metabolism and physiology (Dyar et al., 2014; Lamia et al., 2008; Paschos et al., 2012). However, in these studies it was not addressed whether these animals suffer from inter-tissue desynchrony and if so whether metabolic impairments may be promoted by this. Furthermore, our established dual-zeitgeber desynchrony paradigms may be useful to study the effects of multi-tissue misalignment on physiological and behavioral outcome (Heyde & Oster, 2019, 2022). The advantage of animal over human studies is the accessibility to various tissues to study tissue misalignment. However, translation might not be trivial as animals such as rodents differ in the metabolic rate and might be less prone to develop certain ailments compared to humans. In humans, internal phase can be determined by sampling a limited number of samplings of blood, urine or saliva measuring melatonin, cortisol or a set of genes (reviewed in Dijk & Duffy, 2020). However, these measures are representing the phase of the SCN only. We lack biomarkers for robustly and specifically determining phases of peripheral tissues in a non-invasive, time-saving and non-expensive manner to study inter-tissue (de)synchrony and its consequences among all genders, ages, ethnicities and lifestyles. With the help of such biomarkers, one could investigate to which extent internal desynchrony promotes adverse health effects.
To date, therapeutical approaches to increase internal synchrony are limited and not targeted but instead impact clocks throughout the body which may not in all cases be beneficial. Future studies should investigate whether modulation or weakening of distinct tissue clocks may be beneficial under chronodisruptive zeitgeber conditions. First findings suggest that weakening of the SCN clock or intra-tissue coupling might be beneficial for re-entrainment to shifted LD cycles; however, whole-body homeostasis was not studied (An et al., 2013; Yamaguchi et al., 2013; Pilorz et al., 2014). Bright-light therapy, affecting the SCN clock, reduces the misalignment of geophysical time and internal rhythms. It is shown to improve circadian rhythms and poor sleep quality in elderly, Alzheimer patients and people with major depression (Yamadera et al., 2000; Terman et al., 2001; Neikrug et al., 2012; Lam et al., 2016; Rubiño et al., 2020). Humoral signals impact on specific tissue clocks. However, we know too little about how the different tissue clocks are synchronized. Melatonin increases sleep quality and can entrain blind people to a 24-hour day (Hack et al., 2003; Redman, 1997; Sack et al., 2000). Melatonin-mediated effects were attributed to the inhibiting and phase-shifting effect on SCN activity, but recently its effects on peripheral clock gene expression were shown (Jung-Hynes et al., 2010; Torres-Farfan et al., 2006). Timed administration of melatonin might be beneficial to treat shift work-associated sleep disorders, but it is unknown how the circadian clock system is affected (Carriedo-Diez et al., 2022). One of the best studied hormones affecting tissue clocks are glucocorticoids. Glucocorticoid receptors are expressed in almost all tissues. While most tissue clocks can be reset by glucocorticoids, the SCN clock is unresponsive due to low receptor expression (Rosenfeld et al., 1988; Reddy et al., 2007; Kiessling et al., 2010; Balsalobre et al., 2000). Glucocorticoids also regulate the expression of genes involved in glucose and lipid regulatory pathways in liver and adipose tissue, thereby controlling the maintenance of energy homeostasis (Guia et al., 2014). Time of food consumption has a strong synchronizing effect especially on peripheral tissue clocks. In humans, time-restricted eating was exploited for its beneficial effect on whole body homeostasis in healthy adults and participants with metabolic syndrome (reviewed in Manoogian et al., 2022; Świątkiewicz et al., 2021). In general, eating-intervention decreases body weight – possibly due to decreased energy intake – and in several studies ameliorates metabolism. Time-restricted food intake to the normal active phase obviates body weight gain and metabolic impairments in rats under simulated night shift work (Salgado-Delgado et al., 2010). Recent studies show that time-restricted eating to normal active phase prevent some metabolic impairments in healthy adults under chronodisruptive laboratory settings and shift worker in real life conditions (Chellappa et al., 2021; Grant et al., 2017; Manoogian, Zadourian, et al., 2022). Together, time-restricted eating may be a powerful tool to prevent adverse metabolic outcomes. Energy state-related hormones such as insulin, leptin, glucagon, ghrelin, and adiponectin are capable to impact clocks but mainly control energy homeostasis (Martinez-Merlos et al., 2004; Prosser and Bergeron, 2003; Sun et al., 2015; Tahara et al., 2011; Tsang et al., 2020; Yannielli et al., 2007). Rhythmic administration of adiponectin rescues diurnal variation in gene expression in the MBH and leads to body weight reduction (Tsang et al., 2020). These findings indicate that hormones may be useful to synchronize clocks and clock output in a tissue-specific manner which in turn ameliorates health outcomes. However, caution must be taken since most hormonal receptors are expressed in numerous tissues and, thus, it is impossible to target specific tissue clocks which in turn, may lead to undesirable side effects. Additionally, it is important to note that not all tissues are susceptible to food-related signals but might be impacted by other zeitgebers. Moreover, recent findings demonstrate that non-circadian signals alter tissue transcriptome rhythms without affecting the local clock. For example, elevated thyroid hormone levels result in dampened or abolished rhythms in glucose and lipid metabolism related genes in the liver but have no effect on clock gene expression (de Assis et al., 2022).
Furthermore, despite exploring the potential use of hormones to improve internal synchrony, the efficacy of synthetic clock modulators gained attention in recent years. One of such, a REV-ERB agonist, may be useful to target metabolic impairments but it will also affect clocks throughout the body and side effects are not yet examined (Solt et al., 2012). The ROR agonist nobiletin enhances circadian rhythm amplitude and improves energy homeostasis preventing metabolic syndrome in diet-challenged mice (He et al., 2016). There are many more small-molecule modulators of the circadian clock and the therapeutic efficacy of such is proven or under investigation (reviewed in Ribeiro et al., 2021). To date, the clock machinery is assumed to be the same in all tissues, but there is evidence indicating that it is regulated in a tissue-specific manner (Mure et al., 2018). Global administration of small-molecule modulators of the circadian clock may have a differential effect on intra-tissue synchrony in which it will be beneficial for one tissue clock while worsening the phase relationship for another.
Last but not least, timing of treatment impacts success of therapy (Albuquerque et al., 2021; Montaigne et al., 2018; Ohdo, 2010). Therefore, understanding the complex mechanisms of clock network (de)synchronization, its consequences and how this can be manipulated may also have relevance for medical practice.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: F.A.J.L.S. served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham and Morehouse School of Medicine. F.A.J.L.S. interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. F.A.J.L.S.consultancies are not related to the current work.
Reviewer Expertise: Chronobiology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chronobiology
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
References
1. Wucher V, Sodaei R, Amador R, Irimia M, et al.: Day-night and seasonal variation of human gene expression across tissues.PLoS Biol. 2023; 21 (2): e3001986 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Chronobiology
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
References
1. Montaigne D, Marechal X, Modine T, Coisne A, et al.: Daytime variation of perioperative myocardial injury in cardiac surgery and its prevention by Rev-Erbα antagonism: a single-centre propensity-matched cohort study and a randomised study.Lancet. 2018; 391 (10115): 59-69 PubMed Abstract | Publisher Full TextCompeting Interests: F.A.J.L.S. served on the Board of Directors for the Sleep Research Society and has received consulting fees from the University of Alabama at Birmingham and Morehouse School of Medicine. F.A.J.L.S. interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. F.A.J.L.S. consultancies are not related to the current work.
Reviewer Expertise: Chronobiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 03 Apr 23 |
read | read |
|
Version 1 15 Nov 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)