Keywords
Acacia mangium, hepatitis C virus, infectious disease, medicinal plant, medicine, health
This article is included in the Plant Science gateway.
This article is included in the Pathogens gateway.
Acacia mangium, hepatitis C virus, infectious disease, medicinal plant, medicine, health
We have revised following the reviewer suggestion
1. Abstract section, background, line 3: change word "by" to " with".
2. Abstract section, Method, lines 9, 10, 11 of abstract section
3. Method section: add sentence in cytotoxicity assay section, lines 3-5 of this section
See the authors' detailed response to the review by Ziwen Wang
See the authors' detailed response to the review by Courage Sedem Dzah
See the authors' detailed response to the review by Borris Rosnay T Galani
See the authors' detailed response to the review by Moustafa Sarhan
Hepatitis C virus (HCV) infection is an acute or chronic liver disease. HCV infection has a high prevalence globally, and approximately 71 million people are at risk of liver cirrhosis or hepatocellular carcinoma attributable to chronic infection (Lange et al., 2014). To date, no effective hepatitis C vaccine has been developed because HCV is a commonly mutated virus (Pawlotsky et al., 2015; Pawlotsky Jm et al., 2018).
Hepatitis C treatment has evolved with the availability of direct acting antivirals, which have achieved sustained virological response (SVR) rates exceeding 90% (Segarra-Newnham et al., 2020). However, some low-income countries cannot access those therapies because of their costs, and the combination of interferon-alpha and ribavirin (RBV), which produces an SVR rate of 50%, remains in use. Combination treatment has also been reported to have serious side effects and risks of resistance, making this strategy less effective (Swain et al., 2010). Efforts to develop new agents for HCV are necessary. Further issues to overcome include the development of drugs that can inhibit the virus with fewer side effects and affordable prices for all countries. Therefore, it is necessary to develop affordable, safe, and effective HCV therapies.
Traditional herbal medicine has become a popular treatment, and plants are among the primary components of such medicines. Our previous studies reported medicinal plants possessing anti-HCV activities (Adianti et al., 2014; Wahyuni et al., 2013; Wahyuni et al., 2014). Many medicinal plants have also been reported to inhibit HCV by inhibiting various steps of the HCV life cycle (Hussein et al., 2000; Ravikumar et al., 2011; Wahyuni et al., 2016).
Plants in the Fabaceae family are frequently used by traditional healers to treat liver diseases, including HCV infection. The chemical compounds present in the Fabaceae family include saponins, tannins, flavonoids, proteins, stylbenoid, xanthones, terpenes (triterpenes, diterpenes), phytoalexin, galactonate, lactogenic agents (polyketide), and anthraquinone. Most of those compounds are reported to possess anti-viral, hepatoprotective, and anti-cancer activities (Roy et al., 2016). One genus of the Fabaceae family that has been demonstrated to inhibit HIV is Acacia, and the active species include A. nilotica (50% inhibitory concentration [IC50] = 40.5 μg/mL) and A. confusa (IC50 = 5 μg/mL) (Hussein et al., 2000; Lee et al., 2011).
A. mangium contains alkaloids, flavonoids, polyphenols, glycosides, saponins, steroids, tannins, and terpenoids, and their leaves contain phenolic groups including tannins and flavonoids. A. mangium was reported to contain 2,3-cis-3,4,7,8-tetrahydroxyflavanone and teracacidin (Barry et al., 2005). A. mangium was reported to possess various bioactivities such as anti-oxidant, antibacterial activities, antifungal activities (Batiha et al., 2022; Mihara et al., 2005; Prayogo et al., 2021). Other species of Acacia, Acacia comfisa and Acacia nilotica were reported to inhibit HCV (Lee et al., 2011; Rehman et al., 2011). This current study evaluated the anti-HCV activity of various extracts of A. mangium leaves. In addition, the cytotoxicity of the extracts was evaluated. The mode of action was additionally assayed to determine the part(s) of the HCV life cycle inhibited by the extracts. Moreover, its mechanism of action was examined by Western blotting and combination treatment with current anti-HCV drug. This study was used simeprevir for combination treatment which known as NS3 protease inhibitor. It is particularly effective against HCV genotypes 1 and 4, making it a valuable treatment option for patients infected with those genotypes (Rice et al., 2014).
A. mangium leaves were obtained from an area in Mojokerto Regency, Indonesia. The plant was verified by an expert botanist from Materia Medica Indonesia, East Java (see the Underlying data (Wahyuni, 2022)).
The materials used in the bioassays were as follows: Huh7it-1 cells (Apriyanto et al., 2016); adapted variant of HCV strain (JHF1a) (Yu et al., 2010); Dulbecco’s Modified Eagle’s Medium (DMEM, GIBCO Invitrogen) supplemented with 10% of fetal bovine serum (FBS, GIBCO Invitrogen), 150 μg/mL kanamycin (Sigma-Aldrich), and non-essential amino acids (NEAAs, GIBCO Invitrogen); Dulbecco’s phosphate-buffered saline (GIBCO Invitrogen); formaldehyde (HCHO, Sigma-Aldrich); trypsin-EDTA (Sigma-Aldrich); 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTT, Sigma-Aldrich); bovine serum albumin (BSA, Biowest); Triton X-100 Sigma-Aldrich); 3,3′-diaminobenzidine (DAB, Thermo Fisher Scientific); anti-HCV human antibody and HRP-conjugated goat anti-human Ig antibody (Thermo Fisher Scientific); RIPA buffer; polyacrylamide gel and polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA); β-actin antibody (MBL, Nagoya, Japan); and a chemiluminescence detection system (Bio-Rad; GE Healthcare, UK).
A. mangium leaves (2 kg) were dried, ground into powder, and further extracted with two kind of extraction procesess. First, 200 g of powder was extracted by maceration process with a total of 2 liters of 96% ethanol and another 200 g was successively extracted with 2 liters of n-hexane, dichloromethane, and methanol. Specifically, 10 mg of the extract powder were dissolved in 100 μL of dimethyl sulfoxide to obtain 100,000 μg/mL stock solution (Wahyuni et al., 2013).
Huh7it-1 cells were cultured in DMEM supplemented with 10% FBS, 150 μg/mL kanamycin, and NEAAs in 5% CO2 at 37°C and maintained for bioassay purposes. Cells were incubated at 37 °C for 2 days. Cells which showed more than 80% confluence were used for further bioactivity assay. The detailed protocol for cell passage is available at https://dx.doi.org/10.17504/protocols.io.n92ldpbd7l5b/v1.
Virus stock was obtained by propagating HCV in Huh7it-1 cells. Culture supernatants at day 3, 5 and 7 after virus infection were collected. Virus titers were calculated by titration assay (wahyuni, 2013). In brief the virus harvested was diluted on x5, x25, x125, x625, and x3125 then put onto Huh7it-1 cells and incubated for 4 hours, the remaining virus was removed and refed with new medium for further incubation for 2 days. The infected cells were stained with DAB staining reagent and further calculated. The number of viruses represented as titer virus. The high titer virus stock (higher than 1x105) was chosen for anti-HCV assay. The stock was stored at −80°C until use (Wahyuni et al., 2018).
An anti-HCV assay was conducted using HCV-infected Huh7it-1 cells. Various concentrations of the extracts were mixed with virus solution (multiplicity of infection of 0.1) and inoculated into the cells at a final concentration of 0.01, 0.1, 1, 10, 50, or 100 μg/mL. The cultures were incubated for 48 h with 2 h of virus inoculation and further incubation for 46 hours at 37°C. The viral levels of the supernatants were examined by titration assay. Culture cell supernatant was collected, diluted 10x with medium and inoculated to the Huh7it cells. This was incubated for 2 days and the infected cells were calculated after the immunostaining process. The inhibitory effect of the extracts were calculated compared to the untreated control. The 50% inhibitory activity was conducted by SPSS software version 25 (Wahyuni et al., 2019; Wahyuni et al., 2018). Ribavirin was used as the positive control.
Huh7it-1 cells were incubated with serial dilutions of the supernatant for 48 h. The cells were fixed with formaldehyde, stabilized with triton and subjected to immunostaining with primary antibody (human serum) and secondary antibodies (HRP-conjugated goat anti-human). The detailed immunostaining protocol is available in the Underlying data (Permanasari and Wahyuni, 2022a). DAB staining was performed to visualize the infected cells. The percent inhibitory effect was calculated by comparing the reduction of infected cells to the control (Wahyuni et al., 2018).
The mode of action assay was performed to examine whether A. mangium extract affected the entry or post-entry step of the HCV life cycle. Three parallel experiments were performed. First, the extract was only added to the cells during viral inoculation. Second, the extract was only added to the cells after viral inoculation. Third, the cells were treated with the extract both during and after inoculation. After 48 h of incubation, all supernatants were collected to examine their viral levels by titration assay. Viral supernatant was diluted with medium and inoculated to the Huh7it cells. Infected cells were calculated to further determine the percentage inhibitory against HCV. Anti-HCV activity was expressed by 50% inhibition concentration (IC50) (Hafid et al., 2017).
The MTT (3-(4,5- Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) assay was used to measure cytotoxicity. Huh7it-1 cells were incubated with various concentrations of the extracts for 48 h. After incubation, 10% MTT was added to the cultures for 4 h. The remaining reagent was discarded and DMSO was asses in each wells to dissolved the formazon formation. Absorbance was measured at 560 and 750 nm to calculate the percentage cell viability relative to the control. SPSS software version 25 probit analysis was used to calculate the 50% cytotoxic concentration (CC50) (Wahyuni et al., 2018). The protocol of the MTT assay is available at http://dx.doi.org/10.17504/protocols.io.6qpvr4x5pgmk/v1.
Huh7it-1 cells were treated with mixtures of the extracts (10 or 50 μg/mL) and HCV. After incubation for 2 days at 37 °C, the cells were collected, lysed, and protein levels were determined using a BCA assay kit (Thermo Fisher Scientific). Equal amounts of proteins were subjected to SDS–polyacrylamide gel electrophoresis followed by transfer to a polyvinylidene difluoride membrane. Samples were run in transfer buffer at 0.3 A for 30 minutes followed by processing to SDS running buffer at 0.1 A for 25 minutes. The membrane was applied into a blocking buffer of skim milk and reacted to antibodies. The primary antibody was an HCV NS3 mouse monoclonal antibody (clone H23; Abcam, Cambridge, MA, USA), and the secondary antibody was HRP-conjugated goat anti-mouse immunoglobulin. β-actin (MBL, Nagoya, Japan) served as the internal control (Permanasari et al., 2021; Widyawaruyanti et al., 2021). Membranes were incubated at room temperature for 1 hour in each antibody. NS3 protein expression was detected using an enhanced chemiluminescence detection system (GE Healthcare, Buckinghamshire).
Combination treatment was performed by adding equal volumes of A. mangium extract and simeprevir (Toronto Research Chemical, Canada). Simeprevir was added at 0.25×, 0.5×, 1×, 2×, and 4×IC50 for monotherapy and combination. All treatments were performed for 48 h incubation. The IC50 of simeprevir when used in combination with A. mangium extract and monotherapy were calculated and compared using the SPPS probit assay version 25 (Wahyuni et al., 2020).
All A. mangium leaf extracts strongly inhibited HCV in a dose dependent manner (Figure 1). Inhibition concentrations of 50% of all extracts were calculated by probit analysis. Dichloromethane extract displayed the strongest effects, with an IC50 of 0.2 ± 0.3 μg/mL, whereas the IC50s of the extracts ranged 2.8–4.6 μg/mL (Table 1). While the positive control of ribavirin revealed the IC50 values of 10.4 ± 0.2 μg/mL. All of the extracts were demonstrated to possess stronger activity compared to the positive control of ribavirin. The raw data are available in the Underlying data (Permanasari and Wahyuni, 2022b).
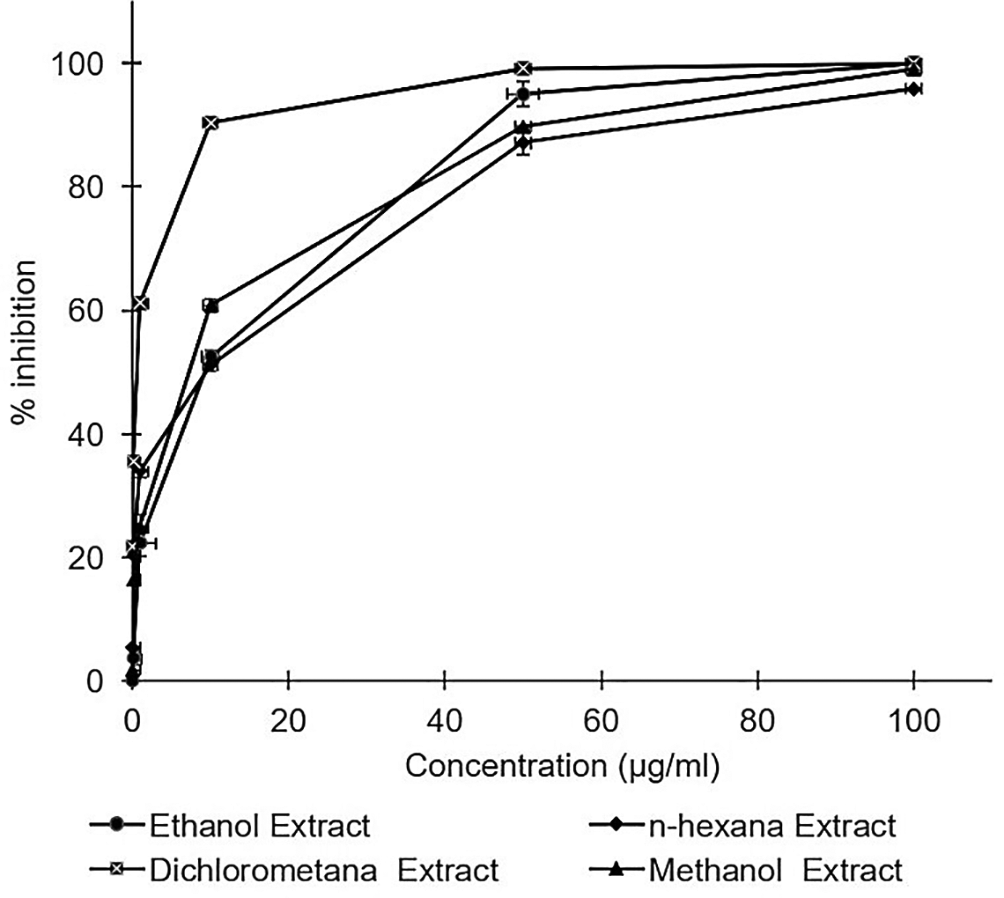
Huh7it-1 cells were cultured and inoculated with mixtures of the virus and each extract at various concentrations. Virus inhibition was calculated relative to the untreated control. The data represent the mean of three independent experiments.
The cytotoxicity assay of the extracts observed no toxic effect in the Huh7it. The percentage of cell viability demonstrated that all extracts possessed cell viability higher than 80% in the concentration of 400 μg/mL. However, n-hexane and dichloromethane extract showed a reduction in the percentage of cell viability at the dose of 400 μg/mL (Figure 2). The raw data are available in the Underlying data (Permanasari and Wahyuni, 2022c).
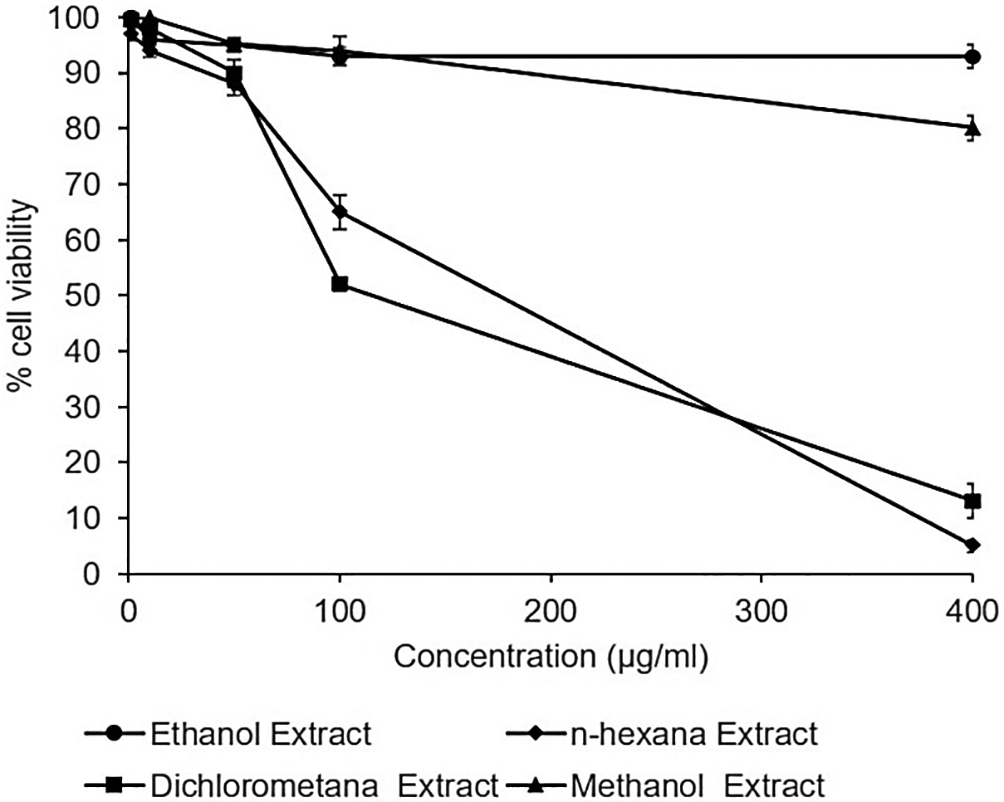
Cells were treated with various concentrations of each extract of Acacia mangium. MTT (3-(4,5- Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) reagent was added after 2 days of incubation, and cell viability was examined using a microplate reader at wavelengths of 450 and 630 nm. The percent cell viability was calculated, and the 50% cytotoxic concentrations were determined by SPSS probit analysis. The data represent the mean of three independent experiments.
The mode of action assay was performed using three series of experiments. The result illustrated that the inhibitory effect was higher on the post-entry step than on the entry step. The percentage virus inhibition of post entry steps revealed more than 20% differences than the entry step (Figure 3). This result suggested that the extract dominantly affected post-infection processes such as virus replication, virus assembly, and virus release. The raw data are available in the Underlying data (Permanasari and Wahyuni, 2022d).
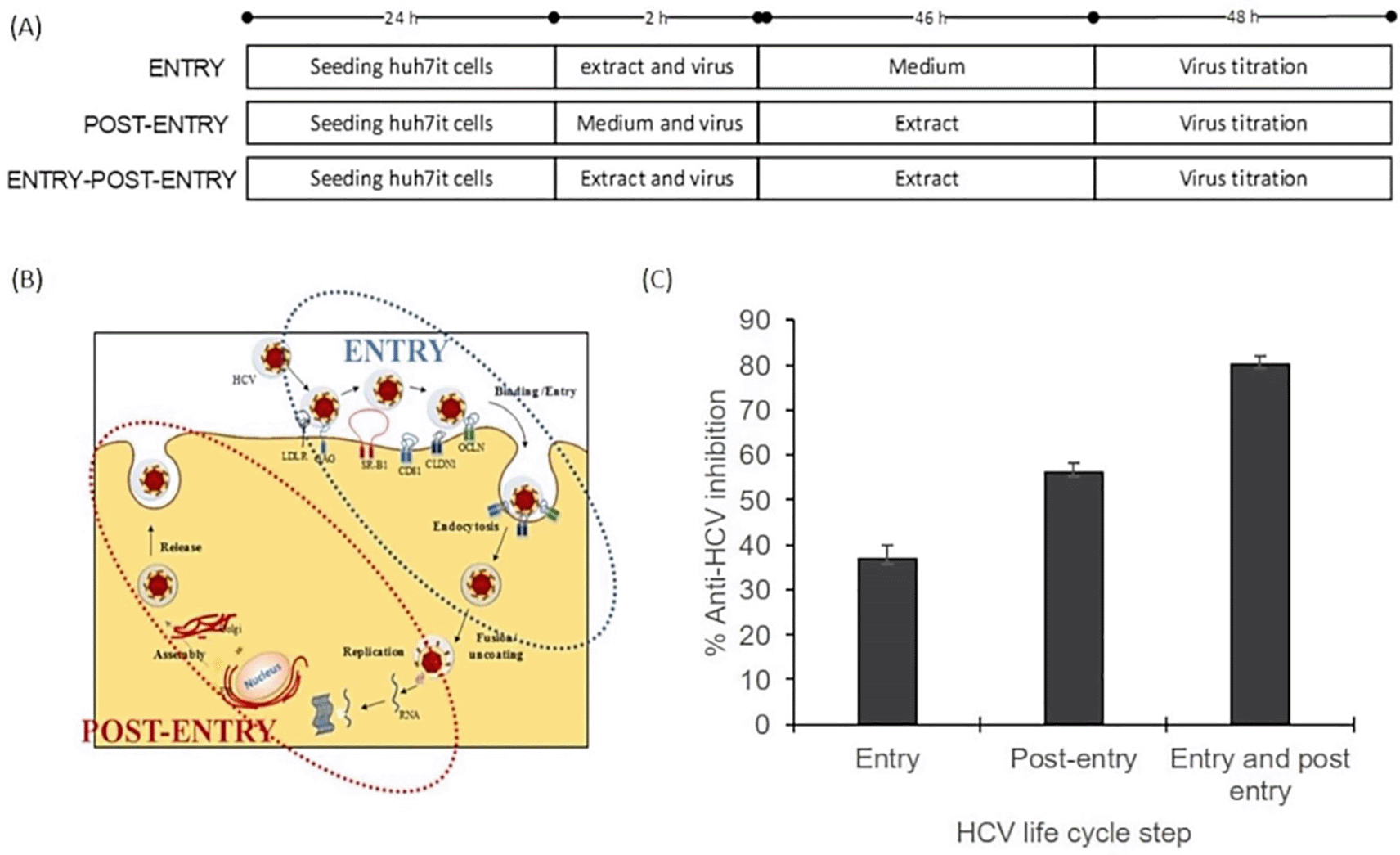
(A) Cells were cultured with the ethanol extract of A. mangium (30 μg/mL) in three parallel experiments. First, cultured cells were treated with the extract only during inoculation (entry step). Second, cells were treated with the extract only after inoculation (post-entry step). Third, cells were treated with the extract both during and after inoculation. (B) The entry step comprises the processes of viral binding to host receptors, viral entry into the cells and endocytosis, whereas the post-entry comprises translation, replication, assembly, and release. (C) Percent inhibition of the entry step, post-entry step, and both steps. The data represent the mean ± SEM of three independent experiments.
Evaluation of the combination effect between the extract and simeprevir showed there is an enhancement of the effects of the extract to simeprevir, which is known as an HCV NS3 protein inhibitor. The addition of A. mangium extract increased the anti-HCV effect of simeprevir, as the IC50 of simeprevir when used in combination with A. mangium extract was reduced by 2-fold compared to that of simeprevir alone (Table 2).
| Sample | 50% inhibitory concentration* |
|---|---|
| Acacia mangium extract alone | 4.75 ± 0.07 μg/mL |
| Simeprevir alone | 19.65 ± 0.49 nM |
| (0.0149 ± 0.0005 μg/mL) | |
| Combination simeprevir and Acacia mangium extract | 9.4 ± 0.2 nM |
| (0.0069 ± 0.00014 μg/mL) |
To examine the mechanism of action of the ethanol extract, western blotting analysis was performed. The result demonstrated a reduction of the NS3 protein level due to the extract intervention. Immunoblotting revealed that treatment with A. mangium extract at 10 or 50 μg/mL decreased the NS3 protein expression by 40% and 95%, respectively, versus the control (Figure 4) (Underlying data, Permanasari and Wahyuni, 2022e).
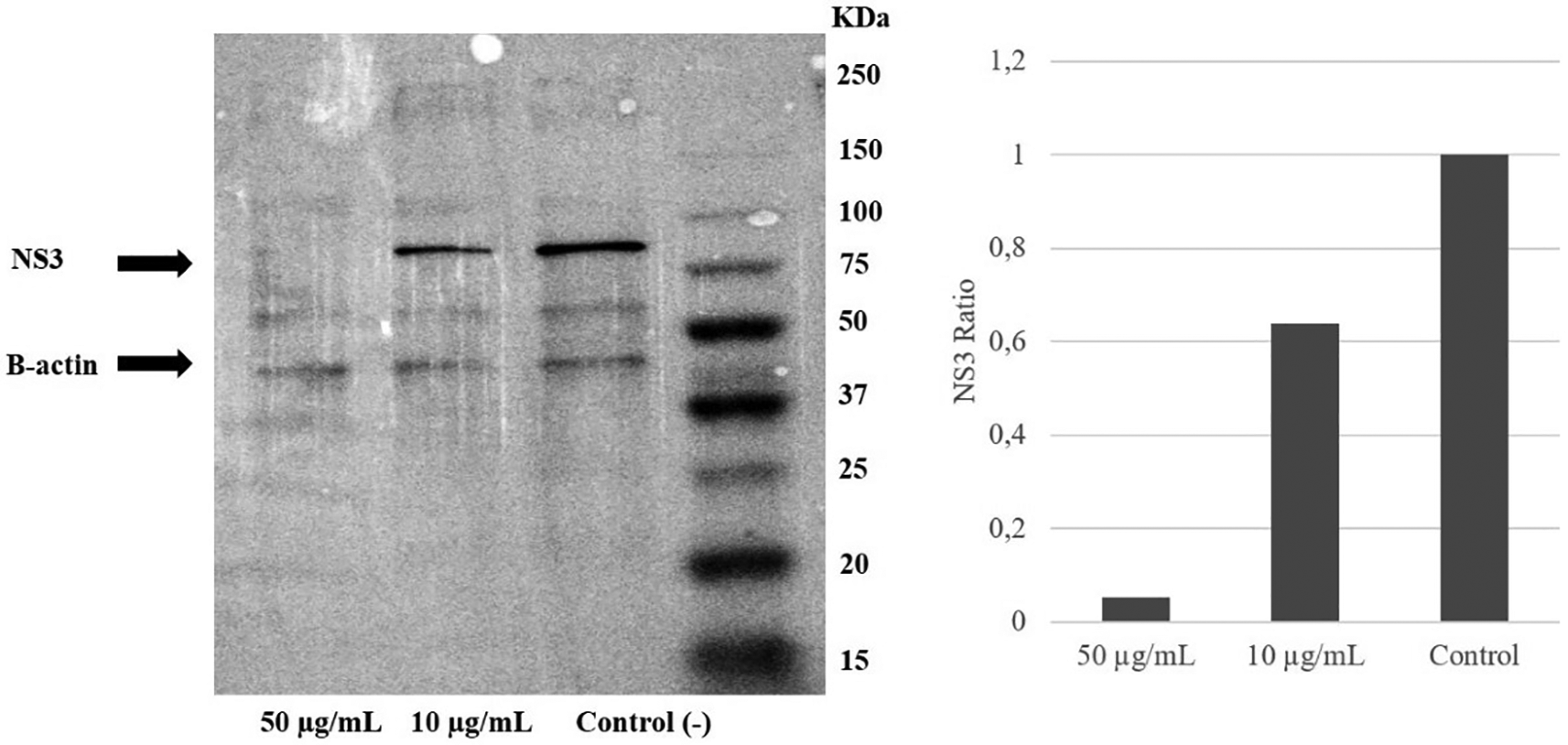
Huh7it-1 cells were treated with a mixture of the extract (final concentration, 10 or 50 μg/mL) and virus. The cells were lysed in RIFA buffer, and an equal amount of proteins were separated by SDS–polyacrylamide gel electrophoresis.
The study found that A. mangium possesses potential anti hepatitis C virus activity through some mechanism. The anti-HCV activities of the A. mangium leaves extracted using solvents of different polarities, namely 96% ethanol, n-hexane, dichloromethane, and methanol was examined. The data illustrated the strong anti-HCV activities of all extracts. Moreover, no toxic effect was observed according to the CC50 and selectivity index.
Ethanol is commonly used as a solvent in traditional drug development. Therefore, the strong anti-HCV activities of the ethanol extract and the lack of cytotoxicity provide necessary information for developing extracts of A. mangium as an alternative or complementary anti-HCV agent. However, we used various solvents with different polarities to further isolate the active anti-HCV compounds. The result demonstrated that the dichloromethane extract of A. mangium possessed the strongest inhibitory activity.
Mode of action analysis was performed as the first screening assay to determine the mechanism of A. mangium extract. The results illustrated that the ethanol extract more strongly inhibited HCV in the post-entry stage than in the entry stage. Virus production starts with binding of the virus to the host cell receptor, followed by virus entry and endocytosis. These processes are included in the entry step. The entry of HCV into hepatocytes is mediated by the viral E1 and E2 glycoproteins, which are the surface proteins of viral particles. HCV infection occurs through complex interactions of viral lipoviral particles with cellular factors, including low-density lipoprotein receptors, glycosaminoglycans, scavenger receptor class B type I, tetraspanin (CD81), claudin-1, and occludin (Dubuisson et al., 2008; Moriishi and Matsuura, 2003). Viral particles enter host cells through clathrin-mediated endocytosis, after which they are sent to the endosome. Meanwhile, the post-entry steps include translation, replication, and assembly. NS3–NS5 form a replication complex that produces new viral genomic RNA. Genomic RNA and HCV core proteins accumulate to form a nucleocapsid, which is excreted through the lumen side of the endoplasmic reticulum. After this excretion, the nucleocapsid can interact with very low-density lipoprotein (VLDL), followed by translocation to the Golgi for maturation. Mature HCV–VLDL complexes are released exocytically via the VLDL secretory pathway. Finally, new cells can be infected by released free HCV particles or by cell-to-cell transmission (Fénéant et al., 2014; Lindenbach and Rice, 2013; Zeisel et al., 2015).
To further clarify the mechanism of the anti-HCV effects of A. mangium extract, Western blotting was performed to evaluate the effect of the extract on NS3 protein. A. mangium extract decreased NS3 protein levels versus the control. NS3 is a non-structural virus protein that plays an important role in replication. It is an attractive target for HCV treatment. Inhibition of NS3 could result in decreased virus production. Moreover, the anti-HCV activity of the extract was evaluated in combination with the NS3 protein inhibitor simeprevir. A. mangium extract was demonstrated to enhance the inhibitory activity of simeprevir against HCV. This suggested that the extract potentiated the effect of simeprevir on secondary targets of HCV.
Chemical compounds play an important role in anti-HCV activities. Further isolation of the active compounds from A. mangium against HCV is needed. However, it has been reported that Acacia species are rich in polyphenols, flavonoids, alkaloids, saponins, and terpenoids. It was reported compounds in the genus Acacia include epicatechin, quercetin, proacaciaside I, and proacaciaside II (Figure 5). Those compounds were previously demonstrated to exhibit bioactivities such as anti-bacterial, anti-fungal, and anti-parasitic effects, which could contribute to anti-HCV properties (Chew et al., 2011; Rangra et al., 2019).
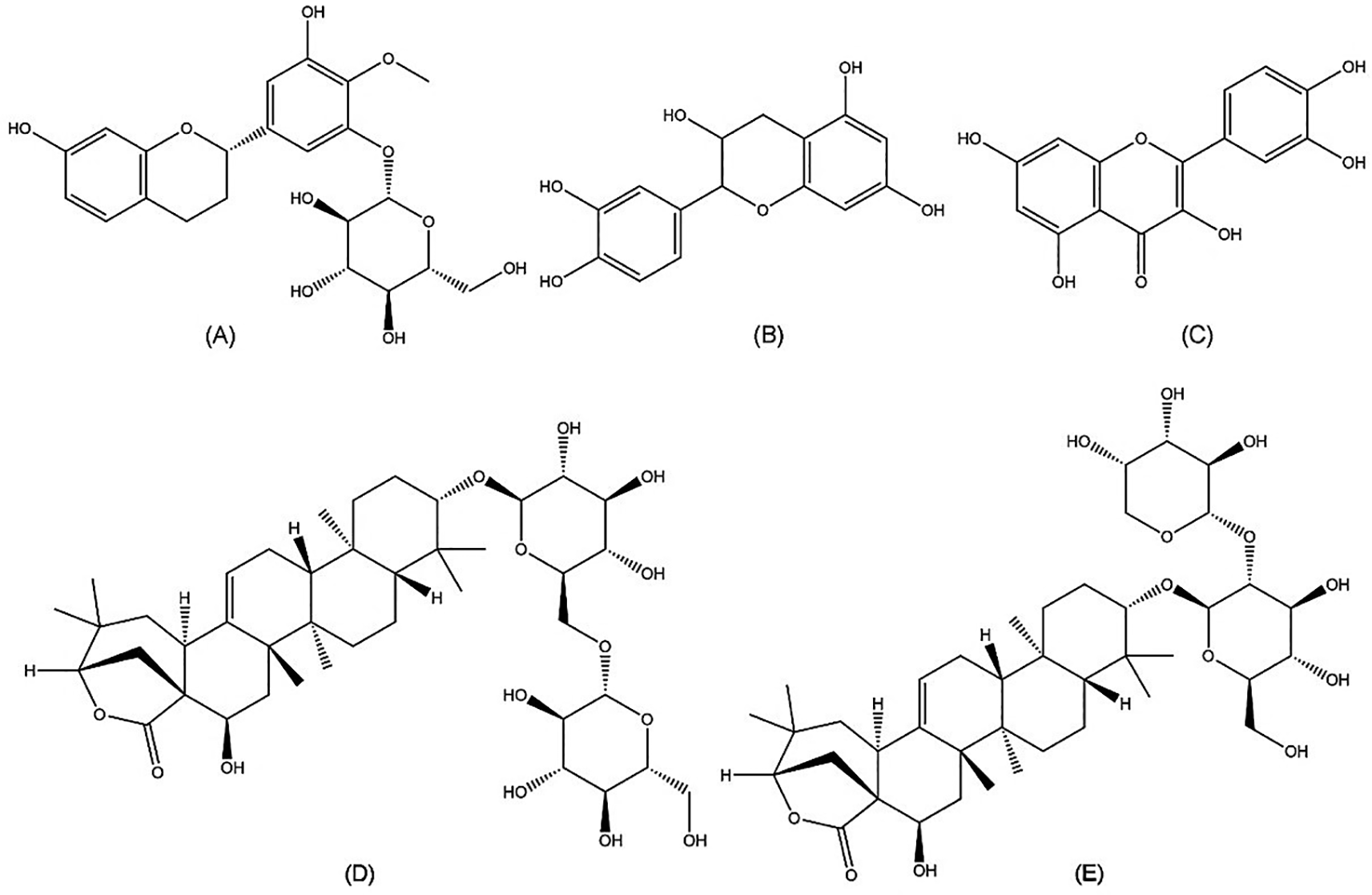
(A) Auriculoside (https://pubchem.ncbi.nlm.nih.gov/compound/Auriculoside), (B) epicatechin (https://pubchem.ncbi.nlm.nih.gov/compound/72276), (C) quercetin (https://pubchem.ncbi.nlm.nih.gov/compound/5280343), (D) proacaciaside I (https://pubchem.ncbi.nlm.nih.gov/compound/102446075), and (E) proacaciaside II (https://pubchem.ncbi.nlm.nih.gov/compound/102446076).
A. mangium leaf extracts possess strong anti-HCV activities without toxic effects. The extracts strongly inhibited the post-entry step, decreased NS3 protein levels, and enhanced the anti-HCV activities of simeprevir. These results suggest that A. mangium could be used to develop complementary and alternative treatments for HCV.
Figshare: Plant Determination of Acacia mangium.jpg (It provided the taxonomy information of Acacia mangium) https://doi.org/10.6084/m9.figshare.20973511 (Wahyuni, 2022)
Figshare: Immunostaing HCV protocol. Immunostaining HCV.docx (It provided the detail steps of immunostainning process.) https://doi.org/10.6084/m9.figshare.20977168 (Permanasari and Wahyuni, 2022a).
Figshare: in vitro activity of A. mangium against HCV. raw data figure 1.docx (It demonstrated the figure of cells with infected cells, the tables which showed number of infected cells and percent inhibition of the three replication of experiments.). https://doi.org/10.6084/m9.figshare.20977714 (Permanasari and Wahyuni, 2022b).
Figshare: Cytotoxic effect of A. mangium extracts. cytotoxicity.docx (It provided the percent viability of extract ethanol, n-hexane, dichloromethane, and methanol https://doi.org/10.6084/m9.figshare.20977939 (Permanasari and Wahyuni, 2022c)
Figshare: Untitled Item. Mode of action.docx (It provided the number of infected cells in three kind of inoculation method,entry, post entry and both.). https://doi.org/10.6084/m9.figshare.20977933 (Permanasari and Wahyuni, 2022d).
Figshare: NS3 Acacia mangium inhibition. NS3 beta actin 4 (1).tif (It provided the raw data of western blotting assay) https://doi.org/10.6084/m9.figshare.21352095 (Permanasari and Wahyuni, 2022e).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank Professor Hak Hotta (Konan Women`s University), Dr. Takaji Wakita (National Institute for Infectious Diseases, Japan), and Dr. Yohko Shimizu (Kobe University) for providing Huh7it-1 cells.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular Biology, Antiviral peptides
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacology, Hepatology and Virology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: 1. Plant bioactive compounds extraction, purification, identification, characterization, bioactivity assessment, bioavailability and potential industrial applications (e.g. bioactive compound-based nanoparticles, nano-emulsions, etc) 2. Food Science and Engineering. 3. Physical processing and treatment of biomass
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Discovery of novel antivirals based on natural products
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 02 Aug 23 |
read | |||
|
Version 2 (revision) 28 Feb 23 |
read | read | ||
|
Version 1 08 Dec 22 |
read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)