Keywords
Grapsus albolineatus, glucogenase, elastase, hyaluronidase, carotenoids.
This article is included in the Plant Science gateway.
Grapsus albolineatus, glucogenase, elastase, hyaluronidase, carotenoids.
Skin is one of the largest organs in the human body and covers almost its entire.1,2 Skin has various functions such as protecting muscles, bones, and internal organs.3 Skin can experience aging that in unavoidable due to intrinsic factors, influenced by age, genetics, hormones, and blood sugar levels.4 While the majority of extrinsic variables are caused by continuous exposure to ultraviolet (UV) rays, pollution, diet, and smoking.5,6
UV radiation is associated with an increase in oxygen radical species on the skin. Excessive reactive radical species such as reactive oxygen species (ROS) and reactive nitrogen species (RNS) can worsen pigmentation and aging of the skin, caused pigmentation disorders, rough skin and skin wrinkling.7 In addition to oxidative damage to the skin, ROS are known to activate hyaluronidase, glucogenase, and elastase enzymes.8 Increased activity of this enzyme can reduce hyaluronic acid, elastasin, and collagen. These components are key elements of the skin that act on elasticity, flexibility, and moisture provision, allowing the skin to seem young and healthy.9,10
Therefore, to prevent premature aging, the skin needs an antioxidant as well as an enzyme inhibitor which acts as a natural inhibitor of enzymes that are activated due to UV radiation. Carotenoids are a class of compounds that have excellent antioxidant activity.11,12 Vegetables and fruits are considered as the most important sources of carotenoids in the human diet; animal products also contain large amounts of carotenoids.13,14
The lightfoot crab from North Sulawesi, G. albolineatus, is known to contain high levels of carotenoids. G. albolineatus is a large crab with a large population. Grapsus albolineatus Latreille has a convex carapace with low tubercles; lateral, rounded margins, with one anterior tooth; the length of the front is equal to the length of the posterior margin of the carapace. The carapace is greenish-blue with paler areas; lateral margins and tubercles white; legs mottled in brown with one orange spot at the tip of meri.15 This species has markedly arched carapace lateral margins; outer infraorbital angle with acute tooth; third maxillipeds with long merus, slightly shorter than the ischium; the male abdomen has five somites about 2.3 times as broad as long; G1 is slightly curved. G. albolineatus is a large-sized species. Its world distribution includes the Indo-West Pacific: East Africa, Mauritius, Somalia, Socotra, Red Sea, Gulf of Aden, Southern Oman, Persian Gulf, Gulf of Oman, Pakistan, India, Sri Lanka, Bay of Bengal, Nicobar Islands, Andaman Sea, Mergui Archipelago, China, Japan, Indonesia, Singapore, Cocos (Keeling) Islands, Australia, and Hawaii. Its habitat is rocky, intertidal substrate.16
It is widely distributed in the seas of the SouthWest Pacific. Male G. albolineatus have been recognized as having varying quantities of carotenoid pigments, while female G. albolineatus have not been studied for the kinds of carotenoid pigments they contain and their separation.17
Therefore, this study aimed to explore the pigment and separation of carotenoids in female G. albolineatus as well as to test carotenoids as anti-aging and natural inhibitors of hyaluronidase, glucogenase, and elastase enzymes using a molecular docking approach.
The researcher submitted a research permit (ethical clearance) to the UB (Universitas Brawijaya) Ethics committee team. Part of this research was conducted at the university.
Ethical clearance was obtained by including a research proposal, in which the method had a 3R stage, namely: 1) Reduction: using 3 animals, 2) Replacement: using 1 gram of the carapace organ, 3) Refinement: To avoid distress in animals, the researcher used 95% alcohol which functions as an analgesic.
This research was funded by the Institution of Research and Community Service Sam Ratulangi University (UNSRAT) with assignment letter Number 673/UN12.13/LT/2019. which was signed by the chairman of Institution of Research and community service UNSRAT, after being passing evaluation by the university reviewer.
Sampling was carried out by exploring the intertidal area at the lowest low tide at night. The sample was put into a container filled with a little seawater, to ensure the crabs did not die. The dorsal carapace of the 3.7 cm crab in that section was smeared with 95% alcohol, when the crab fainted, surgery was carried out and then extraction was carried out.
Crab samples were first injected to obtain blood from G. albolineatus using a five-mile injection and then dissected in order to separate the hepatopancreas and gonads. The next step was to separate the epidermis layer, which is a thin membrane connected to the interior of the carapace, and the black epidermis layer with tweezers and a laboratory knife. The organ carapace was crushed and immersed for five minutes in 2N hydrodrochloric acid. Then we strained it through filter paper into a separator flask,added petroleum ether and distilled water to make two layers. The absorption peak was then calculated using a UV-Vis spectrophotometer with a wavelength range of 380-550 nm.
The absorbance of the carapace pigment extract was measured using a UV-Vis spectrophotometer at 380-550 nm wavelength. The spectrogram’s shape is a curve with the X axis representing wavelength, and the Y axis representing absorbance, and the highest absorption peak at a certain wavelength can be read. Each spectrogram produced can be used to calculate the amount and concentration of G. albolineatus pigments utilized in this investigation.18,19
After that, the entire pigment extract was separated using column chromatography. The n-hexane-acetone was the solvent employed in the mobile phase (70:30). The X fraction was collected, which was then accommodated and examined for absorption with a UV-Vis spectrophotometer at a wavelength of 380-550 nm, yielding the spectrophotometer’s highest absorption peak.18,19
High performance liquid chromatography (HPLC) type LC.20ADVP with photodiode array detector (PDA; SPD-M20A-Shimadzu). The HPLC column used was C30 (YMC carotenoid, 150 × 4.6 mm I.D) with a column temperature of 30°C and a flow rate of 1 mL/min with a gradient system of H2O, methanol and methyl tertiary butyl ether. The sample used was 20 μL. Carotenoids were separated in a gradient form for 50 min using a mixed solution of methanol, methyl tertiary butyl ether, and water (80:5:15) at a flow rate of 1 mL/min. After 10min, the water content was lowered linearly, and the acetone was added to get the acetone proportion up to 20 percent at 50 min after injection. The final composition of the gradient mixture was methanol: methyl tertiary butyl ether (80:20).
The binding mechanism to the active site of three enzymes was investigated using molecular docking: fibroblast collagenase (1CGL), porcine pancreatic elastase (4YM9), and hyaluronidase (1FCV).20–22 These three receptors are available on the Protein DataBank (PDB) website and may be saved as a PDB file.23
Enzyme preparation was accomplished by eliminating water and cofactors from each enzyme. The protein was first optimized by adding polar hydrogen, followed by the addition of atomic charge with Kolman charge and nonpolar hydrogen. The receptor was then stiffened and stored in PDBQT (Protein DataBank, partial charge (Q), and atom type) format.9
The Canonical SmilesY of each ligand utilized in ligand preparation was acquired from Pubchem.24 Subsequently, Canonical Smiles was converted to Chemdraw 2D 16.0 to produce a 2D compound, which was then modified into 3D using Chemdraw 3D and a reduced calculation was done. The file was stored as a PDB file. The ligand’s PDB file was then mixed with polar hydrogen and given the gasteiger atomic charge and torque on each ligand, after which the ligand was stored in PDBQT format.
The AutoDock 4.2.6 software was used to simulate docking.25 At each active site, molecular docking was performed. The active site is determined by verifying the docking of each inhibitor and the corresponding receptor. A grid box was created in the middle of the ligand location to determine the coordinates of the receptor’s active side. Then, for 1CGL and 4YM9 receptors, dimensions of 50×50×50 points were utilized, while for 1FCV, box dimensions of 60×60×60 points were chosen, with a grid point spacing of 0.375 Å. The Lamarckian Genetic Algorithm was the calculation employed in this docking approach. The optimal conformation was chosen based on the lowest bonding energy among the most conformational populations.
To observe the interactions that occur between the ligand and the receptor, docking visualization was performed using Biovia Discovery Studio 2020.26 Interactions are depicted in three and two dimensions, respectively. Hydrogen bonding and hydrophobicity were utilized to analyze intermolecular interactions.
The highest wavelength of the content and concentration of total pigment extract in G. albolineatus crabs, particularly in the carapace, was 474 nm. As a result, the overall pigment extract content and concentration value was 4.33 μg/g, whereas the pigment content in the carapace organ was 4.46 μg/g.
The column chromatography separation was repeated twice with an n-hexane-acetone mobile phase (70:30). A total pigment extract divided into four fractions: F1, F2, F3, and F4. A UV-Vis Spectrophotometer with a wavelength range of 380-550 nm was used to examine the four fractions. The presence of x-carotene pigments was indicated by wavelengths of 426, 448, and 475 nm, whereas the presence of zeaxanthin pigments was indicated by wavelengths of 426, 450, and 475 nm in the second fraction; the pigment types in F3 and F4 were unidentified.27
F1 was not processed through the second round. However, F2, F3, and F4 were separated again using column chromatography: F2.1 was characterized as echinenone and F2.2 as astase. Furthermore, the pigment was not identified in F3.1 and F4.1. Krocoxanthin was found in F3.2, alloxanthin was identified in F3.3, and pyrhoxanthin was identified in F4.2.
To generate 61 maximum absorption peaks, the findings of one column chromatography fraction were separated using HPLC with a propagation time of 50 minutes. The absorption spectra of each peak from the HPLC chromatogram were identified at 463 nm, and those with an area per-centage greater than 3% were investigated by examining the spectral pattern using an HPLC UV-Vis absorption spectrophotometer
The HPLC fraction one findings showed eleven absorption peaks. Table 1 shows the absorption generated at the highest peak. The greatest absorption peak at 473 nm was determined to be a kind of didehydroastaxanthin pigment, while the maximum absorption peak at 476 nm was determined to be a type of tetrahydroastaxanthin. (Tables 1-3). The HPLC separation was carried out at a propagation time of 50 minutes and generated 65 absorption peaks in the findings of the second fraction column chromatography.
| No | Retention time/Peak no. | Maximum absorption peak | Pigment type |
|---|---|---|---|
| 1 | 3.376/8 | 373-467 | - |
| 2 | 6.231/14 | 373-467 | - |
| 3 | 6.907/15 | 477 | Dihydroastaxanthin |
| 4 | 8.281/16 | 425-448-472 | - |
| 5 | 9.643/17 | 420-451-477 | - |
| 6 | 24.701/34 | 481 | Adonixanthin |
At a wavelength of 436 nm, the absorption spectrum region with a larger percentage results in the formation of a maximum absorption peak. The highest absorption in Table 2 helps determine the kind of carotenoid pigment.28
Six spectrum peaks were identified from the separation of nine absorption peaks by HPLC. Only five types of pigments could be identified using the six peak spectral pattern. The HPLC separation resulted in the formation of an absorption peak with a peak area of 3%in Table 3. The greatest absorption peaks were discovered by the absorption of HPLC UV-Vis spectrophotometer, namely the 8th, 14th, 15th, 16th, 17th, and 34th absorption peaks, allowing the kind of pigment to be determined. Figure 1 presents the visualization of the compound structure of the pigment types produced by the HPLC analysis.
The value of the bond energy is generated from the total final intermolecular energy, namely from van der Waals bonds, hydrogen bonds, de-solvation energy, and electrostatic energy, then from the final total internal energy, tor sional energy and unbound system’s energy. This summa tion of energy is usually known as the estimated free energy of binding (EFEB). The inhibition constant was also estimated by using Autodock 4.2.6 at 298 K. The low value of the inhibition constant was related to the biological activity.29
The carotenoids discovered by HPLC (Figure 1) were used as test ligands for binding proteins that contribute to skin aging. According to Table 4, after compared to other carotenoid compounds, adonixanthin had the lowest binding energy with -6.61 kcal/mol and the highest inhibition constant with 14.23 mM. The binding energy of these carotenoids, however, is not better than the native ligand (-7.83 kcal/mol with the lowest inhibition constant of 1.81 mM). The visualization of molecular docking results is shown in Figures 2-4.
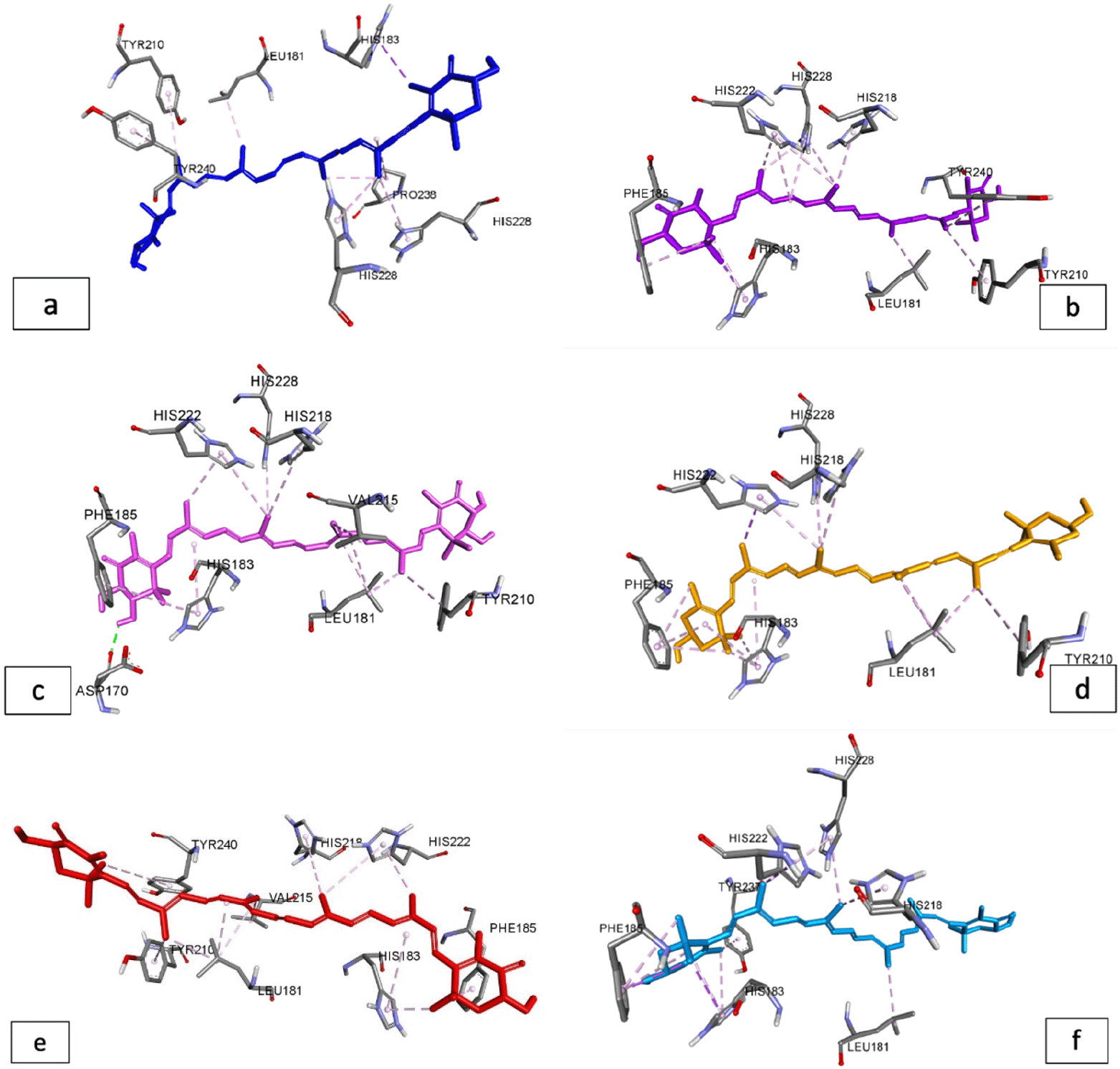
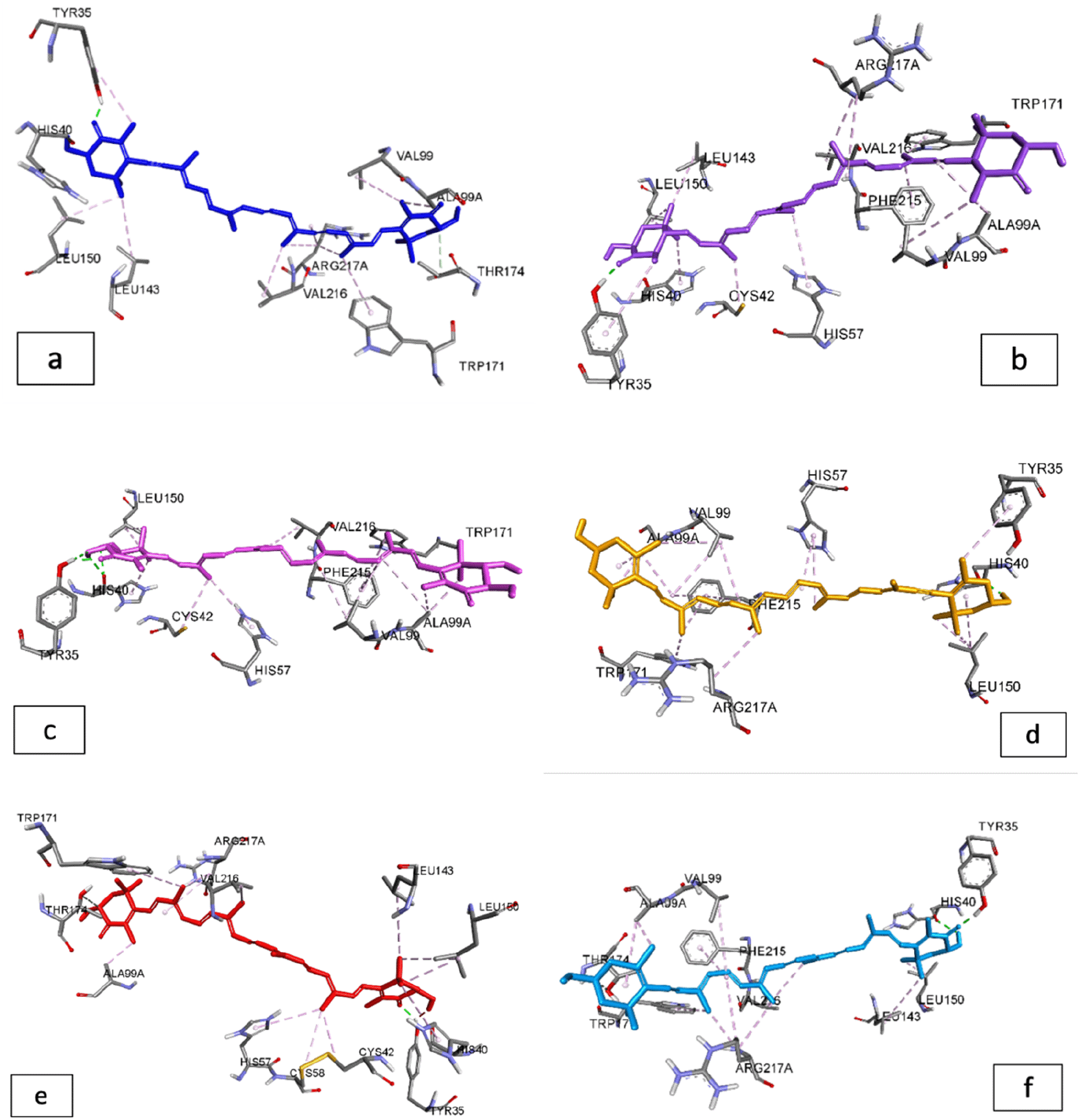
According to the results of the interaction between carotenoids and the protein elastase, carotenoid pigments have a higher binding energy than natural ligands, which only have a binding energy of -5.55 kcal/mol and an inhibition constant of 85.31 mM (Table 5). Adonixanthin and astaxanthin have the highest binding energies, with binding energies of -9.03 kcal/mol and -9.01 kcal/mol, respectively.
The carotenoid compounds from G. albolineatus had a lower binding energy than the natural hyaluronidase ligand, which had a binding value of -4.17 kcal/mol. Astaxanthin had a binding energy of -2.27 kcal/mol and is only bound to four amino acid residues, whereas didehydroastaxanthin had a binding energy of -7.71 kcal/mol (Table 6).
G. albolineatus is a large crab with a large population along the rocky intertidal zone.30 G. albolineatus can be found off the coast of Kalasey, Manado Bay, North Su-lawesi, in Indonesia. Carotenoid pigments, such as carotene, echinonen, and cantaxanthin, are found in hemocyanin, hepatopancreas, epidermal layer, and outside the carapace in male G. albolineatus from Indonesia.17
Based on HPLC results from the carapace of the female G. albolineatus, several types of carotenoids were found, including didehydrodiastaxanthin, tetrahydroastaxanthin, dihydroastaxanthin, diatoxanthin, astaxanthin, and adonixanthin. Identification of carotenoids from female G. albolineatus is a novelty.
Carotenoids provide effective antioxidant protection against peroxyl radicals during the photo-oxidation process. Carotenoids have a maximum wavelength of 450 nm due to their compound structure.11,31 Several studies have shown that people with high levels of carotenoids in their skin have skin that looks younger than their age, while those with low levels of carotenoids have skin that looks older.32 This is presumably because skin aging is linked to UV radiation, and carotenoids are thought to be effective radical scavengers.33
Skin exposition to high levels of free radicals can activate collagenase, elastase, and hyaluronidase enzymes which cause a decrease in collagen, elastin, and hyaluronic acid, causing aging of the skin. Molecular docking studies have been used to figure out how carotenoids suppress the enzymes collagenase, elastase, and hyaluronidase, and Table 5 show the binding energy of each component. Figures 1-3 show an image representation of the bonding that occurs. Native inhibitors had a higher binding energy in collagenase protein (1cgl) than carotenoids derived from G. albolineatus. This is because the native nhibitors interact with the amino acid residues His222, His218, and His228 via hydrogen interactions.29,34
Meanwhile, the interaction between these residues in the interaction between carotenoid compounds and the collagenase receptor is only found in hydrophobic interactions. Torsion energy is another factor that influences binding energy. A positive value for the torsional energy between carotenoids’ interactions that is too large is thought to have an effect on the small binding energy produced. However, adonixanthin (Table 4) has a relatively high binding energy and an inhibitory value of 14.23 mM, which determines the bioavailability of this component for use as a drug29 (Figure 2 and 3).
According to the literature, elastases are serine proteases whose main function is the cleavage of peptide bonds of many proteins, including elastin, which is responsible for the elasticity of connective tissues and is primarily found in the lungs, arteries, and ligaments.35,36 It has been identified that binding to Tyr35, His40, and Val216 has a significant influence on the strength of the binding energy between the ligand and the target receptor in the molecular docking interaction between carotenoids and elastase. The docking results revealed that astaxanthin and adonixanthin had the highest binding energies, i.e. -9.01 and -9.03 kcal/mol, respectively. Based on binding energy results, the carotenoid compounds from G. albolineatus in Table 6 can be used as elastase inhibitors when compared to native inhibitors (Figure 3).
In the interaction that occurs between the complex ligand and protein hyaluronidase, from the results when compared with native inhibitors, residues Tyr227, Phe46, and Pro18 are thought to have an effect on increasing the binding energy of the complex formed, and hydrophobic bonds are preferred. The absence of interaction between native and Pro18 reinforces that the interaction that occurs has a major influence on the resulting bond energy. In addition, astaxanthin only produced a binding energy of -2.27 kcal/mol. As a result of this discovery, it is possible to conclude that astaxanthin has an excessively large inhibition constant, resulting in a low bioavailability value.9 Meanwhile, other carotenoids with high binding energy, such as didehydroastaxanthin, tetrahydroastaxanthin, diatoxanthin, and adonixanthin, have the potential to be used as natural inhibitors of hyaluronidase protein. Because the test ligands’ interactions are anchored in the same location as the active site, these compounds can be used as competitive inhibitors of collagenase, elastase, and hyaluronidase proteins.
The pigment content of the carapace was 4.46 mg based on quantitative measurement of total pigment extraction in female G. albolineatus. Qualitatively, the carapace was recognized as containing carotenoid pigments such as didehydroastaxanthin, tetrahydroastaxanthin, dihydroastaxanthin, diatoxanthin, and astaxanthin based on HPLC. Carotenoids are naturally occurring antioxidants that play a vital role in skin regeneration. According to molecular docking results, carotenoid compounds from G. albolineatus are stronger inhibitors than native inhibitors, which competitively bind to the same catalytic residues on elastase and hyaluronidase protein. However, in vivo and in vitro studies are required to confirm this approach so that this data may be utilized as a contender for medication prospects, particularly in the cosmetic area.
Figshare: Potential Carotenoids as Anti-aging from Female Grapsus albolineatus, https://doi.org/10.6084/m9.figshare.19947758 37
This project contains the following underlying data:
- hasil docking-Dr. darus.xlsx
- dock1cgl-native.dlg
- dock4ym9-native.dlg
- dockasta1cgl.dlg
- dockasta4ym9.dlg
- dockbeta1cgl.dlg
- dockbeta4ym9.dlg
- dockdehydro1cgl.dlg
- dockdehydro4ym9.dlg
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication)
We express our deepest gratitude for the financial support of Sam Ratulangi University, Manado, Indonesia, through the Excellent University Fundamental Research (RDUU) Scheme, Fiscal Year 2019
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chemistry of natural products and their biological applications
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 2 (revision) 14 Apr 23 |
read |
|
Version 1 08 Dec 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)