Keywords
Quality of life, Cholangiocarcinoma, medicinal cannabis, Northeastern Thailand
This article is included in the Oncology gateway.
Quality of life, Cholangiocarcinoma, medicinal cannabis, Northeastern Thailand
Cholangiocarcinoma (CCA) is a rare liver tumor worldwide (incidence <6 cases per 100,000), but, is highly prevalent in parts of Thailand.1 The Northeastern (Isaan) region of Thailand has > 85 cases per 100,000, especially in Khon Kaen Province with 118.5 cases per 100,000 populations. Which is 100 times higher than the global rate.2 Typically, CCAs are asymptomatic in the early stages, and are consequently not diagnosed until late stage when the cancer has already metastasized, severely limiting effective therapeutic options and becoming a major cause of mortality.3 The median post-surgery survival of CCA patients is about 4–5 months4; 5 and the 6-month survival is only 35%.5 The major surgical treatments are surgical resection and/or liver transplantation, whereas chemotherapies are virtually palliative given the typical late-stage diagnosis and marked chemo-resistance of this cancer.6
Quality of life (QOL) is a broad multidimensional concept which includes subjective evaluations of both positive and negative aspects of life7 Health Related QOL (HRQoL) scales can be used as clinical assessments for new treatment efficacy or for well-being before and after an intervention, and have been used with hepatobiliary cancer.8
Medical cannabis has been used in palliative care to alleviate pain, relieve nausea and stimulate appetite,9 and has been shown to attain good symptom control and reduce the number of palliative care drugs used.10 A recent oncology study on the short-term outcomes of medical cannabis showed significant improvement in multiple symptoms between baseline and one-month follow-up, including reductions in pain intensity, affective and sensory pain, improved sleep quality and duration and reduced cancer distress, and both physical and psychological symptom burden.11 However, no studies have yet compared the quality of life of patients with advanced CCA treated with, either, a standard palliative-care treatment protocol, or, with medical cannabis.
Thailand was the first country in Southeast Asia to approve cannabis for medical treatment.12 Currently, in Thailand, there are two palliative care treatment protocols allowed for cancer patients; standard treatment regime, and medical cannabis treatment. As there is no comparative Thai research yet on HRQoL from the perspective of patients, this study was designed to compare the perceived quality of life outcomes between the two treatment protocol options.
This was a prospective cohort study using face-to-face interview questionnaire data from 72
The EORTC QLQ-C30 was used to assess patients with palliative cholangiocarcinoma. In the group with similar characteristics to the sample of both groups of 15 people each, the data were the mean and the variance, entering the aforementioned formula. by doing a pilot study
∆ = different QOL both broup = 10.0
α = α error = 0.05 (1.65)
β = β error = 0.2 (test power 0.80) (0.84)
Samples were used in each study group of 30 people.
When calculating the total sample size, which was the sample in the 4th month data collection, but because it was a forward study and the selection of target groups to participate in the study was Liver and bile duct cancer patients with a PPS score greater than or equal to 50 predictive of life expectancy greater than or equal to 60 days and from the literature review, it was found that The median survival for cholangiocarcinoma patients after palliative treatment was 4 months (95%CI = 3.6-4.6). Survival rates after palliative treatment were 3 and 6 months. equal to 59 percent (95%CI = 55.5-62.6), 39 (95%CI = 35.1-42.1) (Nat Thanyahan, 2013) The researchers estimated the likelihood of the target group dying while collecting data in the fourth month was 50%. Therefore, the study sample size was increased to 30 plus 15 per group equal to 45 people in the first sample data collection. that have completed until the end of the data collection and analysis of the study results must have a sample of more than or equal to 30 people (Figure 1).
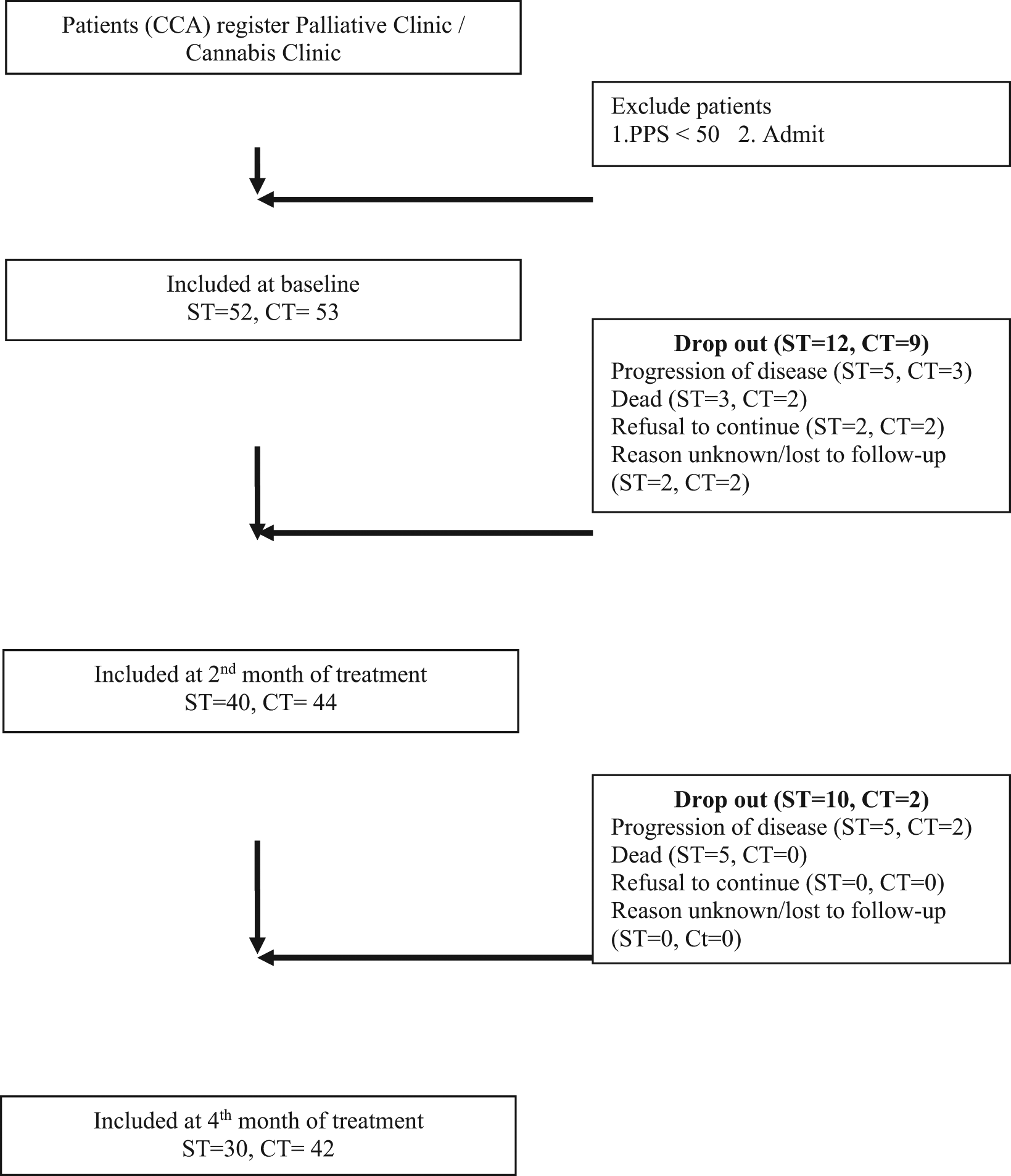
CCA = Cholangiocarcinoma; PPS = Palliative performance scale; ST = Standard treatment; CT= Cannabis treatment.
CCA out-patients receiving either a standard palliative care treatment regimen, or medical cannabis treatment. Participants were recruited between September 2019 to 31st October 2020 from the four tertiary hospitals and two of the secondary hospitals serving five provinces of Northeast Thailand (Roi-Et Regional Hospital, Burirum Regional Hospital, Surin Provincial Hospital, Sawang Dandin Crown Prince Hospital, Panna Nikhom Hospital and Pana Hospital). They were recruited by clinicians upon late-stage CCA diagnosis, based on a diagnostic result from at least one of six procedures namely; ultrasonography (U/S), computerised tomography (CT), magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangio -pancreatography (ERCP), and histology.13 and PPS score > 50. Patients had treatment plan options explained to them, such as chemotherapy, surgery, radiation therapy, morphine/other pain relief, or medical cannabis treatment and made their own selection. Depending on their choice, patients were then referred to either the hospital’s palliative care clinic or the medical cannabis clinic for treatment management. Medical cannabis clinic services are available in all hospitals affiliated with the Ministry of Public Health in Thailand. Doctors and patient care teams are trained and registered to prescribe using cannabis pre-assessment, treatment, and follow-up side effects and adverse reactions guidelines (http://www.lpnh.go.th/files/cmorph/SR.pdf). The medical cannabis treatment protocol has 3 regimens: 1) THC (1 ml = THC 12.5 mg), 2) THC: CBD 1:1 (1 ml = THC 27mg: CBD 25mg), 3) DTAM GANJA OIL (DEJA) (1 ml = THC 2 mg ± CBD 0.5 mg) (https://cannabis.fda.moph.go.th/) A doctor, or Thai traditional medicine practitioner is the one who prescribes treatment according to the guidelines.
Ans = Initiation of medical cannabis extracts in end-stage patients with various diseases who have never have been extracted from cannabis before (cannabinoids naive) is a small dose of medicinal cannabis extract to stimulate. The endocannabinoid system is based on three dosing principles (Reference: Cyr C, et al. Cannabis in palliative. care: current challenges and practical recommendations. Ann Pall Med 2018;7:463-77.)
1. Start low: no exact starting dose. depending on the characteristics of each patient
Initiate the smallest amount of medication that can be divided into individual products. The frequency or number of doses depends on how many hours can each medicinal cannabis extract be active in the body to manage pain, patients must be given medicinal cannabis extracts. to be effective continuously for 24 hours (around the clock)
2. Go slow: adjusting the drug slowly until the appropriate dose is obtained. with symptom assessment designated as indications for individual use of medicinal cannabis extracts according to advance care plans If still does not improve, increase the dose gradually every 1-2 days (titration) until the effective treatment of the symptoms of interest. This should be adjusted. Medical cannabis extract dosage causing the patient to feel that they have a satisfactory quality of life within the period about 2 weeks If the patient has symptoms of dizziness, loss of coordination, slow heartbeat, abnormal blood pressure, disorientation, agitation, hallucinations from medical cannabis extracts stop increasing the cannabis extract dosage and reverse the dose. Go down one step and assess the symptoms that are indicated as indications. Case of cannabis extract used causing side effects ineffective in treating the symptoms according to the indications prescribed or not likely to improve at all within a period of time of the 2-week dosage adjustment. The use of medicinal cannabis extracts in patients should be discontinued by a slow reduction in dosage every 1 to 2 days (tape off)
3. Stay low: maintain dosage and dosage of medicinal cannabis extract at patient levels. Feeling that the symptoms that are specific to the individual indication have decreased The patient had a satisfactory quality of life. If the symptoms are indicative. If recurring, consider taking a 48-hour break from medicinal cannabis extracts, known as drug holidays. By reducing the dose slowly over about 1 week to prevent the side effects of stopping the cannabis extract. Sudden medical After stopping the drug for approximately 48 hours, resume using the medical cannabis extract. Again in small quantities and gradually increase the drug with the same method
Procedure for prescribing cannabis extract in end stage palliative patients
1. Patients with terminal diseases who are likely to benefit from the use of cannabis extracts When there is no prohibition to use is metastatic or recurrent cancer, code C00-C96, emphysema (COPD), ICD-10, code J44.and full AIDS patients, code B22-B24, undergo a drug risk assessment screening at a cannabis clinic. no risk found
2. Consulting the palliative care team
3. Palliative care team make family meeting and advance care plan. Evaluate 11 Edmonton Symptom Assessment System (ESAS) symptoms and side effects.from cannabis extract Scored according to the patient's feelings at the time of assessment, from 0-10, the system must force the assessment. Every symptom, every time, the system collects data for each symptom in numerical base. and displayed in colors or symbols for convenience to adjust medication
1. There is no exact starting dose for each cannabis product. The appropriate dosage depends on characteristics of each patient and adjusted for each product. Start with a low dose and increase the dose slowly until the dose is reached. suitable for the highest therapeutic effect and the lowest side effects Low doses are less likely to cause side effects (http://www.mhso.dmh.go.th/fileupload/20210129308824218.pdf)
We assessed quality of life with the EORTC QLQ-C30, QLQ-BIL-21 before treatment commenced and re-evaluated at the end of the 2nd and 4th month of treatment. We excluded patients when Palliative Performance Scale (PPS) scores were less than 30 (PPS<30 median survival time of 13 day)14 at the 2nd and 4th month, or, when patients were admitted to hospital, patients died, refused to continue, or, were lost to follow-up for unknown reasons.
The QOL data was collected using the EORTC QLQ-C30 (version 3.0), and the QLQ-BIL21. The EORTC QLQ-C30 (v 3.0) is one of the most widely used patient self-report quality of life outcome scales. The EORTC QLQ-C30 assesses five functional domains: ambulation ability level, extent of disease, self-care, food and fluid intake, and level of consciousness. PPS is measured in 10% decrements from 100% (fully ambulatory and healthy) to 0% (death). It is simple and practical for palliative care patients15 and one of the most studied prognostic tools in palliative care, and is in common use in Thailand.16 The QLQ-BIL21 contains: 3 single item assessments relating to treatment side effects, difficulties with drainage bags/tubes and concerns regarding weight loss, in addition to 5 proposed scales: eating symptoms (4 items), jaundice symptoms (3 items), tiredness (3 items), pain symptoms (4 items) and anxiety symptoms (4 items).17 The scores were linearly transformed to a score between 0 and 100. For the functional and global QoL scales, a higher score indicates better health. For the symptom scales, a higher score indicates more symptom burden.18
Patients were divided into two groups according to their scores; those who scored < 33 on the functional scales and on the global QoL were considered to have low QoL, while patients who scored > 66 were considered to have higher QoL. For symptom scales, the scoring was reversed, i.e., those who scored < 33 were considered in better health and the patients who scored >66 had high symptom burden.19
The QOL data was collected by specially trained research assistants in each hospital at the time of enrollment into the study, and by the researcher in hospital, or, in the community among survivors at 2 and 4 months’ post-treatment. Research monitoring and quality control procedures included the researcher observing research assistants’ first interview before treatment and researcher carrying out follow-up interviews at 2 and 4 months post-treatment commencement.
Descriptive statistics was used to present patients’ demographic characteristics. Percentages were used to describe categorical data and means with standard deviations or medians with ranges were used to describe continuous data. The Mann-Whitney U-test was used to compare quality of life scores between the two patient groups and Wilcoxon signed rank test performed to compared QoL score within groups at pre-treatment, 2, and 4 months. All calculations were carried out using SPSS (v.24).
This research was approved by the Maha-Sarakham University Human Research Ethics Committee (Reference NO.204/2563) and Roi-Et Regional Hospital, and Burirum Regional Hospital (Reference RE064/2563) Ethics Committees for Human Research, based on the Declaration of Helsinki and the ICH Good Clinical Practice Guidelines Written informed consent was obtained from all patients.
Initially, 52 patients chose a standard treatment (ST), and 63 patients chose medical cannabis treatment (CT), all with Palliative Performance Scores (PPS) > 50. However, by the four-month follow-up point, there were only 72 patients remaining (21 dropped out at the 2nd month, and a further 12 at 4 months), leaving 30 patients in the ST group and 42 in the CT group, respectively. In total, 30 patients were in the ST group (15 males and 15 females) and 42 patients in the CT group (27 males and 15 females). Their mean ages were 66.03 (S.D. = 11.46) and 65.80 years (S. D. = 10.55), respectively. Most patients were single/widowed (66.64%/59.52%) and worked in agriculture (50.0%/52.38%). Mean PPS scores were 79.33 (S.D. = 5.83) and 80.23 (S.D. = 12.78), respectively. Table 1 displays fuller demographic characteristics of the 72 CCA patients.
Comparison of variables in EORTC QLQ-C30, QLQ-QLQ-BIL21 at the pre-treatment, 2nd and 4th month treatment (Table 2).
| variables | Pre-treatment | 2nd month treatment | 4th month treatment | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard | Cannabis | P value | Standard | Cannabis | P value | Standard | Cannabis | P value | |
| PPS | 79.33(5.83) | 80.23(12.78) | 0.813 | 59.00 (9.59) | 80.24(12.94) | <0.001* | 41.66 (18.76) | 68.09(26.79) | <0.001* |
| Min, max | 70, 90 | 60, 100 | 50, 80 | 60, 100 | 20, 80 | 20, 100 | |||
| EORTC QLQ-C30 | |||||||||
| Global health status/QoL | 67.77(8.39) | 67.26(14.06) | 0.831 | 58.05(9.15) | 67.85(19.17) | 0.010* | 41.66(11.58) | 65.27(27.16) | <0.001* |
| Functional scales | |||||||||
| Physical functioning | 84.00(12.81) | 78.73(22.19) | 0.541 | 60.22(17.19) | 67.93(33.93) | 0.096 | 26.66(25.37) | 62.06(41.68) | 0.002* |
| Role functioning | 92.77(11.31) | 86.11(18.73) | 0.143 | 73.89(18.40) | 74.60(32.35) | 0.355 | 39.44(33.18) | 66.26(40.73) | 0.007* |
| Emotional functioning | 76.94(14.79) | 73.01(25.88) | 0.831 | 75.55(12.36) | 80.75(21.81) | 0.035* | 76.66(15.53) | 85.11(18.90) | 0.017* |
| Cognitive functioning | 93.88(10.24) | 86.50(16.14) | 0.046* | 86.66(12.68) | 80.15(23.92) | 0.459 | 62.22(25.86) | 78.57(26.36) | 0.007* |
| Social functioning | 68.88(17.90) | 75.00(22.16) | 0.263 | 61.66(17.03) | 76.54(23.89) | 0.007* | 48.33(24.89) | 76.98(27.77) | <0.001* |
| Symptom scales | |||||||||
| Fatigue | 28.51(5.59) | 33.86(25.02) | 0.301 | 52.59(14.57) | 29.36(26.06) | <0.001* | 78.14(21.13) | 27.77(33.22) | <0.001* |
| Nausea & vomiting | 2.22(7.23) | 8.53(18.85) | 0.215 | 2.22(7.23) | 4.36(15.20) | 0.964 | 1.66(5.08) | 2.38(11.38) | 0.454 |
| Pain | 26.66(10.35) | 28.57(23.07) | 0.999 | 13.88(14.57) | 21.42(21.55) | 0.186 | 17.22(16.65) | 17.85(16.20) | 0.950 |
| Dyspnea | 7.77(14.33) | 11.90(16.16) | 0.236 | 18.88(16.79) | 21.42(24.21) | 0.839 | 37.77(24.34) | 17.45(25.75) | 0.004* |
| Insomnia | 17.77(22.71) | 32.53(33.03) | 0.540 | 36.66(20.24) | 20.63(23.22) | 0.004* | 50.55(27.85) | 15.87(22.37) | <0.001* |
| Appetite loss | 31.10(21.32) | 30.15(20.57) | 0.841 | 34.44(8.45) | 23.80(25.80) | <0.001* | 82.22(25.86) | 22.22(27.21) | <0.001* |
| Constipation | 16.66(16.95) | 14.28(22.22) | 0.364 | 43.33(19.86) | 14.28(19.67) | <0.001* | 81.11(24.26) | 13.49(19.56) | <0.001* |
| Diarrhea | 4.44(11.52) | 5.55(14.56) | 0.847 | 9.99(15.53) | 6.34(15.15) | 0.139 | 4.44(11.52) | 3.96(10.92) | 0.888 |
| Financial difficulties | 22.22(23.70) | 26.19(28.06) | 0.686 | 29.99(25.29) | 24.60(26.60) | 0.265 | 41.11(35.75) | 23.01(27.03) | 0.026* |
| EORTC QLQ- BIL 21 | |||||||||
| Eating scale | 20.00(13.05) | 25.00(20.82) | 0.422 | 45.55(14.79) | 25.79(20.14) | <0.001* | 81.38(18.52) | 33.72(28.80) | <0.001* |
| Jaundice scale | 5.55(11.94) | 8.20(10.71) | 0.114 | 4.07(10.30) | 8.99(14.14) | 0.069 | 2.22(6.12) | 9.78(18.39) | 0.052 |
| Tiredness scale | 28.51(11.18) | 26.45(24.77) | 0.316 | 53.33(14.99) | 29.09(27.32) | <0.001* | 88.51(18.10) | 34.12(32.22) | <0.001* |
| Pain scale | 16.38(10.37) | 19.04(14.98) | 0.399 | 18.61(8.38) | 18.25(16.38) | 0.551 | 23.61(9.04) | 19.64(19.36) | 0.120 |
| Anxiety scale | 26.37(15.93) | 35.91(26.89) | 0.148 | 35.27(11.72) | 32.53(26.21) | 0.314 | 39.99(16.58) | 32.73(27.38) | 0.114 |
| Treatment side-effects | 29.99(20.24) | 35.71(29.80) | 0.426 | 26.66(16.14) | 30.95(30.70) | 0.854 | 16.66(20.99) | 29.36(28.70) | 0.63 |
| Drainage bags/tubes | 2.22(8.45) | 4.76(13.91) | 0.43 | 5.55(12.63) | 7.14(15.67) | 0.729 | 4.44(11.52) | 9.52(19.87 | 0.452 |
| Weight loss | 21.11(16.33) | 23.80(31.48) | 0.674 | 41.10(14.34) | 19.84(24.48) | <0.001* | 72.22(19.73) | 20.63(26.49) | <0.001* |
At baseline, there were no statistically significant PPS, QoL, or symptom differences between the two groups on QLQ-C30, and QLQ-BIL21 except for higher cognitive functioning in the ST group over the CT group (p = 0.046).
All global health status and functional scores were >66, indicating higher QoL, while all symptom scores were moderate to low. Higher symptom scale scores indicate lower QoL.
The CT group had statistically higher scores for both PPS and QoL than the ST group after 2 months (80.24 ± 12.97; 59.00 ± 9.59, p = 0.010).
In the functional domain, emotional and social function scores were also statistically significantly higher than for the CT group over the ST patients. The CT group now also had statistically significantly lower scores (better QoL) on 7 symptom scales: fatigue, insomnia, appetite loss, constipation, eating, tiredness and weight loss.
Four months after treatment commenced the CT group again had statistically significantly higher PPS and QoL scores than the CT group (68.09 ± 26.79; 41.66 ± 18) (p < 0.010). Most notably, the CT group now also had significantly higher scores on all five Global Health Status QoL function scales: physical, role, emotional, cognitive and social functioning. The CT group also had statistically significantly lower scores (better QoL) on 8 symptom scales: fatigue, dyspnea, insomnia, appetite loss, constipation, eating, tiredness and weight loss.
To our knowledge, this is the first multi-center study comparing the effects of standard palliative care versus medicinal cannabis treatment on QoL on patients with advanced CCA. The two groups had little difference at baseline but at 2- and 4-month follow-ups the CT group consistently self-reported higher global health status and functional behaviors, and lower illness/symptom-related scores than the standard palliative-care group.
The CT regimen for our CCA patients was associated with meaningful improvements in health-related QoL consistent with previous improvements seen in pain reduction, quality of life, social life, and activity levels with other chronic pain patients.20 From the second month of treatment our CT patients rated improvements in fatigue levels, insomnia, appetite loss, constipation, eating tiredness and weight loss. There was also a significant improvement in dyspnea at the fourth month, maybe due to cannabis’ beneficial effect on appetite, sleep and rest.21 Other cannabis studies have shown more than 10% weight gain,22 and cancer patients feeling refreshed, less fatigued and having reduced side-effects such as nausea, vomiting, and loss of appetite from treatment.23 Cannabinoids can also stimulate a therapeutic response via immune response enhancement.24 This is relevant due to current standard chemotherapy treatments which increase inflammation in all body systems with consequent intellectual impairments including attention, memory, decision making, risk management, fatigue, lack of motivation and peripheral nerve inflammation.25 Cannabis or its derivatives have been widely used by patients with advanced cancer to help with cancer symptoms and treat side effects24 and patients affirm its use for pain, anxiety, depression, and significantly prefer it over antianxiety medications.26 It is also reported as useful for nausea, sleep, appetite stimulation,26 and even at one-month follow-up, most parameters have been shown to improve significantly from baseline, including pain intensity, affective and sensory pain, sleep quality and duration, cancer distress, and both physical and psychological symptom burden.11
We found no inter-group differences in pain ratings between the second- and fourth-month follow-ups. Pain is one of most common symptoms associated with cancer27 and is one of the symptoms patients fear most.28 Unrelieved pain denies patients comfort and greatly affects their activities, motivation, social interactions, and overall quality of life.28 Pain management goals are, therefore, is to reduce pain to a level that allows for an acceptable quality of life, and both CT and ST treatments appeared to do this satisfactorily. The benefit of pain relief must be balanced against the risk of adverse side-effects and overdose. Opioid analgesics are essential for the adequate treatment of moderate and severe cancer pain, however, despite good opioid control of baseline pain, some patients do have short-term, short-lived, intense pain episodes. A recent study on cannabis palliative care in oncology patients, had similar findings, suggesting that medical cannabis reduced chronic or neuropathic pain in advanced cancer patients29 with significant improvements in other assessed parameters, including reduced pain intensity, improved sleep, and a decrease in pharmaceutical analgesics consumption.11
There was no inter-group difference in anxiety ratings between the second- and fourth- month follow-ups for our patient groups. Anxiety is common in cancer patients and greatly influences survival rate, adherence to treatment, and quality of life. Advanced stage cancer patients, and those with metastasis, are more likely to have higher levels of anxiety than those with no sign of metastasis.30 Some research on cannabis use in surviving cancer patients has found it alleviated 41.6% of anxiety symptoms,44 however, research on cannabis use for anxiety and depression is currently limited, and there are many confounding factors. In addition, depression and anxiety are both normal and common responses to a cancer diagnosis, and therefore diagnosing clinical levels of anxiety and studying treatments to address these levels in the context of cancer is difficult.31
In addition to quality of life outcome differences between ST and CT, research also shows a range of potential patient CT palliative-care access issues. Most patients need oncologist or primary care physician consultations. Thus, the primary care physician needs to be qualified and have good knowledge and a positive attitude towards the use of cannabis treatment among these patients. In an Australian study, most doctors felt their own knowledge was inadequate and only 28.8% felt comfortable discussing medicinal cannabis with patients. GPs generally rated their medical cannabis knowledge as poor and shared care arrangements with a specialist, though supported medical cannabis use in chronic cancer pain and palliative care.32 Four themes were found to underpin reluctance to authorize medical cannabis in Canada; presumed lack of evidence, indications for therapeutic use, discomfort with therapeutic use and practitioner’s openness to emerging evidence.33 Importantly, patients deciding to use cannabis to alleviate cancer symptoms, desired the approval from their medical team. While some patients found their physicians were willing to prescribe cannabis, or, refer them to a medical cannabis expert, some found their physicians were unwilling to discuss a cannabis option for managing their cancer symptoms34 and, were not ready, or, did not want to answer patients’ questions about medical cannabis.35 Some clinicians feel hampered by a lack of clinically relevant information about cannabis use, efficacy, side-effects and have difficulty discussing the medicinal benefits of cannabis in a clinical settings.36 Another study found only 30% of oncologists felt sufficiently informed to make recommendations regarding medical cannabis treatment.21
Most patients with advanced cancer experience symptoms throughout the disease trajectory, often with greater intensity as death approaches. If poorly managed, such symptoms can have a considerable impact on patients’ ability to function, their quality of life and ability to comply with anticancer treatments and use of health care resources.37 Medical cannabis is another option to relieve symptoms caused by the cancer itself, direct or indirect consequences of the cancer, early or late adverse effects of treatment, and/or comorbid conditions cancer treatment, especially late stage cancer. Medical cannabis has been shown to relieve symptoms caused by cancer, to reduce chronic or neuropathic pain in advanced cancer patients38 and to improve patients’ quality of life outcomes. Past medical cannabis treatment research evidence has been inconsistent and generally limited by poor quality, with large variations in cannabis-based products limiting the ability to make direct comparisons,39 as well as studies lacking statistical power and with small subject sizes, and recommendations against the use of medical cannabis as a first or second line option for palliative cancer pain or when other treatments have failed.40 Our study, found beneficial outcomes in a range of quality of life and symptom measures for cannabis treatment over standard palliative care protocols, using standardized medical cannabis products and treatment protocols.
This study has several limitations. One is the number of patients who dropped out before study completion likely due to rapid disease progression. Most patients were elderly and suffered from advanced CCA. Newly diagnosed CCA patients typically have a poor prognosis and short-term survival due to late-stage diagnosis. Registration for the standard palliative care clinic and/or cannabis clinic in each hospital also differs across physicians. Decision-making across patients, families, at different stages of disease, organ metastasis, and for methods of treatment also varies.
To the best of our knowledge, this is the first study to compare quality of life of CCA patients who received ST or CT and were monitored before treatment commencement and at 2nd and 4th month follow-ups and, across 8 hospitals, and 5 provinces. Medical cannabis products and usage was standardized under the Thai Food and Drug Administration regulations. The side effects, safety, benefits and harms of this cannabis have been reviewed and certified for patient treatment, by trained and Thai registered prescribers of medical cannabis.
Figshare: QOL of patients with cholangiocarcinoma _ DATA, https://doi.org/10.6084/m9.figshare.17162621.v1.41
Figshare: QOL_document information, https://doi.org/10.6084/m9.figshare.17203922.v1.42
This project contains the research information sheet.
Figshare: QOL_Inform consent, https://doi.org/10.6084/m9.figshare.17203931.v1.43
This project contains the consent form.
Figshare: QOL_questionnaire, https://doi.org/10.6084/m9.figshare.17203934.v1.44
This project contains the questionnaire.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank all patients and family colleagues in the Faculty of Medicine, Mahasarakham University especially the University Hospital Center of Excellence Team palliative clinic and cannabis clinic) for their invaluable help and encouragement throughout the course of this research. Finally, the authors express their appreciation for the participation of all patients in this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Nursing and Biostatistics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Apr 25 |
read | read |
|
Version 1 10 Jan 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)