Keywords
COVID-19, Cancer, outcome, oncology, Indonesia
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Oncology gateway.
This article is included in the Coronavirus (COVID-19) collection.
COVID-19, Cancer, outcome, oncology, Indonesia
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 It is shown that 2.1% of patients with confirmed COVID-19 were reported also to have cancer.2 A meta-analysis revealed that the mortality rate for COVID-19 patients with cancer was 21.1%. As many as 45.4% of cancer patients with COVID-19 have severe or critical symptoms.2
Cancer patients are a uniquely susceptible population because most of these patients may be in a suboptimal physical condition while also requiring cytotoxic drugs that can reduce immunity. In addition to the symptoms of COVID-19, these patients' cancer treatment may also be delayed during the pandemic.3
Most observational studies have exposed that COVID-19 patients with cancer tend to have worse prognosis compared to non-cancerous COVID-19 patients.4,5 Previous research revealed that patients with hematologic cancers (HC) experienced more severe COVID-19 symptoms and higher CFR (case fatality rate) side to those with solid cancers (SC).6 More severe manifestation and higher CFR are found in hematologic cancer patients compared to solid cancer patients.
Previous research has suggested that the presence of haematological malignancies may reduce COVID-19 severity progression due to an attenuated inflammatory response.7–9 Other studies have reported that solid tumors were a worse prognosis predictor.10,11 The variation between studies and the lack of publications have encouraged us to analyze if patients with hematologic cancer and those with solid tumors would fare differently in the setting of COVID-19 infection.
The Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol PRISMA) guidelines were used to guide this study.22
This review included clinical studies (clinical trial, retrospective, or prospective) of all cancer patients who had COVID-19 infection based on polymerase chain reaction (PCR) test. Articles published between December 2019 to January 2021 in English were considered. For inclusion, the published articles must have had documentation of COVID-19 infection in both solid cancer and hematological cancer patients. Proceeding, commentaries, and editorials without a peer-review process were excluded.
We systematically searched databases to identify eligible articles using PubMed and Google Scholar for articles published from December 2019 to January 2021 using the search strategy in Table 1. We also researched references lists of relevant articles to identify additional primary studies and minimize bias.
All articles from the search strategy were screened further for eligibility. The titles and abstracts were independently screened and reviewed by three authors (FP, AR, RM). The article's technical uncertainties were resolved through discussion between all authors (FP, AR, RM, JH, RI, IB, MJ). Study assessment was based on the following criteria: 1) published in English, 2) prospective or prospective study on cancer patients with COVD-19 infection; 3) sufficient data relating to PICO (participants/interventions/comparisons/outcomes) criteria (Table 2) from COVID-19 patients with hematological and primary solid cancer.
COVID-19=coronavirus disease 2019.
The data collected were demographic details (e.g., age, race, comorbidities), type of cancer (primary solid tumors and hematological malignancy), patient's anti-cancer therapies (described as surgery, chemotherapy, radiotherapy of immunotherapy), clinical outcomes (developing severe events, hospitalization rates, intensive care unit (ICU) admission rates, 30-days mortality rate and case-fatality rate). Three authors (FP, AR, RM) extracted the data, jointly reconciled, and discussed technical uncertainties. The authors then appraise the studies using the Newcastle-Ottawa scale (NOS) for cohort studies (Table 3).2
The study selection for this review can be seen in Figure 1. In total, 20 articles were included in the analysis.
In 2020, DeMelo et al. reported that age, advanced malignancy stage, and number of metastases were associated with clinical fragility and higher risk of death in COVID-19 patients.10
A previous study explained that COVID-19 symptoms in cancer patients ranged from mild symptoms (55% of cases), which required outpatient management only (fever, cough, fatigue, myalgia, etc.) to moderate to severe symptoms (45% of cases). From these patients, 42% were admitted to the ICU.10 According to a study, a complication such as secondary infection and acute respiratory distress syndrome occurred in a majority of cancer patients (63%).4 Another study in 2020 found that patients with malignancy were prone to having COVID-19 with severe manifestation (54% vs. 35%; P=0.003). The study mentioned that severe COVID-19 symptoms upon admission are a significant risk of in-hospital death (hazard ratio=28.2).11 Two other studies reported similar findings which support that cancer is associated with worse outcome.12,13
Regarding type of cancer, Dai and colleagues reported the hematologic cancer and lung cancer group had the highest and second highest severity and death rates compared to other cancer groups. The patients with hematological malignancy have reduced immunity and are more prone to infection, which can exacerbate COVID-19 infection.8 Previous studies showed that leukemia, lymphoma, and myeloma as hematological cancer groups could increase death rate, ICU admission, critical manifestation, and invasive mechanical ventilation requirement.8
A previous study showed that patients with and without cancer had similar COVID-19 severity. In the said study, the hematological and solid tumors groups showed non-significant trends for immediate manifestation of severe events (hematological group cohort = 30% vs. solid group cohort = 61.4%).16 However, another study from Canada investigated 252 cancer patients with COVID-19 and showed that 28% of adult patients had a high mortality rate, whereas none of the patients in the pediatric cohort had a significant illness. In hospital-acquired patients with COVID-19, overall survival (OS) was shorter than those with community-acquired infection.17 Similarly, a study from the UK reported that patients with hematological cancer have a greater risk of severe COVID-19 clinical manifestation, which needs more intensive supportive interventions and poses a greater risk of death than non-cancer patients.5 Tremblay and colleagues explained that the hematologic malignancies group of patients might be vulnerable to COVID-19. The preliminary study also suggests that hematological cancer patients have higher mortality than the general population.18
Different cancer treatments including surgical, radiotherapy and COVID-19-specific medication done within 60 days before COVID-19 infection did not affect the death risk.10 Two studies reported an increased death rate in patients who received immunotherapy, surgery and chemotherapy.8,19 Robilloti demonstrated that lung cancer patients treated with immune checkpoint inhibitors (ICI) correlated with worse COVID-19 infection outcomes.17 On the other hand, patients with lung cancer who had COVID-19 had better outcomes despite having immunotherapy.13
Rivera et al. analyzed the treatments of COVID-19 in patients with cancer. High-dose corticosteroids combined with other therapies were correlated to higher mortality than positive and negative controls. Hydroxychloroquine combined with other drugs also demonstrated similar results, in which when combined, the risk of all-cause mortality every 30-day was increased when compared with the positive control (OR=2.15). On the other hand, remdesivir showed potential benefit as lower 30-day all-cause mortality compared to positive group (OR=0.41).19
Table 4 shows a summary of the included studies.
| Author | Country | Type of Study | Age (Median years) | Type of cancer | Case-fatality rate | ||
|---|---|---|---|---|---|---|---|
| Hematological cancer (%) | Primary solid cancer (%) | Hematological cancer (%) | Primary solid cancer (%) | ||||
| Antrim 202023 | US | Retrospective Study | 60.5 | 11 | 36 | 3 | 2 |
| Kuderer 202024 | US, Canada, Spain | Retrospective Study | 66 | 204 | 728 | 24 | 76 |
| Dai 20208 | China | Retrospective Study | 64 | 9 | 96 | 3 | 6 |
| deMelo 202010 | Brazil | Retrospective Study | 67.5 | 34 | 138 | 8 | 52 |
| Ferrari 202111 | Brazil | Retrospective Study | 61 | 31 | 167 | 5 | 33 |
| Fillmore 202020 | US | Prospective Study | 65 | 176 | 1483 | 30 | 200 |
| Jazieh 202025 | Saudi Arab | Retrospective Study | 66 | 9 | 10 | 9 | 7 |
| Lennard 20205 | UK | Prospective Study | 70 | 224 | 801 | 81 | 229 |
| Li 202012 | China | Prospective Study | 63 | 9 | 50 | 2 | 16 |
| Rivera 202019 | US | Retrospective Study | 67 | 470 | 1781 | N/A | N/A |
| Rüthrich 202026 | Germany | Retrospective Study | N/A | 124 | 256 | 31 | 156 |
| Shoumariyeh 202015 | Germany | Retrospective Study | 73 | 10 | 29 | 8 | 23 |
| Wang 202027 | US | Retrospective Study | 41.5 | 170 | 640 | N/A | N/A |
| Yang 20204 | China | Retrospective Study | 63 | 22 | 183 | 9 | 31 |
| Robilotti 202017 | China, Italy | Retrospective Study | 64 | 102 | 321 | N/A | N/A |
| de Joode 202028 | Dutch | Prospective Study | 70 | 111 | 208 | 43 | 62 |
| Meng 202013 | China | Retrospective Study | 64.5 | 16 | 92 | 8 | 24 |
| Elkrief 202016 | Canada | Retrospective Study | 73 | 66 | 179 | N/A | N/A |
| Tremblay 202018 | US | Prospective Study | 69 | 14 | 10 | N/A | N/A |
| Stroppa 202014 | Italy | Retrospective Study | 67 | 2 | 22 | 2 | 7 |
In total, 14 studies included detailed case fatality rates of hematological cancer and primary solid cancer groups. Overall, the case-fatality rate in the hematological cancer group was 1.22 fold higher than the primary solid cancer group (263/976 vs. 852/4373; RR 1.22; CI 95% [1.08-1.37]; P<0.001) (Figure 2).
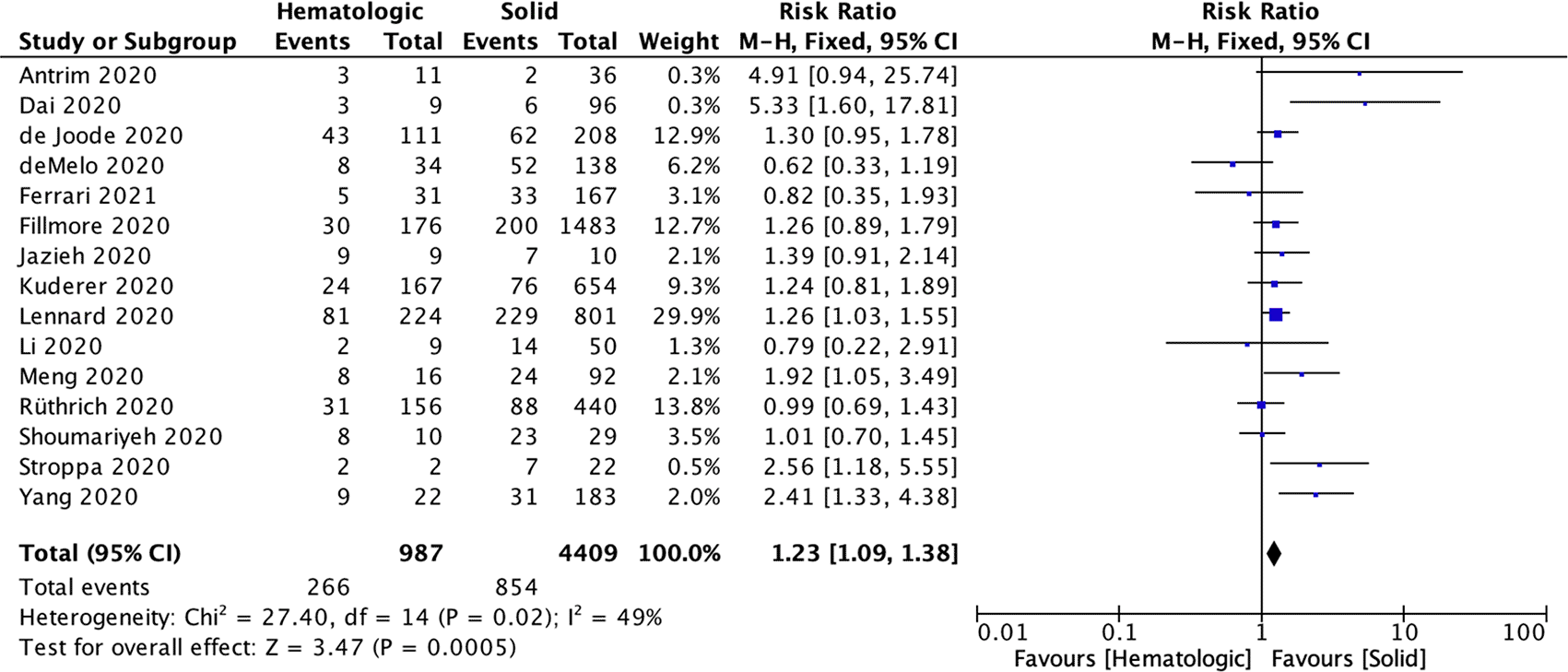
CI=confidence interval, df=degrees of freedom, M-H=Mantel Haenszel method.
We performed two sub-analyses on case-fatality rate, to determine the correlation with comorbidities and age. Two studies provided data on the patients' comorbidites (two or less comorbidities and more than two comorbidities group).10,24 Overall, the case-fatality rate in patients with two or fewer comorbidities group was 0.57-fold lower than patients with more than two comorbidities group (97/694 vs. 65/327; RR 0.57; CI 95% [0.42-0.76]; P<0.001) (Figure 3). We also calculated the pooled proportion of case-fatality rate in the cardiovascular disease group (42.5%) (Figure 4), hypertension (36.8%) (Figure 5), and diabetes mellitus (36,8%) (Figure 6), as those three were deemed the most prevalent comorbidities.
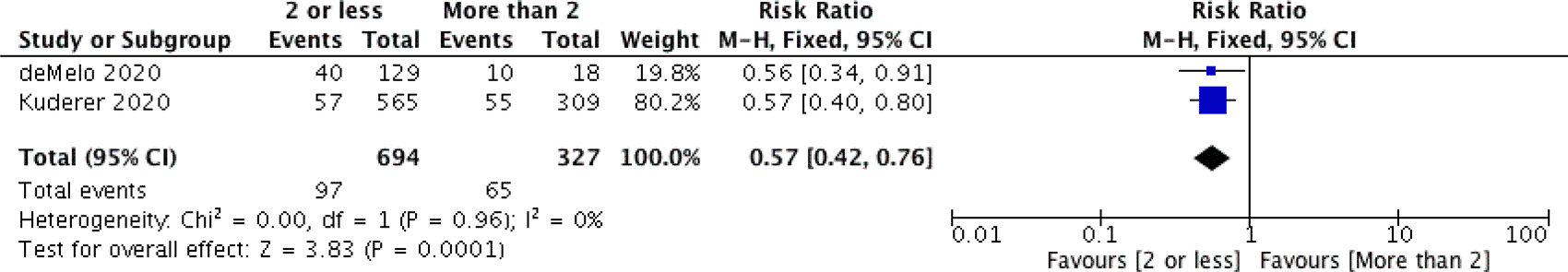
CI=confidence interval, df=degrees of freedom, M-H=Mantel Haenszel method.
In total, six studies included detailed data of elderly patients (under 75 y.o. and 75 y.o. or older) in both cancer groups. Overall, the rate of death in patients under 75 y.o. group was 0.53 fold lower than patients under 75 y.o. group (250/1350 vs. 154/465; RR 0.53; CI 95% [0.36-0.80]; P=0.002) (Figure 7).
Overall, five studies included detailed data of patients who developed critical events in the hematological and primary solid cancer groups separately. Overall, the rate of critical care events in the hematological cancer group was 1.65 fold higher than the primary solid cancer group (140/371 vs. 585/2312; RR 1.65; CI 95% [1.22-2.23]; P=0.001) (Figure 8).
To our best knowledge, the severity of COVID-19 can be worsened by cancer. The risk of death may also increase due to cancer. Patients with hematologic malignancy have an immunocompromised state which may induce co-infection and thus aggravate COVID-19 clinical presentation.4,5,8–14,17 Our meta-analysis shows that the rate of mortality and critical care events were higher in the hematologic group than in the primary solid cancer group. At the same time, the case-fatality is higher in patients who had more than two comorbidities and patients aged 75 or older. Thus, our analysis showed a tendency toward publication bias for case-fatality rate (P=0.03) (Figure 9) likely to the presence of small sample size studies.
Our analysis on critical care events seemed to differ from the rest of the study. The COVID-19 diagnosis test might cause this as both PCR and anti-SARS-CoV-2 IgG/IgM antibody tests12 were used in Li's study, whereas other studies included in the meta-analysis only used PCR for diagnostic testing. Moreover, This was a retrospective study with relatively few subjects yet with an enormous number of controls.12
The hematological cancer group had more severe COVID-19 manifestation.12 However, this finding requires further verification through multi-center studies. Based on a previous study, delaying surgery or chemotherapy for patients with cancer during the COVID-19 pandemic is not required, especially in areas with fewer COVID-19 patients.16
From our review, several studies from China, Europe, and North America reported that cancer patients with COVID-19 infection who received chemotherapy, immunotherapy, and ICI treatment had a higher death risk.4,5,8,17,20 A meta-analysis in the US reported that active cytotoxic chemotherapy was associated with a high risk of adverse outcomes from COVID-19.21 At the same time, Stroppa et al. revealed a better prognosis of COVID-19-infected lung cancer patients treated with immunotherapy.14 Similarly, Fillmore et al. reported a lower risk of infection was correlated with ICI treatment.14 A meta-analysis by Yekedüz et al. revealed that cancer treatment was not associated with severity and mortality risk of COVID-19 within the last 30 days before diagnosis.22
A COVID-19 and Cancer Consortium Cohort Study in US revealed that corticosteroids in high dose administration combined with any other therapies, and hydroxychloroquine combined with other drugs or given alone were associated with higher 30-day all-cause mortality risk in cancer patients with COVID-19 infection. While remdesivir has shown to be a potential treatment, the all-cause mortality rate in 30 days decreases.19
Limitations of this review include that the review section may have been influenced by the authors' personal viewpoints, gaps in literature searching practices may have led to the omission of relevant research, and errors in the translation of data from the primary literature to summarization in the review. There were also missing data points from some studies. Given these limitations, we encourage conducting multi-center registries (web/online-based) to obtain all the data from every individual case of cancer patients with COVID-19 infection.
Hematological malignancy, older age (75 years) and the number of comorbidities are predictors for worse prognosis in COVID-19 infection. The therapy protocol for cancer patients with COVID-19 infection and COVID-19 therapy is still debatable. Future research needs to evaluate these treatments in prospective randomized controlled trials (RCTs), address disparities, and promote studies evaluating potential anti-COVID-19 therapies.
All data underlying the results are available as part of the article and no additional source data are required.
Figshare: PRISMA checklist for 'Comparison of Clinical Outcome in Hematological Cancer Compared to Primary Solid Cancer Patients With COVID-19 Infection: a Systematic Review and Meta-Analysis, https://doi.org/10.6084/m9.figshare.17122541.29
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: neuroscience
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Systematic review, prediction model. colorectal diseases, surgery, guideline.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 16 Feb 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)