Keywords
COVID-19; critically ill; D-dimer; dose-response relationship; mortality
This article is included in the Emerging Diseases and Outbreaks gateway.
COVID-19; critically ill; D-dimer; dose-response relationship; mortality
Currently, the coronavirus disease 2019 (COVID-19) is one of the most common diseases affecting society globally, causing a sizeable number of deaths as of 2022.1 Although the majority of patients had only mild-moderate clinical manifestations, a small but significant proportion developed life-threatening complications, for example, multiple organ failure.2–4 This is especially true in patients with comorbidities, such as diabetes mellitus (DM), chronic kidney disease (CKD), and cerebrovascular and cardiovascular diseases, that are associated with increased severity and mortality from COVID-19.5–11 The cases of COVID-19 remain high and threaten to overwhelm the healthcare system, therefore risk stratification is required for prudent allocation of resources. Several biomarkers have been evaluated or repurposed to attain this objective.12–14
The importance of simple laboratory values, such as complete blood count, coagulation parameters, and inflammatory biomarkers, cannot be underestimated during the ongoing COVID-19 pandemic.15–18 These values can help predict the severity of the disease course and often indicate the need for intensive care unit (ICU) admission or mechanical ventilation. Coagulopathy is among the most frequently found complications, which is characterized by the elevation of D-dimer level and changes in fibrinogen levels and platelet counts.19,20 Although increased D-dimer levels have been consistently observed in severely and critically ill patients, the optimal cut-off level and prognostic values remain unknown.21 This study aimed to investigate the dose-response relationship between elevated D-dimer level and mortality in critically ill COVID-19 patients.
This study was conducted in accordance with the ethical standards of the Helsinki Declaration. The Ethics Committee of the Faculty of Medicine, Universitas Indonesia (date 15.06.20, No. 0593/UN2.F1/ETIK/PPM.00.02/2020) approved the study protocol. Informed consent was not obtained because the study was retrospective observational in nature.
This study was a retrospective observational study in Cipto Mangunkusumo Hospital and Universitas Indonesia Hospital, Indonesia. There were 261 critically ill COVID-19 patients requiring intensive care unit (ICU) admission between March and December 2020. COVID-19 diagnosis was based on a reverse transcriptase-polymerase chain reaction (RT-PCR) examination. We did consecutive enrollment to address any potential sources of bias. Data on the patients’ baseline clinical and laboratory characteristics were collected in a list form from the medical records in December 2020 and then analyzed using IBM SPSS Statistics version 25. We used a multivariate analysis to adjust the possible effect of confounders.
The outcome of this study was mortality, defined as clinically validated death/non-survivor. The outcome was ascertained from the medical record and confirmed by the death certificate. An independent investigator of the data collection process and patient care performed the statistical analysis. We compared the mortality rate between the patients with elevated D-dimer level and those without.
We performed the statistical analysis using SPSS 25.0 (Armonk, US) and STATA 14.0 (College Station, TX, US). We tested continuous data for normal distribution; t-test was used for normally distributed data, and Mann-Whitney test was used for abnormally distributed data. Chi-square test or Fischer-Exact test was performed for categorical variables. Normally distributed continuous data were presented as mean and standard deviation (SD); while abnormally distributed continuous data were reported as the median and interquartile range (IQR). Bivariate analysis was performed to evaluate variables. Continuous variables were compared using either independent T or Mann-Whitney U test. Categorical variables were tested by using chi-square test. Significant variables (P<0.05) were included in the logistic regression analysis. ROC curve analysis was performed to determine the optimal cut-off points for the D-dimer level. The sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-), and area under the curve (AUC) were calculated. Fagan’s nomogram was plotted to determine the posterior probability of mortality for the cut-off points determined by ROC curve analysis. The dose-response relationship between D-dimer level and mortality in the patients was explored and reported in the form of a restricted cubic spline.
This study enrolled 261 critically ill COVID-19 patients between March 2020 and December 2020 in this study. Two patients were excluded due to missing data, so 259 patients who met the inclusion criteria were included in the analysis. The mortality rate was 40.9% (106 patients). Demographic characteristics, preoperative laboratory parameters, and comorbidities of the patients were presented in Table 1. Patients in the non-survivor group were older than the survivor group. The mean ages of the non-survivor group and survivor group are 56.04 years and 46.73 years, respectively (p<0.001). There was no significant difference in gender distribution between groups. Non-survivors were more likely to have higher D-dimer levels, leukocyte levels, C-reactive protein levels, and lower thrombocyte levels. Comorbidities such as hypertension, coronary artery disease, and chronic kidney disease were more frequently found in the non-survivor group.
Median D-dimer level was higher in the non-survivor group (10,170 ng/mL vs 4,050 ng/mL, p=0.028). This association remained significant after logistic regression multivariate analysis (p=0.046), as shown in Table 2.
| Variables | P value |
|---|---|
| Age | <0.001 |
| D-dimer level | 0.046 |
| Leukocyte | 0.189 |
| Thrombocyte | 0.070 |
| C-reactive protein | 0.014 |
| Hypertension | 0.787 |
| Coronary artery disease | 0.177 |
| Chronic kidney disease | 0.315 |
The optimal cut-off level for D-dimer to predict mortality in critically ill COVID-19 patients was determined to be 9,020 ng/mL (OR 3.73 [95% CI 1.91 – 7.28], p<0.001) through ROC curve analysis. It was associated with 52% sensitivity, 78% specificity, LR+2.31, and LR- 0.62. The AUC was 0.657 (95% CI 0.573-0.740), p=0.001 [Figure 1]. In our patients, a D-dimer level of >9,020 ng/mL gave 67% posterior probability of mortality, and a D-dimer level of <9,020 ng/mL had 35% probability of mortality [Figure 2]. There was a non-linear dose-response relationship between D-dimer level and mortality with Pnonlinearity=0.004 [Figure 3].
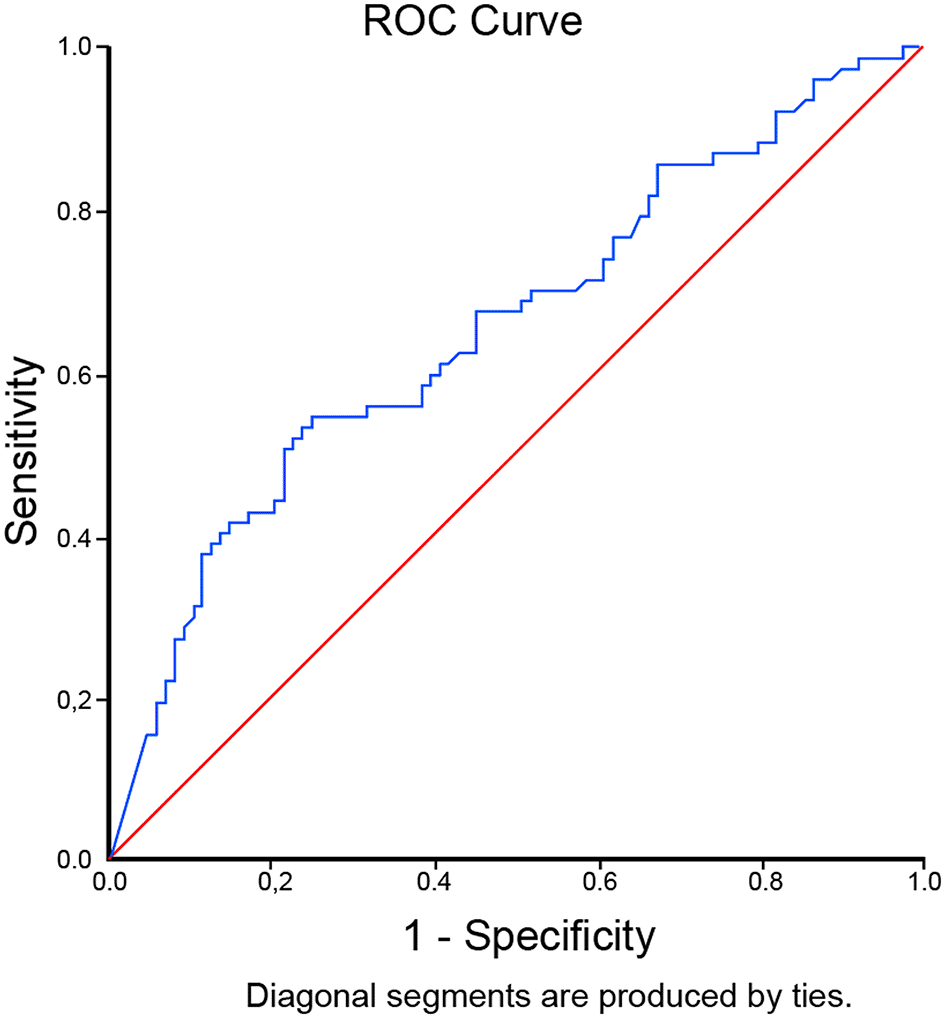
This study showed that D-dimer level has a non-linear dose-relationship with mortality in critically ill COVID-19 patients. The optimal D-dimer level cut-off point was 9,020 ng/mL. An elevation beyond the cut-off point confers to 67% posterior probability of mortality, and D-dimer level of <9,020 ng/mL had 35% probability of mortality.
D-dimer is a fragment produced when plasmin cleaves fibrin during clot breakdown. In clinical practice, D-dimer assays are frequently used to exclude a diagnosis of deep vein thrombosis (DVT) and pulmonary embolism (PE). Abnormal coagulation function, specifically blood clotting, is often indicated by an elevation in D-dimer level, reflecting a severe viral infection. Elevated D-dimer level is associated with COVID-19 progression as well as a greater death rate from community-acquired pneumonia (CAP).17,22 For this reason, testing D-dimer level on admission could be useful to stratify patients with COVID-19 early and to determine the treatment plan. A high on-admission D-dimer level might suggest a prothrombotic state, increased fibrinolysis, bleeding, and thrombotic events, and indicate cytokine storm, tissue damage, or impending sepsis.17,23
Elevated D-dimer level has been shown to be associated with mortality in COVID-19 patients.17 This study showed that D-dimer level can be used for prognostication in critically ill COVID-19 patients and also showed the non-linear dose-response relationship of D-dimer level and mortality. Elevation of inflammatory cytokines and biomarkers such as interleukin (IL)-2, IL-6, IL-7, granulocyte-colony stimulating factor, macrophage inflammatory protein 1-α, tumor necrosis factor-α, C-reactive protein (CRP), ferritin, procalcitonin (PCT), and D-dimer level were found in the systemic hyperinflammation phase.17,24 Excessive hyperinflammation consequently leads to multi-organ failure24–26; therefore D-dimer level may reflect the severity of inflammation. Additionally, SARS-CoV-2 can induce hypercoagulability,27,28 causing thrombosis in cardiovascular systems as well as myocardial injuries.29 Coagulopathy and cardiac injury results in higher mortality in patients with COVID-19,3,26,30,31 which may explain the relationship between D-dimer level and mortality in COVID-19 patients.
There is a crosstalk between blood coagulation and inflammation. The release of pro-inflammatory cytokines results in the upregulation of tissue factor (TF) expression on the endothelial cells and monocytes surfaces, which further promotes procoagulant activity.32 Moreover, hypoxia in COVID-19, CAP, or other illnesses may stimulate thrombosis by upregulating the hypoxia-inducible transcription factors which modulate the expression of various coagulation and fibrinolytic factors such as TF, tissue factor pathway inhibitor, and plasminogen activator inhibitor-1 (PAI-1).33 In addition, neutrophil extracellular traps, in which neutrophil infiltrates lung capillaries and cause fibrin deposition and acute inflammation, may exacerbate COVID-19 progression by causing organ damage and promoting thrombosis.34
International Society of Thrombosis and Haemostasis (ISTH) guideline states that an elevated D-dimer levels indicates increased thrombin production. COVID-19 patients with markedly elevated D-dimer level may require hospitalization despite the severity of clinical presentation.17,35 Prophylactic anticoagulant is recommended in hospitalized patients with COVID-19 in the absence of contraindication.
A limitation of this study was the small sample size of patients from two centers that needed further external validation to support the clinical practice. This research began at the beginning of the pandemic, hence there were adjustments to the system for recording and storing medical records for COVID-19 patients. Secondary data was taken from the medical records, which had a risk of selection, recall, or misclassification bias. This study is a cross-sectional descriptive study, which was influenced by several data biases such as data availability, different follow-up examination, and different given therapies because several patients underwent another different study as this study was held.
D-dimer level was associated with mortality in critically ill COVID-19 patients in the non-linear dose-response relationship.
figshare: Data Set (D-Dimer).xlsx. https://doi.org/10.6084/m9.figshare.17125520.v5
This project contains the following files:
- Data Set (D-Dimer) – Outcome.xlsx (Outcome of the subjects whether died or stepped down from ICU)
- Data Set (D-Dimer) – Characteristics.xlsx (Characteristics of the subjects, such as gender, age, body weight, comorbidities, and lab result)
- Data Set (D-Dimer) – Lab.xlsx (Lab result from each subject)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Zolfaghari Emameh R, Falak R, Bahreini E: Application of System Biology to Explore the Association of Neprilysin, Angiotensin-Converting Enzyme 2 (ACE2), and Carbonic Anhydrase (CA) in Pathogenesis of SARS-CoV-2.Biol Proced Online. 2020; 22: 11 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Biotechnology, Bioinformatics, Carbonic anhydrase, SARS-CoV-2, COVID-19
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Anesthesiology and intensive care
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 10 Jan 23 |
read | read | |
|
Version 1 03 Mar 22 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)