Keywords
Primaquine; relapse; severe malaria
This article is included in the Manipal Academy of Higher Education gateway.
Primaquine; relapse; severe malaria
Malaria is a major global health problem, with around 228 million reported cases alone in 2018, most due to Plasmodium falciparum (Pf).1 Consequently, most reports on malaria concentrate on Pf. Traditionally, Pf has been described as the causative agent for severe malaria. However, recent reports have shown that malaria caused by Plasmodium vivax (Pv) can also be severe. Although India represents a small percentage of the overall global malaria cases, it is responsible for nearly half of the total cases of Pv.2,3 Despite a decline in the number of Malaria cases in India, the major roadblock to elimination is the tendency of Pv to relapse frequently, mainly when primaquine (PQ) is not prescribed or prescribed in sub-therapeutic dosage.4,5 Therefore, the objective of the study was to calculate the incidence of recurrence in patients with Pv malaria and find the impact of PQ prescription practices on recurrence.
A prospective observational cohort study was conducted at Kasturba Hospital, Manipal in Udupi district of Karnataka State, India, for two years, from October 2016 to August 2018. The study was commenced after taking approval from the Institute's Ethical Committee (IEC 636/2016). All patients of either sex above 18 years of age who presented during the study period with fever and had malarial parasites on the quantitative buffy coat (QBC) examination were included in the study after taking written informed consent. The article was reported according to the STROBE guidelines and all the criteria in the STROBE checklist were met. The sample size was calculated as 206 cases of Pv, considering recurrence prevalence as 31.5%, 95% level of confidence and 6.5% precision.6
They were categorized into Pv, Pf or mixed based on the results of peripheral smear. The patients were classified as having severe disease if they met the criteria for severity laid down by World Health Organisation.7 A detailed history (including comorbidities), physical examination, and laboratory parameters were noted in a predefined case study form. In addition, the worst value of the variables during hospitalization was recorded. Since the study aimed to record the prescription practices of treating physicians, the study objectives were not disclosed to them to avoid bias. The diagnosed cases were treated by the treating team. Glucose-6 Phosphate dehydrogenase (G6PD) levels were requested by the treating physician’s discretion. The enzyme activity was quantified by the manual spectrophotometric kinetic 'gold standard' method in the institutional biochemistry laboratory. G6PD deficiency was defined as less than 30% of mean G6PD activity.
The treating physicians decided the dosage of antimalarials, including PQ. The details of treatment, supportive care hospitalization days and mortality during hospital stay were noted. The primary outcome was microbiologically-confirmed recurrence at the end of the study period. Individuals were followed up telephonically every two months until the end of the study period for the development of fever recurrence. Additionally, individuals were asked to report if the fever recurred and were classified as recurrence if they were microscopically proven to have malaria again.
Statistical analysis was performed using Statistical Package for the Social Sciences version 23.0 (SPSS, RRID:SCR_002865, http://www-01.ibm.com/software/uk/analytics/spss/). Continuous variables were summarized as mean with standard deviation (SD) or median with interquartile range (IQR) (in skewed data). Categorical variables were summarized as the frequency with proportion. Overall, patients with Pv were divided into four groups according to PQ dosage- no PQ, weekly PQ, low dose daily PQ (0.25 mg/kg/day), and high dose daily PQ (0.5 mg/kg/day). The number of recurrences in each group were calculated. A Kaplan-Meier survival plot was generated to determine the survival function of recurrences according to PQ categories until 23 months' follow-up duration. Log-rank test was used to compare the survival function. A Chi-square test was used to compare qualitative variables, whereas an independent t-test was used to compare quantitative variables. A p-value of less than 0.01 was considered significant.
A total of 294 malaria cases were enrolled during the study period, of which 206 (70%) were Pv, 79 (27%) were Pf, and 9 (3%) were mixed (pv+pf). A total of 29.6 % (87/294) cases had severe malaria. The proportion of severity, the requirement of supportive care, and mortality were comparable in both groups and summarized in Figure 1. The baseline clinical and laboratory features of patients with Pv and Pf malaria have been summarized in Table 1.
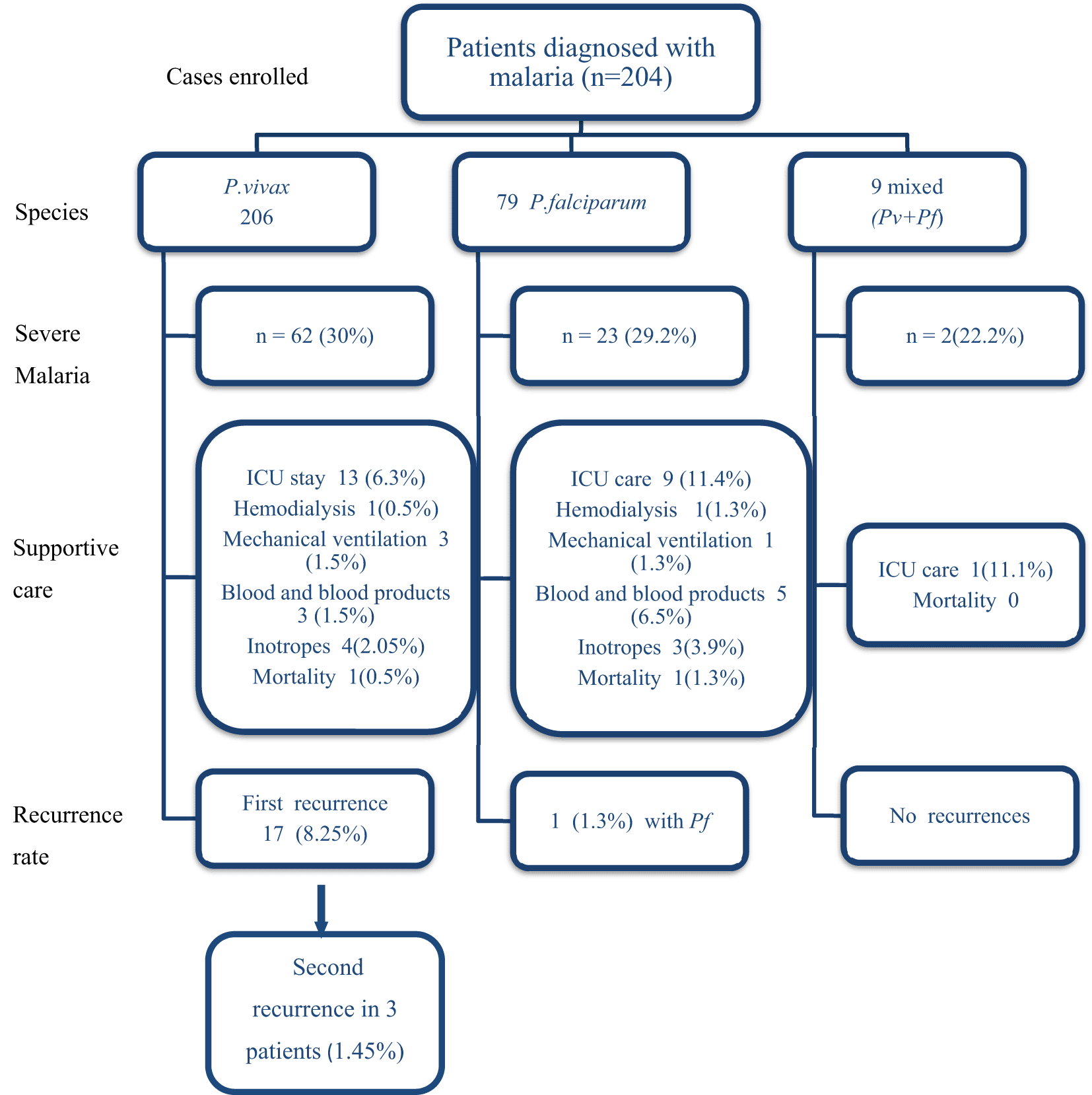
Pv: Plasmodium vivax; Pf: Plasmodium falciparum; ICU: Intensive care unit.
| Plasmodium vivax (N=206) | Plasmodium falciparum (N=79) | |||||
|---|---|---|---|---|---|---|
| Non-severe (n=144) | Severe (n=62) | P-value* | Non-severe (n=56) | Severe (n=23) | P-value* | |
| Age (years) | 36.1±14.2 | 40.6±14.1 | 0.76 | 34.4±14.6 | 38.59±13.1 | 0.41 |
| Male gender | 121(84%) | 55 (88.5%) | 0.38 | 48 (85.7%) | 21 (95.5%) | 0.22 |
| Fever in days | 4 (3,7) | 4 (3,6) | 0.83 | 4 (3,6) | 6 (4,7) | 0.01 |
| Diabetes mellitus | 15 (10.45%) | 13 (21%) | 0.04 | 5 (9%) | 1 (4.3%) | 0.48 |
| Hypertension | 14 (9.72%) | 10 (16.1%) | 0.18 | 5 (9%) | 3 (13.04%) | 0.58 |
| Pulse rate (beats/min) | 88±14 | 92±16 | 0.22 | 88±11 | 87±12 | 0.47 |
| Respiratory rate (breaths/min) | 19±2 | 20±5 | 0.007 | 18±2 | 21±6 | 0.001 |
| ARDS | 5 (3.5%) | 0 | 0.001 | 3 (5.4%) | 0 | 0.005 |
| Systolic blood pressure (mmHg) | 120±14 | 114±20 | 0.001 | 121±17 | 113±15 | 0.8 |
| Diastolic blood pressure (mmHg) | 77±8 | 73±12 | 0.002 | 77±9 | 73±13 | 0.15 |
| Shock | 7 (3.4%) | 0 | <0.001 | 4 (7.1%) | 0 | 0.001 |
| Pallor | 5 (3.5%) | 6 (9.8%) | 0.07 | 5 (8.9%) | 4 (18.2%) | 0.28 |
| Icterus | 44 (30.6%) | 0 | <0.001 | 17 (30.4%) | 0 | <0.001 |
| Impaired consciousness | 3 (2.1%) | 0 | 0.009 | 1 (1.8%) | 0 | 0.108 |
| Convulsion | 1 (0.7%) | 0 | 0.136 | 1 (1.8%) | 0 | 0.108 |
| Metabolic acidosis | 3 (2.1%) | 0 | 0.010 | 1 (1.8%) | 0 | 0.108 |
| Renal failure | 10 (6.9%) | 0 | <0.001 | 3 (5.4%) | 0 | 0.005 |
| Splenomegaly | 17 (11.8%) | 14 (23%) | 0.04 | 11 (19.6%) | 8 (36.4%) | 0.15 |
| Hepatomegaly | 8 (5.6%) | 15 (24.6%) | <0.001 | 6 (10.7%) | 4 (18.2%) | 0.41 |
| Hemoglobin (g/dL) | 13.4 ± 1.9 | 12.8 ± 2.5 | 0.01 | 12.9 ± 2.1 | 12.2 ± 3.2 | 0.03 |
| Hematocrit (%) | 39.7 ± 5.6 | 37.8 ± 7.3 | 0.02 | 38 ± 6.5 | 35.7 ± 9 | 0.12 |
| Total Leukocyte count (cells/mm3) | 5655 ± 2154 | 5813 ± 2978 | 0.008 | 5049 ± 1804 | 7632 ± 4516 | 0.001 |
| Platelet count (cells/mm3) | 74500 (49250,113250) | 47000 (30750,79500) | 0.001 | 75000 (48500,136250) | 39000 (16000,96500) | 0.007 |
| Plasma Glucose (mg/dL) | 132±54 | 149±60.1 | 0.13 | 139±70.8 | 132±44.5 | 0.15 |
| Blood Urea (mg/dL) | 25 (20,31) | 32 (23,45.5) | <0.001 | 24 (19, 30) | 32 (22, 65) | 0.01 |
| Serum Creatinine (mg/dL) | 0.98±0.27 | 1.17±0.48 | <0.001 | 1.01±0.42 | 1.7±2.01 | 0.01 |
| Total Bilirubin (mg/dL) | 1.49 ± 0.62 | 3.8 ± 2.9 | <0.001 | 1.5 ± 0.6 | 6.8 ± 7.56 | <0.001 |
| Direct Bilirubin (mg/dL) | 0.6 ± 0.3 | 2.08 ± 2.42 | <0.001 | 0.6 ± 0.4 | 4.23 ± 5.16 | <0.001 |
| Aspartate transaminase (IU/L) | 33.5 (24,43) | 49 (30,65.5) | <0.001 | 36 (25, 58.5) | 47.5 (37.3, 96) | 0.02 |
| Alanine transaminase (IU/L) | 34 (22,53) | 43.5 (27.2,87.7) | 0.01 | 43 (24, 70) | 54.5 (31.7, 103.2) | 0.2 |
| Alkaline phosphatase (IU/L) | 75 (60,94) | 99 (76.3,144.7) | <0.001 | 93 (61, 115.8) | 122.5 (76.3, 181.5) | 0.02 |
* Categorical variables are summarized as the frequency with proportion whereas continuous variables are summarized as either mean (±SD) or median (IQR). Chi-square or Fischer's exact test and Independent sample t-test or Mann Whitney U test were performed, p-value less than 0.05 shows the statistically significant difference and shown in bold font. ARDS: Acute Respiratory Distress Syndrome.
Of 294 cases included in the study, there were 21 recurrences in 18 (6.12%) patients. All patients with recurrent disease had non-severe malaria with good clinical recovery. Twenty recurrences (20/206, 9.7%) belong to the Pv group and 1 (1/79, 1.3%) patient from the Pf group. Of the 20 recurrences in the 17 patients were in the Pv group, three patients had a recurrence for the second time. The median time to follow-up was 388 (293–567) days. The median time to the first recurrence in the Pv group was 83 (66.5–242.5) days.
Of the 206 patients with Pv, G6PD levels could be done in 196 patients only, out of which nine patients were found to have low G6PD levels (Table 2). No case of PQ-induced hemolysis was noted in our cohort. The dose of PQ was significantly associated with recurrences on the Chi-square test (p<0.001). The percentage of first-time recurrences were highest in the no PQ group (25%), followed by the weekly PQ group (20%), low dose daily PQ (8.2%) group, and high dose daily PQ group (3.1%) (Table 2). A Kaplan-Meier curve was plotted to compare the median time to recurrence in each of the PQ-based groups, and the difference was found to be significant on the log-rank test (p=0.009) (Figure 2).
Udupi district has a population of 1,177,908 with an area of 3,582 sq. km and is located 13°32′ 24.43′′ N latitude and 74°52′26.78′′ E longitude, with typical tropical climatic conditions. The monsoon in this region starts in June and extends till October, with an average rainfall of more than 4000mm every year. The catchment area of our hospital encompasses both the rural and urban populations of coastal and interior Karnataka, Goa and Kerala. Pv is the largest infecting species in this region, followed by Pf.8,9 The same trend is noted in other parts of India.10,11
In this study, around 30% of the cases had severe malaria with a similar incidences of severity in Pv and Pf. In the last decade, the severity of malaria has ranged from 6.5% to 48% across the world.12,13 Classically, Pf is supposed to be one with a higher frequency of severe manifestation, whereas Pv is apparently the benign form.14 This dogma has been challenged more and more as reports emerge from Pv endemic areas. Like other reports, hepatic and renal dysfunction were the commonest manifestation of severity in our study.14 Central nervous system (CNS) manifestations, which were initially thought to be exclusive to Pf, were seen in Pv and Pf in our study. This study reiterates that severe Pv malaria cases presented with similar phenotypic features as Pf malaria. Although previous studies have reported variable mortality with malaria cases, mortality in our study was low, with one mortality each in Pv and Pf patients.10
As expected, all but one recurrence were seen in patients with Pv. The percentage recurrence in Pv cases was close to 10%, which was considerably lower than recurrences reported in the previous series (24–38%).15,16 Like a previous study, all recurrent cases had mild symptoms, presumably due to the development of acquired immunity from the previous episode.17 The median time to recurrence was 83 days in our study, similar to previously published studies.15 Those patients for whom PQ was not used had higher rates of recurrence. Interestingly, the 16 patients for whom no PQ was used, only one patient had proven low levels of G6PD. In all the other cases, the levels were either not done or were normal. This reflects the need to reinforce the importance of PQ prescription in patients with Pv. The rates were lower in the daily PQ group even when they were used at a lower dose. Similar results were observed in other studies as well.18 Since the study was done in a tertiary care hospital where G6PD levels and specialist referrals are available, the study cannot be generalized to primary care settings. Similar widespread prescription audits are required all over the country to understand the practices and pattern of recurrences in patients with Pv.
Self-limiting intermittent recurrences that are asymptomatic could not be ruled out as symptom-based screening for recurrence was done. The genotyping of recurrences could not be done to discern relapse and reinfection. The possibility of non-compliance cannot be ruled out as PQ therapy was unsupervised.
The study reiterates that Pv is the dominant species in this part of India with similar frequencies of severity. Moreover, it is associated with recurrences, especially when PQ prescription is inappropriate. Therefore, there is a need for improving prescription practices amongst primary care physicians through regular educational interventions.
Data cannot be shared due to ethical and security concerns, however a de-identified dataset with all the details can be shared with reviewer or readers at reasonable request to corresponding author.
All authors have read and approved the final manuscript. The requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Divya Gandrala: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing-original draft preparation, Writing-Review & Editing
Nitin Gupta: Formal analysis, Validation, Writing-original draft preparation, Writing-Review & Editing
Alekhya Lavu: Data curation, Formal analysis, Investigation, Methodology
Vishnu Teja Nallapati: Writing-original draft preparation, Writing-Review & Editing
Vasudeva Guddattu: Formal analysis, Software, Writing-original draft preparation, Writing-Review & Editing
Kavitha Saravu: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Validation, Writing-original draft preparation, Writing-Review & Editing
Authors gratefully acknowledge the seed grant funding and publication support from Manipal Center for Infectious Diseases, Prasanna School of Public Health, Manipal Academy of Higher Education, Manipal, India.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plasmodium vivax, radical cure with 8-aminoquinolines, G6PD deficiency
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious Diseases, Tropical Infections
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 06 Apr 22 |
read | |
|
Version 1 04 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)