Introduction
Age-related macular degeneration (AMD) is the most common cause of blindness in developed countries1. For the neovascular form of AMD (nAMD; aka “wet” AMD), the standard of care is the management of the disease by intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) drugs with a primary goal of reducing the progression of the disease2. However, the magnitude of “real-life” vision improvement is typically small, leaving many patients still with significant vision impairment3. Other retinal disorders, including non-neovascular AMD (aka “dry” AMD) and diabetic macular edema (DME) also lead to significant vision impairment. The purpose of the present study is to describe the corneal photovitrification (CPV) corneal laser procedure and outcomes of the procedure that provide additional and significant vision improvement in patients with nAMD and other retinal disorders involving central vision loss. All of these patients have received and continue to receive standard of care routine treatments such as anti-VEGF injections. A preliminary version of this work was presented, in part, at the Association for Research in Vision and Ophthalmology Annual Meeting on 1–7 May 20214.
Methods
Study design
This retrospective observational cohort study (registered with ClinicalTrials.gov NCT 04693702 on 5 January 2021) was completed in conformance with ethical principles of the World Medical Association Declaration of Helsinki. The study protocol (Pro00048890) was approved on 16 February 2021 by an institutional review board (Advarra. Aurora, Ontario, Canada). Written informed consent, after explanation of the nature and possible consequences of the study and with a provision for release of medical records, was obtained from each patient. Treatments had been completed by one physician (Robert G. Devenyi, MD, FRCSC – a retinal specialist.)
The methods seen in the following section are based upon a previous study completed by the authors5. However, the current study is concentrated on the treatment of nAMD rather than dry AMD which was the focus of the previous study5.
Participants
Examinations were analyzed for 72 eyes [53 nAMD, 9 dry AMD, 3 diabetic macular edema (DME), 3 macular hole, 2 myopic macular degeneration (MMD), and 2 branch retinal vein occlusion (BRVO)] of 54 patients [31F, 23M; mean ± standard deviation (SD) age: 79.1 (± 10.7) y] with retinal disorders with central vision loss that received one treatment in each eye using the same device and protocol. Eyes were either pseudophakic or phakic with no visually significant cataract. All patient eyes had vision impairment, with mean ± SD best spectacle-corrected distance visual acuity (BCDVA) of 20/303 (1.18 ± 0.33 logarithm of the minimum angle of resolution (logMAR) ; 26.0 letters). At the time of CPV treatment, most (42 of 53) of the nAMD eyes were continuing to receive anti-VEGF injections using aflibercept; a few (5 of 53) eyes were continuing to receive ranibizumab injections; one was receiving bevacizumab injections and the remaining (5 of 53) eyes were no longer receiving injections. Eyes with nAMD had received anti-VEGF injections as needed, based on optical coherence tomography (OCT) measurements of choroidal thickness, over a mean ± SD period of 5.8 ± 3.3 years prior to CPV treatment.
Inclusion criteria included patient age 50 years or greater and, in the eye to be treated, diagnosed retinal disorders involving central vision loss with moderate to profound BCDVA impairment (in the range of 20/63 to 20/2000); all treated eyes were pseudophakic or phakic with no significant vision loss due to cataract. Exclusion criteria included previous corneal surgery and visually significant ocular disease other than AMD and other retinal disorders involving central vision loss.
Examinations on both treated and untreated eyes included slit-lamp biomicroscopy; subjective manifest refraction (SMR); BCDVA using early treatment diabetic retinopathy study (ETDRS) eye charts; and potential visual acuity (PVA)6 using Gonzalez-Markowitz charts (Precision Vision, Woodstock, IL) at 50 cm examination distance.
CPV treatments were completed using a Clear-K® Low Vision Aid System (Optimal Acuity Corporation, Austin, TX, USA) to deliver pulsed laser energy simultaneously to the cornea in 4 spots of 0.5 mm diameter arranged symmetrically 90° apart and located on a 6.0 mm diameter ring centered on the pupillary centroid. Laser parameters included 2 µm wavelength, 150 ms pulse duration and 48 to 50 mJ energy per spot. Laser light was transmitted from the console through an optical fiber array terminated by a handpiece that docks onto a sapphire applanation window/suction ring (SAWSR) assembly mounted on the eye. Laser energy was delivered through the SAWSR onto the eye in order to provide a fixed location of treatment spots with epithelial protection (by the sapphire window acting as a heat sink) from thermal damage. Patients were reclined to a supine position, given a drop of topical anesthetic in the eye to be treated, and then treated.
Data collection
Data was collected from patient records between 1 September 2019 and 21 September 2021. Data was only collected on patients that had been treated and initially examined (pre-treatment (Tx)) prior to 15th January 2021. Patients were not enrolled in a clinical study; instead, patient exams were obtained during standard office visits to a low vision specialist.
Statistical analysis
Statistical significances of paired outcomes were assessed by Wilcoxon signed rank tests. OD and OS logMAR values for correlated bilateral treatments were averaged for BCDVA at baseline and at each follow-up time (1 month, 3 months, 6 months and 12 months post-Tx) in order to calculate statistical significances of post- vs. pre-Tx differences7. Statistical significances of the correlations between treatment efficacy and other variables were determined by shuffling the data and calculating the fraction of shuffled trials with correlation greater than the observed data. Microsoft Excel 365 (Microsoft, US; RRID: SCR_106137) and Igor Pro 8 (Wavemetrics, Lake Oswego, OR, USA) (version 8.04; RRID: SCR_000325) functions were used for statistical analyses.
Results
Safety
No clinically significant complications or serious adverse events occurred.
Efficacy
Outcomes for treated and untreated eyes are shown in Figure 1 in terms of mean BCDVA letters of vision gained on ETDRS eye charts vs. follow-up time for three groups of eyes (nAMD, non-nAMD treated eyes and untreated eyes). Table 1 summarizes descriptive statistics of outcomes (BCDVA letters gained) for nAMD eyes [with bilateral treatments (calculated with intereye correlations7), with unilateral treatments, and with all treatments], for other (non-nAMD) treated eyes, for all treated eyes and for untreated eyes.
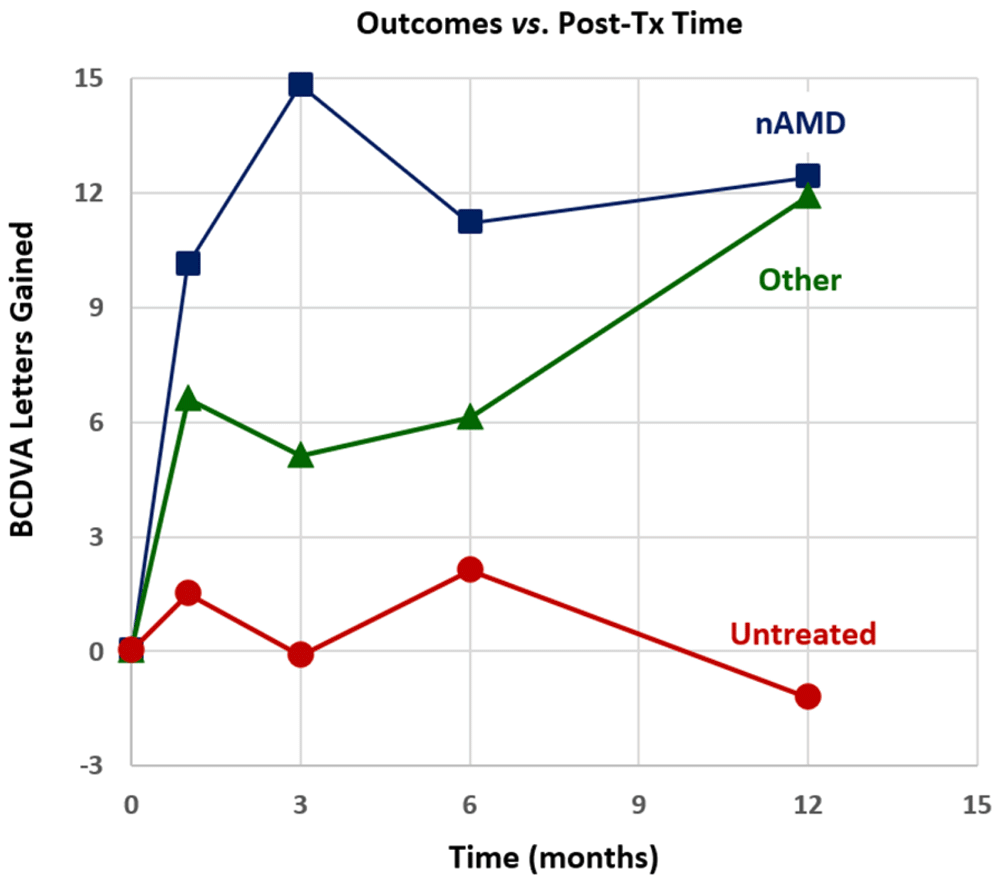
Figure 1. Mean best-corrected distance visual acuity (BCDVA) Early Treatment of Diabetic Retinopathy Study (ETDRS) chart letters gained for neovascular age-related macular degeneration (nAMD) post treatment (post-tx) in treated eyes (top), other treated eyes (middle) and untreated eyes (bottom).
Table 1. Top neovascular age-related macular degeneration (nAMD), second from top (non-nAMD), third from top (all treated eyes), bottom (untreated): Best spectacle-corrected distance visual acuity Early Treatment of Diabetic Retinopathy Study letters gained.
Each entry contains the mean, standard deviation, sample size (n) and p-value. n/a: not applicable due to small sample size
| Treated nAMD Eyes | 1m | 3m | 6m | 12m |
|---|
Bilateral treatments (Txs)
with intereye correlation | 10.5 ± 8.4 n=11
p=0.005 | 15.6 ± 14.5 n=6
p=0.03 | 8.4 ± 9.6 n=9
p=0.012 | 12.7 ± 15.7 n=5
p=n/a |
| Unilateral Txs | 9.7 ± 15.7 n=21
p = 0.005 | 12.8 ± 18.4 n=15
p = 0.012 | 15.0 ± 7.8 n=13
p = 0.005 | 13.6 ± 15.7 n=10
p = 0.066 |
All Txs without intereye
correlation | 10.1 ± 12.3 n=43
p = 0.000014 | 14.1 ± 16.5 n=27
p = 0.0001 | 11.2 ± 13.8 n=31
p = 0.00003 | 12.4 ± 15.2 n=20
p = 0.006 |
| Treated non-nAMD Eyes | 1m | 3m | 6m | 12m |
|---|
All Txs without intereye
correlation | 6.6 ± 11.5 n=14
p = 0.046 | 5.1 ± 12.0 n=15
p = 0.041 | 6.1 ± 13.1 n=7
p = 0.50 | 10.4 ± 13.5 n=9
p = 0.14 |
| All Treated Eyes | 1m | 3m | 6m | 12m |
|---|
All Txs without intereye
correlation | 9.3 ± 12.1 n=57
p = 0.000001 | 11.1 ± 15.8 n=42
p = 0.00005 | 10.2 ± 15.6 n=38
p = 0.00013 | 11.6 ± 14.7 n=29
p = 0.0009 |
| Untreated Eyes | 1m | 3m | 6m | 12m |
|---|
| All Untreated Eyes | 1.5 ± 3.8 n=25
p = 0.32 | -0.1 ± 8.7 n=20
p = 0.55 | 2.1 ± 9.3 n=8
p = 0.57 | -1.2 ± 11.4 n=12
p = 0.59 |
For treated nAMD eye groups, outcomes are statistically significant at the p<0.05 level for most post-Tx times with the exception of the bilateral Tx and unilateral Tx groups at 12m post-Tx; the bilateral Tx group at 12m post-Tx has too small a sample size (n=5) to calculate statistical significance and the unilateral Tx group at 12m post-Tx has near statistically significant improvement (with p = 0.066)8. The largest mean (±SD) gain of 14.1 ± 16.5 letters in BCDVA was achieved at 3m post-Tx for the nAMD Txs group. 34.9% (15 of 43) of treated nAMD eyes gained 15 or more letters (3 or more lines) of BCDVA by 1m post-Tx and this success percentage increased to 40% (8 of 20 eyes) at 12m post-Tx. Figure 2 shows the percentages of changes in lines of BCDVA at 12m post-Tx for treated nAMD eyes; 10% (2 of 20 eyes) lost three or more lines of BCDVA, 10% (2 of 20 eyes) were unchanged and 80% (16 of 20 eyes) gained one or more lines of BCDVA.
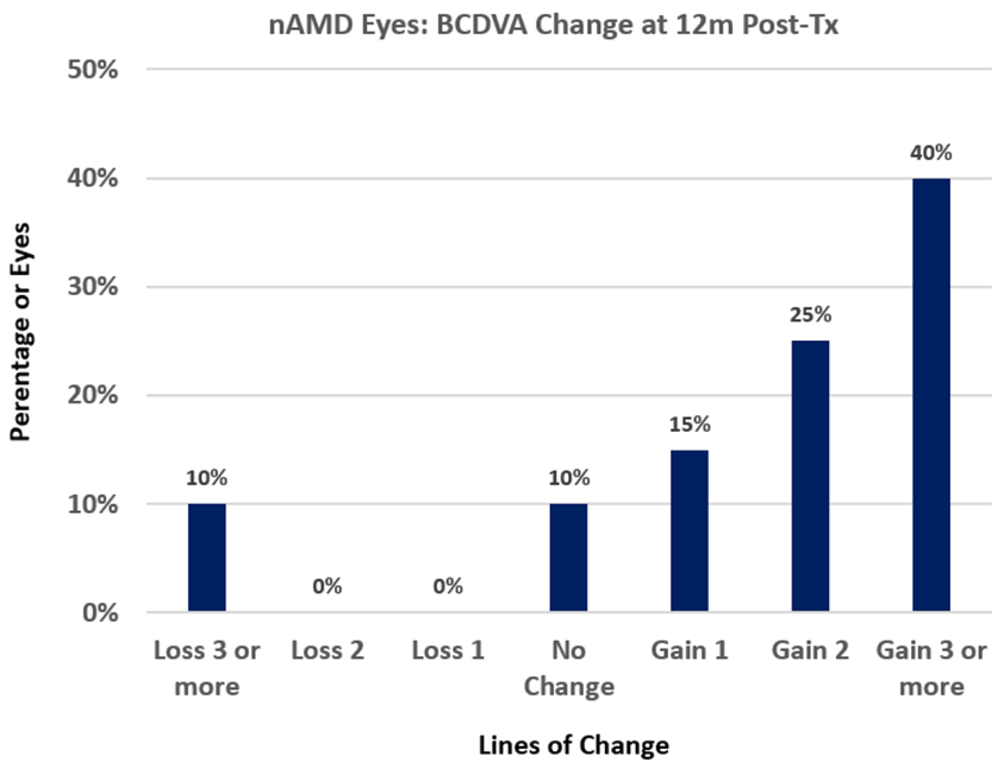
Figure 2. Best-corrected distance visual acuity (BCDVA) changes from baseline for neovascular age-related macular degeneration (nAMD) treated eyes at 12m post-treatment (post-Tx).
The histogram shows the percentage of eyes that lost lines of BCDVA, were unchanged, or gained 1, 2, or 3 or more lines of BCDVA.
For treated non-nAMD eyes [i.e., eyes with dry AMD (n=9), DME (n=3), macular hole (n=3), MMD (n=2) and BRVO (n=2)] BCDVA improvement outcomes are statistically significant (p<0.05) at 1m and 3m post-Tx but not at 6m and 12m post-Tx, probably due to small sample sizes at later post-Tx times. The BCDVA improvements for the non-nAMD group were less than the improvements for the nAMD group at 1m through 6m post-Tx times. However, the 12m post-Tx outcome of 10.4 ± 13.5 letters improvement for the non-nAMD group was comparable to the nAMD group outcome. Outcomes at 6m (for n=5 eyes) and at 12m (for n=6 eyes) for the dry AMD group were similar to previous dry AMD outcomes at those post-Tx times5.
Figure 3 shows mean 1m post-Tx BCDVA letters gained vs. mean pre-Tx BCDVA letters for all treated eyes. The data range for mean pre-Tx BCDVA letters in Figure 3 is from 5 letters (1.60 logMAR, 20/800 Snellen) to 60 letters (0.50 logMAR, 20/63 Snellen). The negative correlation in Figure 3 is statistically significant (p = 0.033). Table 2 lists percentages of treated eyes with 1m post-Tx outcomes of ≥10, ≥15 and ≥20 letters improvement for cohorts of pre-Tx BCDVA values. For all treated eyes with both 1m and 12m post-Tx exams (n=23), the mean (± SD) BCDVA gains increased from 2.0 (± 2.4) lines at 1m to 2.6 (± 2.6) lines at 12m, without statistical significance (p=0.13). The outcomes were nearly constant over the 1m through 12m post-Tx period.
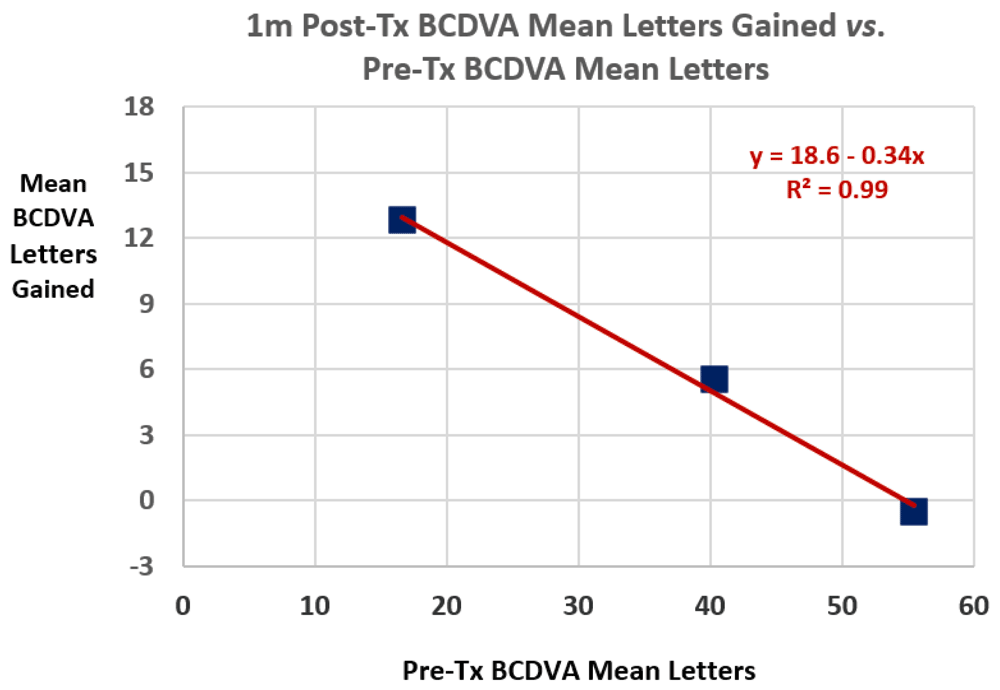
Figure 3. Mean 1m post-treatment (post-Tx) best-corrected distance visual acuity (BCDVA) letters gained vs.
mean pre-Tx BCDVA letters. A linear regression fit is shown. R² is the coefficient of determination for the fit.
Table 2. Percentages of treated eyes with 1 month (1m) best-corrected distance visual acuity ( BCDVA) gains of ≥10, ≥15 and ≥20 letter for pre-treatment (pre-Tx) BCDVA cohorts.
Pre-Tx BCDVA logarithm
of the minimum angle of
resolution (Snellen) | n | ≥ 10 letters
gain | ≥ 15 letters
gain | ≥ 20 letters
gain |
|---|
| 1.6 to 1.4 (20/800 to 20/500) | 21 | 62% | 38% | 24% |
| 1.3 to 1.1 (20/400 to 20/250) | 15 | 60% | 60% | 27% |
| 1.0 to 0.8 (20/200 to 20/125) | 14 | 50% | 7% | 7% |
| 0.7 to 0.5 (20/100 to 20/63) | 6 | 17% | 17% | 0% |
Untreated eyes gained 1.5, -0.1, 2.1 and -1.2 mean BCDVA letters at 1m, 3m, 6m and 12m post-Tx, respectively; no outcome was statistically significant.
Pre-Tx PVA measurements6 demonstrated variable improvements compared to pre-Tx BCDVA measurements, with a range of 0 to 45 letters improvement and a mean (± SD) improvement of 20.6 ± 10.6 letters. Figure 4 shows mean 1m post-Tx BCDVA improvements as a function of mean PVA gains (compared to pre-Tx BCDVAs) for three groups of treated eyes with three ranges of PVA gains: Group A with PVA gains between 0 to 17 letters, Group B with PVA gains between 20 to 31 letters and Group C with PVA gains of ≥35 letters. The positive correlation in Figure 4 is statistically significant (p = 0.0018). Overall, the mean 1m post-Tx BCDVA improvement is 0.45 times the mean PVA gain for the 57 treated eyes that had PVA measurements. The Pearson correlation coefficient (Pcc) for 1m post-Tx BCDVA improvements vs. PVA gains is 0.99. The corresponding Pccs for 3m, 6m and 12m post-Tx BDVA improvements vs. PVA gains are 0.97, 0.99 and 0.89, respectively. Overall, the mean BCDVA improvements are 0.52, 0.51 and 0.50 times the mean PVA gains for the cohorts of treated eyes at 1m, 3m and 6m post-Tx, respectively. For Group A (PVA gains between 0 and 3.3 lines), the success percentages of obtaining 1 or more lines of BCDVA gain is 58.3% (14 of 24) at 1m and 62.5% (5 of 8) at 12m post-Tx. For Groups B+C (PVA gains of 4 or more lines), the success percentages improve to 78.8% (26 of 33) at 1m and 85.7% (18 of 21) at 12m post-Tx.
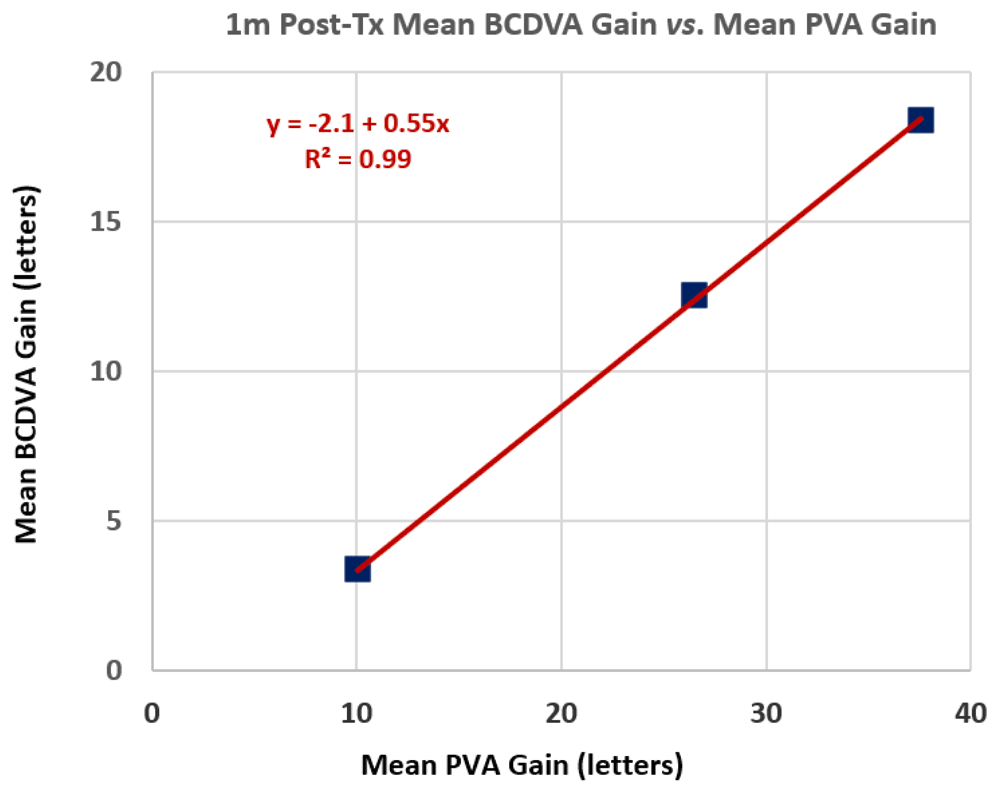
Figure 4. Mean 1m post-treatment (post-Tx) best-corrected distance visual acuity (BCDVA) letters gained vs. mean potential visual acuity (PVA) letters gained for three ranges of PVA gains (0 to 17, 20 to 31 and ≥35 letters).
A linear regression fit is shown. R² is the coefficient of determination for the fit.
Discussion
Laser irradiation of the cornea by CPV treatment produces modifications of corneal shape and corneal refraction as described previously5. Corneal refraction changes redistribute the pattern of light that irradiates the retina, a process termed retinal irradiance distribution modification (IDM).
The proposed mechanism of action has two components with different timescales (see Ref. 5 for more discussion):
1 – Optical (prompt) – corneal shape and refractive changes produce prompt retinal IDM that redistributes visible light, in part, from dysfunctional to functional regions of the retina. Visual acuity may also be improved, in part, by a change in preferred retinal locus and improved fixation stability initiated by retinal IDM.
2 – Neuroadaptation (progressive and long-term) – the visual cortex recognizes and processes new retinal information to achieve better perception and to improve eye movement strategy as a function of post-Tx time.
The laser is “eye safe” meaning that the laser light is completely absorbed in the cornea; none of the laser light propagates through the cornea to irradiate the lens or the retina9. The cornea is intact after treatment – no corneal tissue is removed, cut or punctured and the corneal epithelium is protected from thermal damage. Indentation of corneal tissue in Tx spots is caused by reduction of water content as demonstrated by laser Raman spectromicroscopy measurements10. The resulting tissue compaction in Tx spots produces CPV in which the modulus of corneal tissue is increased, changing treated anterior corneal stroma from a gel-like state to a more glass-like state, as demonstrated by atomic force microscopy measurements10. The Tx spots had the same appearance as previously described5. Each eye received only a single treatment.
There were no clinically significant safety problems seen in any of the patients, such as severe adverse events or clinically significant complications..
The “standard of care” for nAMD eyes is management by anti-VEGF intravitreal injections2. Pivotal randomized clinical trials (RCTs) demonstrated the efficacy of ranibizumab (Lucentis®)11 and aflibercept (Eylea®)12 injections in reducing the progression of nAMD: at 12m post-Tx, only 4%12 to 5%11 of the treated eyes lost 15 or more ETDRS letters compared to ca. 38% of sham (untreated) eyes11. However, BCDVA gains were modest: at 12m post-Tx, ≤ 33.8%11 and ≤ 37.5%12 of treated eyes gained 15 or more ETDRS letters with mean gains of ≤ 7.211 and ≤ 10.912 letters. “Real-life” outcomes of anti-VEGF therapy for nAMD eyes are significantly worse than RCT outcomes: in 2227 eyes at 1 and 2 years after initiation of anti-VEGF therapy, the mean gains were only 2.4 and 0.6 letters, respectively3. Extrapolation of “real-life” outcomes to 5.8 years after initiation of anti-VEGF therapy (the mean time for nAMD eyes in this study) provides an estimate of net loss of ≥5 letters. In contrast to those anti-VEGF monotherapy results, the present combination therapy (of anti-VEGF injections plus CPV Tx) at 12m post-CPV Tx adds onto “real-life” outcomes: 40% of eyes gained 15 or more letters, with a mean gain of 12.4 letters. (The two cases that lost 15 or more ETDRS letters had gains at earlier post-Tx times; the causes of losses at 12m post-Tx are due to unknown, but probably not CPV Tx, reasons.) Combination therapy appears to be greatly beneficial to nAMD patients for vision improvement. BCDVA improvements for nAMD patients in this study are similar to previous BCDVA improvements for dry AMD patients5 at 12m post-Tx: 12.4 ± 15.2 (nAMD) vs. 11.1 ± 13.1 (dry AMD) mean BCDVA letters gained.
Treatment efficacy has a significant negative correlation with initial visual acuity (cf. Figure 3), such that patients who initially had poorer vision experienced a larger gain in acuity after treatment than patients who started out with better vision. The negative correlation may be due, in part, to a “ceiling effect” associated with the greatest visual acuity that can be achieved for a retina with central vision loss; the foveal and parafoveal regions of the retina provide the greatest visual acuity, so if these regions are dysfunctional, only reduced visual acuity functional regions are available.
PVA gain measurements6 for treated eyes in this pilot study correlate very well with post-Tx BCDVA improvements (cf. Figure 4). On average, the PVA test appears to be an excellent indicator of mean BCDVA improvements; it can be used as a screening test and as an input to both patient and physician expectations. However, individual PVA test measurements and their correlations with BCDVA gains may vary considerably from average values.
As in a previous study on dry AMD5, CPV patients typically experienced rapid and comfortable Txs with no post-Tx requirements for new medications or visual rehabilitation training. The present CPV study involved both unilateral and bilateral Txs, depending on whether one or both eyes needed vision improvement.
This study demonstrated that patients benefit by combination therapy for nAMD eyes in which anti-VEGF injections reduce the progression of the disease and CPV Txs provide very significant vision improvements. “Real-life” rates of discontinuation of anti-VEGF treatments by patients within 2 years after initial injections have been reported to range from 15.7%3 to 71%13. There are many reasons for discontinuation, including treatment failure. Combination therapy involving CPV Tx may help to reduce treatment failure and hence treatment discontinuation.
Limitations of the present pilot study are:
1 – smaller than expected sample size (with reduced accountability due to COVID-19 lockdowns),
2 – retrospective analysis of outcomes,
3 – follow-up of only 12 months post-Tx and
4 – the eligibility criteria could lead to bias.
Conclusions
As in our previous study involving eyes with dry AMD5, the availability of a corneal laser procedure for vision improvement in eyes with nAMD and other retinal disorders involving central vision loss offers a new modality to benefit patients. Although only a few cases of eyes with DME and other non-AMD disorders have similarly benefited by CPV treatment, it is likely that CPV treatment will be broadly useful. The PVA test may be useful for estimating BCDVA gains.
Data availability
Underlying data
Dryad: Data from: Corneal laser procedure for safety and efficacy in vision improvement.
https://doi.org/10.5061/dryad.m905qfv2r7.
This project contains the following underlying data:
- F1000Rearch_Dataset_1_-_Devenyi_et_al_-_Corneal_laser_procedure.xls – (Vision improvement outcomes BCDVA and PVA for treated eyes).
- F1000Rearch_Dataset_2_-_Devenyi_et_al_-_Corneal_laser_procedure.xls – (Vision improvement outcomes BCDVA and PVA for untreated eyes).
Data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Author contributions
Study concept and design (RGD, SNM, MJB); data collection (RGD, SNM); analysis and interpretation of data (SNM, MJB II, MJB); writing the manuscript (MJB II, MJB); critical revision of the manuscript (RGD, SNM, MJB II, MJB).
Acknowledgement
The authors gratefully acknowledge Nicole McLaren for supplying patient demographics and treatment documentation.
Faculty Opinions recommendedReferences
- 1.
Wong WL, Su X, Li X, et al.:
Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis.
Lancet Glob Health.
2014; 2(2): e106–e116. PubMed Abstract
| Publisher Full Text
- 2.
Holekamp NM:
Review of neovascular age-related macular degeneration treatment options.
Am J Manag Care.
2019; 25(10 Suppl): S172–S181. PubMed Abstract
- 3.
Holz FG, Tadayoni R, Beatty S, et al.:
Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration.
Br J Ophthalmol.
2015; 99(2): 220–226. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 4.
Berry M, Devenyi R, Markowitz S, et al.:
Corneal laser procedure for vision improvement in patients with late-stage age-related macular degeneration and other retinal disorders.
Invest Ophthalmol Vis Sci.
2021; 62: 303. Reference Source
- 5.
Stein RM, Markowitz SN, Berry MJ II, et al.:
Corneal laser procedure for vision improvement in patients with late stage dry age-related macular degeneration - a retrospective observational cohort study [version 1; peer review: 2 approved with reservations].
F1000Res.
2020; 9: 1500. Publisher Full Text
- 6.
González EG, Tarita-Nistor L, Markowitz SN, et al.:
Computer-based test to measure optimal visual acuity in age-related macular degeneration.
Invest Ophthalmol Vis Sci.
2007; 48(10): 4838–4845. PubMed Abstract
| Publisher Full Text
- 7.
Murdoch IE, Morris SS, Cousens SN:
People and eyes: statistical approaches in ophthalmology.
Br J Ophthalmol.
1998; 82(8): 971–973. PubMed Abstract
| Publisher Full Text
| Free Full Text
- 8.
Berry MJ, Devenyi RG, Markowitz SN, et al.:
Corneal Laser Procedure for safety and efficacy in vision improvement.
Dryad.
Dataset, 2022. http://www.doi.org/10.5061/dryad.m905qfv2r
- 9.
Franks JK:
What is eye safe?
Proc SPIE.
1991; 1419: 2–8. Publisher Full Text
- 10.
Serdarevic O, Heller D, Berry M:
Corneal photovitrification – basic science experiments.
Lasers Surg Med.
2017; 49: 466–467.
- 11.
Rosenfeld PJ, Brown DM, Heier JS, et al.:
Ranibizumab for neovascular age-related macular degeneration.
New Engl J Med.
2006; 355: 1419–1431. Publisher Full Text
- 12.
Heier JS, Brown DM, Chong V, et al.:
Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration.
Ophthalmology.
2012; 119(12): 2537–2548. PubMed Abstract
| Publisher Full Text
- 13.
Lad EM, Hammill BG, Qualls LG, et al.:
Anti-VEGF treatment patterns for neovascular age-related macular degeneration among Medicare beneficiaries.
Am J Ophthalmol.
2014; 158(3): 537–543e2. PubMed Abstract
| Publisher Full Text




Comments on this article Comments (0)