Keywords
4’-fluorouridine, SARS-CoV-2, antiviral drug, COVID-19, RNA-dependent RNA polymerase
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Pathogens gateway.
This article is included in the Coronavirus (COVID-19) collection.
4’-fluorouridine, SARS-CoV-2, antiviral drug, COVID-19, RNA-dependent RNA polymerase
In the revised version, we have corrected and revised our article to ensure that all data are now correct based on the updated information. We have updated our manuscript using the current available data related to the COVID-19, and the drugs. We have revised our article title into: 4’-fluorouridine as a potential COVID-19 oral drug?: a review. We also have revised our abstract to provide better fact with the latest information. We have updated the information of molnupiravir stating that it has been approved by FDA under emergency use authorization (EUA). The stereochemistry has been added under the figure 2. We have provided the pharmacokinetics and pharmacokinetic parameters in to a table from animal study. We also have provided the toxicity of 4'-fluorouridine from animal study under the evidence from in vivo study heading.
See the authors' detailed response to the review by Slobodan M. Janković
See the authors' detailed response to the review by Steven De Jonghe
Coronavirus disease 2019 (COVID-19) is a respiratory disease that emerged in December 2019. The World Health Organization (WHO) declared COVID-19 as a pandemic, that is still ongoing, caused by the novel, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 SARS-CoV-2 is enclosed, positive-single-stranded RNA virus that could infect humans, as well as a variety of other animals.2 SARS-CoV-2 enters into host cells via the spike (S) glycoprotein, which has a receptor-binding domain (RBD) that mediates direct contact with a cellular receptor, angiotensin-converting enzyme 2 (ACE2).2 The S glycoprotein, which consists of two subunits, S1 and S2, is an inactive precursor that must be cleaved to mediate membrane fusion.3 Accordingly, the S1/S2 polybasic cleavage site is proteolytically cleaved by cellular cathepsin L and transmembrane protease serine 2 (TMPRSS2)4,5 to facilitate viral entry.
COVID-19 was initially detected as a zoonotically transmitted disease in Wuhan, China and has since spread throughout the world and caused high burdens in society.6,7 The disease has a broad spectrum of symptoms, ranging from mild and common symptoms, such as fever and cough, to the more severe presentations of pneumonia and even organ failure.8 Based on WHO COVID-19 Dashboard, more than 757 million cases of COVID-19 have been reported as of February 20, 2023, with over 6.8 million deaths.9 The causative agent, SARS-CoV-2, is easily transmitted through the air via respiratory droplets, direct contact with contaminated surfaces, and fecal–oral transmission,10 making it spread rapidly with a reproduction number (R0) of 2.69 and a case fatality rate (CFR) of 2.67.11 Along with its rapid spread, it has a high mutation rate,12,13 making the fight against this virus a race between drugs and vaccines and the virus itself.14
The repurposing of existing drugs is currently being extensively explored, as these drugs have the potential to significantly accelerate the prevention of viral spread and transmission, as well as the development of new COVID-19 therapies.15,16 Some of antiviral have been tested against COVID-19 with some strength and limitation.17 For example, molnupiravir, a potent ribonucleoside analog that inhibits viral replication, has been shown to reduce the risk of hospitalization or death in COVID-19 patients18 by inserting itself into the viral genome during its synthesis by RNA-dependent RNA polymerase (RdRp).19 However, there are some concerns regarding its mechanism of action and its possible detrimental effects on human cells.20 Amid these concerns, there is rising hope in the emergence of 4′-fluorouridine (4′-FIU), whose mechanism of action involves the inhibition of RdRp activity. 4′-FIU was reported to inhibit the replication of the virus in cells without impairing cell metabolism.21 Additionally, it inhibited SARS-CoV-2 and the agents of several other pandemic-potential viral infections, such as avian influenza.21 In this review, the antiviral activity of 4′-FlU and its The promising benefits of 4′-FlU against COVID-19 are highlighted in this review.
The genome of SARS-CoV-2 comprises approximately 30,000 nucleotide-long single-stranded positive-sense RNA, similar to the genomes of other human coronaviruses.22,23 The genome is packed with viral nucleocapsid (N) proteins and surrounded by an envelope membrane composed of lipids and viral proteins: S (spike), M (membrane), and E (envelope) proteins.24–26 The viral genome encodes a 7096-residue polyprotein with a variety of structural and non-structural proteins (NSPs). The large portion of the genomic content is composed of sequences encoding two non-structural proteins, followed by ORF1a, and ORF1ab, and subsequently the sequences encoding the structural proteins. ORFs1a and 1b encode the polyproteins pp1a and pp1ab, respectively, with the polyprotein pp1ab being encoded by the ribosomal frameshift mechanism of gene 1b. These polyproteins are then digested by virally encoded proteinases, which results in the production of 16 proteins that are highly conserved across all coronaviruses of the same family.27 The viral genome has a guanine-cytosine (GC) content of 38%28 and 11 protein-coding genes, with 12 expressed proteins. The hemagglutinin-esterase gene, which is found in several beta-coronaviruses, is absent in SARS-CoV-2.23 The genomic arrangement of the ORFs is strikingly similar to that observed in SARS-CoV and Middle East respiratory syndrome coronavirus (MERS-CoV).29–32 The ORFs are organized into replicase and protease genes, as well as genes encoding the important S, E, M, and N proteins, which are present in a regular 5′–3′ order and are important drug/vaccine targets. The products of these genes facilitate viral entry, fusion, and survival in host cells.32,33 The SARS-CoV-2 genome, similar to the genomes of other human coronaviruses such as SARS-CoV and MERS-CoV, has a m7G-cap structure, with m7GpppA1, at the 5′ end, and an approximately 30–60 nucleotide long poly A tail at the 3′ end.34
SARS-CoV-2 is a member of genus Betacoronavirus (β-CoV) in the family Coronaviridae of the order Nidovirales.35,36 SARS-CoV-2 is relatively more infectious than SARS-CoV and MERS-CoV. Other mammalian species may have served as intermediate or amplifying hosts, with eventual ecological isolation resulting in the acquisition of some or all of the mutations required for efficient human transmission.37
Since its discovery, the SARS-CoV-2 genome has shown genomic variability.38 In a recent study, 48,635 complete genomes of SARS-CoV-2 were compared with the reference Wuhan genome NC_045512.2 and were evaluated to exhibit an average of 7.23 mutations per sample.39 The study demonstrated single nucleotide transitions to be the most common type of mutations prevalent in SARS-CoV-2 isolates globally.39 Another study also found that 5,775 distinct genomic variants of SARS-CoV-2 emerged compared to Wuhan genome NC_045512.2 with multiple mutations including in-frame deletions, non-coding deletions and insertions, and frameshift deletions and insertions.40 The host RNA editing machinery, in which adenosine deaminase acts on RNA (ADAR deaminase (APOBEC) targets dsRNA for adenosine deamination to inosines (A-to-I) and apolipoprotein B mRNA editing enzyme catalytic subunit) deaminates cytosines to uracils (C-to-U) on ssRNA or ssDNA, and may play a role in the induction of the SARS-CoV-2 genome mutations and modifications found during viral infection.22
While mutations in the viral genome are natural, during this pandemic, mutations in the SARS-CoV-2 genome have been a scourge. This is because of the numerous mutations that have enabled this virus to repeatedly evade antibody neutralization.41,42 This is especially true when there are multiple mutations in the gene that encodes the S glycoprotein.43 Special attention should be given to mutations in genes encoding non-structural proteins, as they may impair the effectiveness of antiviral drugs. Even though some variations are rare in specific regions, the C14408T and A23403G mutations on the Nsp12 and S proteins, respectively, have been found to be the most common worldwide and both induce missense mutations.44 Accordingly, emerging variants of SARS-CoV-2 are very influential and pose a significant threat. Even if individuals have been exposed to SARS-CoV-2 previously, they can still become infected with other variants of SARS-CoV-2 and contract COVID-19, indicating that prior exposure to the virus does not guarantee complete immunity against the disease.45
The structural and non-structural proteins of SARS-CoV-2 are all potential drug/vaccine targets.26 The main protease (Mpro), a key enzyme for the viral polyprotein proteolytic process, and RdRp, a key viral enzyme that is critical in mediating viral replication and transcription, are two attractive drug targets for SARS-CoV-2.27,46,47
RdRp (Figure 1) catalyzes the replication of RNA from an RNA template. It catalyzes the production of complementary RNA strands for a given RNA template. It differs from the traditional DNA-dependent RNA polymerases, which are found in all species and catalyze the transcription of RNA from a DNA template.48 RdRp is encoded in the genomes of the majority of RNA viruses that do not have a “DNA stage,” including SARS-CoV-2.49,50 Structural modeling of RdRps from positive-sense RNA viruses (HCV and SARS-CoV-2) and negative-sense RNA viruses (i.e., influenza) have confirmed the differences between the two types of RdRps.51
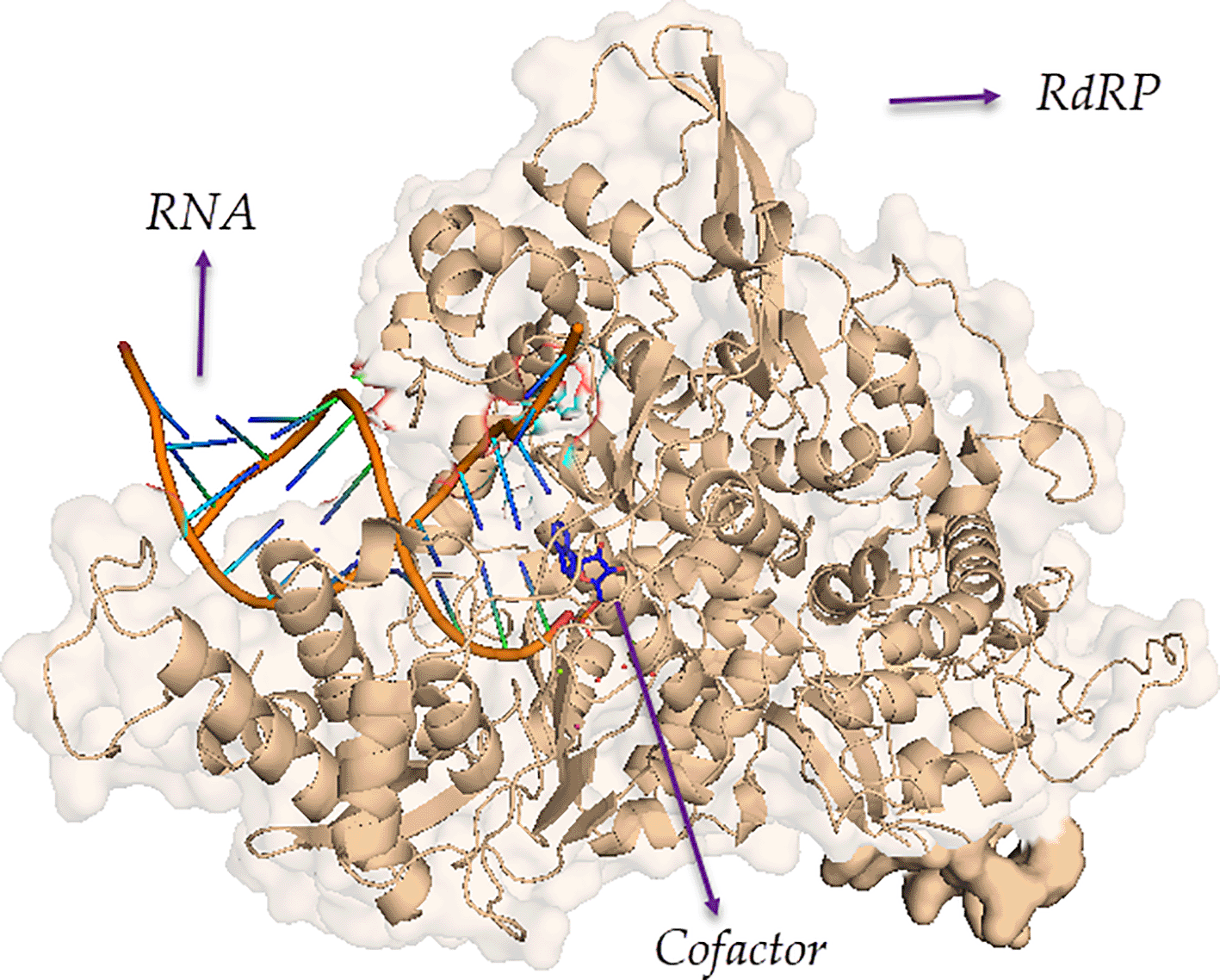
Protein Data Bank (PDB) ID of RdRP: 7BV2.
RdRps are not required for the survival of eukaryotic cells. As a result, they have the potential to be used as therapeutic targets against viral infections. The replication of RNA from a template strand is prevented when RdRp is inhibited, although DNA-dependent RNA polymerases continue to function. While the RdRp of SARS-CoV-2 differs from the RdRp of SARS-CoV and MERS-COV27 the RdRps of all three coronaviruses are highly conserved, suggesting that RdRp is a promising broad-spectrum antiviral target for coronaviruses.52,53
Some antivirals against SARS-CoV-2 such as remdesivir and molnupiravir targeted RdRp.54,55 Remdesivir has been demonstrated to shorten recovery time in COVID-19 patients56,57 and is currently licensed for clinical usage.58 RdRp can use GS-441524 triphosphate (an active metabolite of remdesivir) as a substrate, thus causing premature chain termination and inhibition of viral replication. Remdesivir is a nucleotide analog that prevents further elongation of the RNA polynucleotide by covalently binding to and interfering with the termination of the nascent RNA either early or late, or by inhibiting further elongation of the RNA polynucleotide. As a result of the premature termination, nonfunctional RNA is produced, which is then degraded via the normal cellular functions of the body.57,59 Molnupiravir, another RdRp, has been reported to reduce the risk of hospitalization or mortality in at-risk patients by 50%.18 Molnupiravir, another RdRp inhibitor, is a highly effective ribonucleoside analog that prevents viral replication.55 4′-FIU is another nucleotide analog that has been tested to have antiviral activity against SARS-CoV-2 by causing the stalling of RdRp.21 This compound has been shown to have antiviral activity against several viruses.21,60,61
Uridine, a ribonucleoside that forms nucleic acids, is a glycosylated pyrimidine analog of uracil that is attached by a –N1-glycosidic bond to a ribofuranose moiety.62 Uridine replaces thymidine in the RNA. Uridine analogs are often developed as antiviral for RNA viruses. The general hypothesis is that these uridine analogs interfere with or inhibit the replication of RNA viruses in host cells. One such example is the use of molnupiravir, a nucleoside analog, that mimics the cytidine or uridine.63 The possibility of 4′-fluorouridine being a new oral drug for COVID-19 with a different mechanism of action than molnupiravir is being investigated. It is a uridine nucleoside analog that contains uridine and fluorine. The 4′-FIU structure and its similarity to uridine described in Figure 2 were created using ChemDraw.64 The antiviral activity of 4′-FlU has led to the suggestion that this is due to the small atomic radius and strong stereo-electronic effect of fluorine. This configuration may have an effect on the conformational flexibility of the backbone, thereby increasing metabolic stability.21,65
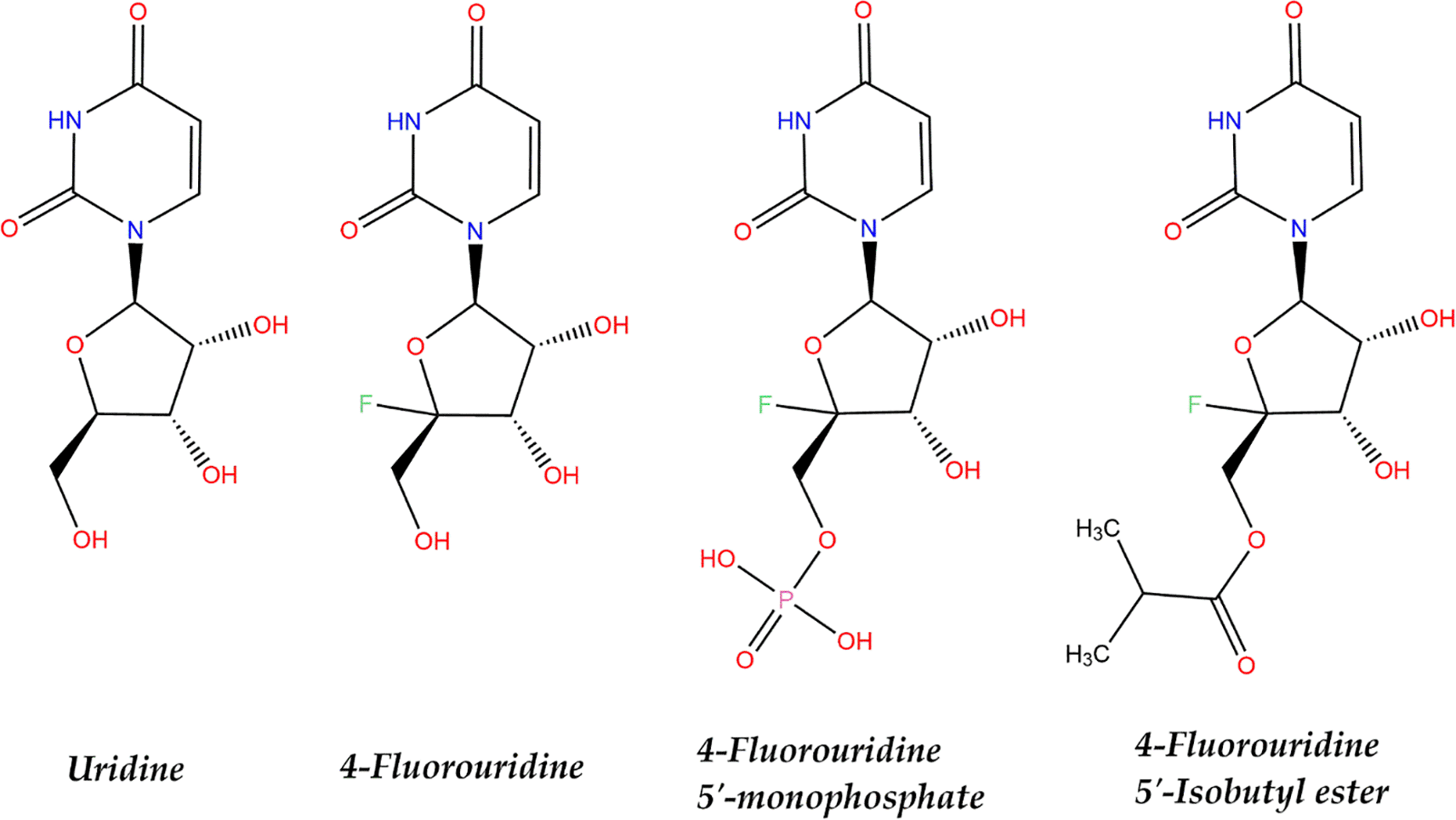
The only difference between uridine and 4′-FlU is the substitution of a hydrogen atom with a fluorine atom at the 4′ position of the sugar moiety. In terms of stereochemistry, both uridine and 4′-FlU have a beta-D-ribofuranosyl configuration, with the ribose sugar adopting a five-membered ring conformation in which the 2′-hydroxyl group is in the axial position and the 3′- and 5′-hydroxyl groups are in the equatorial positions. The figures presented above depict several compounds that share an uracil base with an R configuration at the C2 and C4 positions. In the case of 4′-fluorouridine-5′-monophosphate, a phosphate group is covalently bound to the 5′ carbon of the ribose sugar. Conversely, 4′-fluorouridine-5′-Isobutyl ester features an isobutyl group that is attached to the 5′ carbon of the ribose sugar. Fluorouridine PubChem ID: 90233147 and uridine PubChem ID: 6029. The 4′-fluorouridine-5′-monophosphate and 4′-fuorouridine-5′-Isobutyl ester were created according to the 4′-fluorouridine structure (Created in ChemDraw Professional 17.0).
However, several studies have stated that 4′-FIU is quite unstable66,67 compared to its derivatives.67 4′-FIU derivatives have been proven to have high enough stability for biological studies.67 Examples of 4′-FIU derivatives are 4′-fluorouridine-5-monophosphate and 4′-fluorouridine 5′-O-triphospate.61,66 Two patents, WO2019173602A1 and WO2021137913A2, showed that 4′-FIU, 4′-fluorouridine 5′-triphosphate (EIDD-02991) and 4′-fluorouridine-5′-isobutyl ester (EIDD-02947) (Figure 2) have antiviral activity against positive- and negative-sense RNA viruses by blocking their RdRp.
A study revealed that 4′-FIU was active at submicromolar concentrations against paramyxovirus, rhabdovirus, measles virus (MeV), human parainfluenza virus type 3 (HPIV3), Sendai virus (SeV), vesicular stomatitis virus (VSV), and rabies virus (RabV).21 The compound rapidly accumulates intracellularly in human airway epithelial (HAE) cells, resulting in 3.42 nmol/million cells after 1 h of exposure. Additionally, the metabolic activities of tested cell lines such as Hep-2, Madin-Darby canine kidney (MDCK) cells, hamster kidney fibroblasts (BHK-T7), and human bronchial epithelium cells (BEAS-2B) when exposed with 500 μM 4′-FIU remains unaffected, implying that the antiviral effect is due to cytotoxicity.21
According to patent WO2019173602A1, 4′-FIU has a half maximal effective concentration (EC50) of 1.86 μM in the Vero E6 cell line, cytotoxicity with a CC50 of 380 μM, and stability in human plasma against Junin virus. CC50 is the concentration of a drug required to achieve a 50% reduction in cell viability. A study found revealed that 4′-FIU has effective antiviral activity against strains of Arenaviridae and inhibits lymphocytic choriomeningitis virus (LCMV) with an EC50 of 7.22 μM in the Vero E6 cell line.60 This compound had a significant action against recombinant respiratory syncytial viruses (RSV) A2-line19F and clinical RSV isolates, with EC50 values ranging from 0.61 to 1.2 μM.68 In cell-based minireplicon systems, 4′-FIU inhibited RSV RdRp complex activity during the initial mechanistic characterization. In a human tissue model, HAE cells, including ciliate and mucus-producing cells, with 4′-FIU added to the basolateral chamber after RSV infection, showed that this compound can reduce apical viral shedding with an EC50 of 55 nM.68 Confocal microscopy confirmed the efficacy of 4′-FIU at the basolateral position after the pseudostratified organization of the epithelium with tight junctions and the observation of a rare positive strain of RSV antigen.68 In addition to RSV, 4′-FIU can also reduce paramyxovirus RdRp complex activity in cell-based minireplicon systems, and in a Nipah virus (NIV) minireplicon reporter test, 4′-FIU effectively suppressed the RdRp activity of NIV.21
Several studies on 4′-FIU derivatives have also revealed that they possess antiviral properties. The 4′-FIU 5′-O-triphosphate was shown to effectively inhibit the key hepatitis C virus (HCV) enzymes, nucleoside triphosphate (NTP)-dependent RNA polymerase NS5B, and NTP-dependent NTPase/helicase NS3. This compound successfully inhibited NTP-dependent RNA polymerase with an IC50 value of 2 μM. For the NTP-dependent NTPase/helicase NS3, 4′-FIU 5′-O-triphosphate was found to be a substrate for the NTPase reaction and did not inhibit helicase activity, but this reaction was found to be slightly weaker than adenosine triphosphate (ATP).61
An in vitro study on 4′-FIU-5′-triphosphate showed that RSV RdRp recognized and incorporated 4′-FIU-5′-triphosphate in place of uridine triphosphate (UTP) during the synthesis of the genome. Full addition of cytidine triphosphate (CTP) and 4′-FIU caused limited elongation and not the full-length expected product, indicating that 4′-FIU delayed polymerase stalling.21 Another study showed that 4′-FIU derivatives potently inhibited HCV infection. The IC50 of the 4′-FIU derivative in the polymerase NS5B assay was as low as 27 nM. Human DNA and RNA polymerase also showed little inhibition by the 4′-FIU derivative.69
However, while several studies have shown that 4′-FIU derivatives have antiviral activity, they are different from 4′-FIU analogs. A study conducted to determine the antiviral activity of 4′-FIU analogs for the varicella-zoster virus (VZV) and human cytomegalovirus (HMCV) showed that there was no antiviral activity with an EC50 value >100 μM.70 Another study showed that SARS-CoV-2 was particularly sensitive to 4′-FIU, with EC50 values ranging from 0.2 to 0.6 μM.21 Additionally, this study demonstrated that when 4′-FIU 5′-triphosphate was incorporated in place of UTP in SARS-CoV-2 RdRp, the enzyme did not immediately stall, but it stalled after multiple incorporations and was prominent when 4′-FIU 5′-triphosphate was incorporated. This is a distinctive mode of action in comparison to molnupiravir, which induces lethal viral mutagenesis once it is incorporated into the viral genomic RNA.21
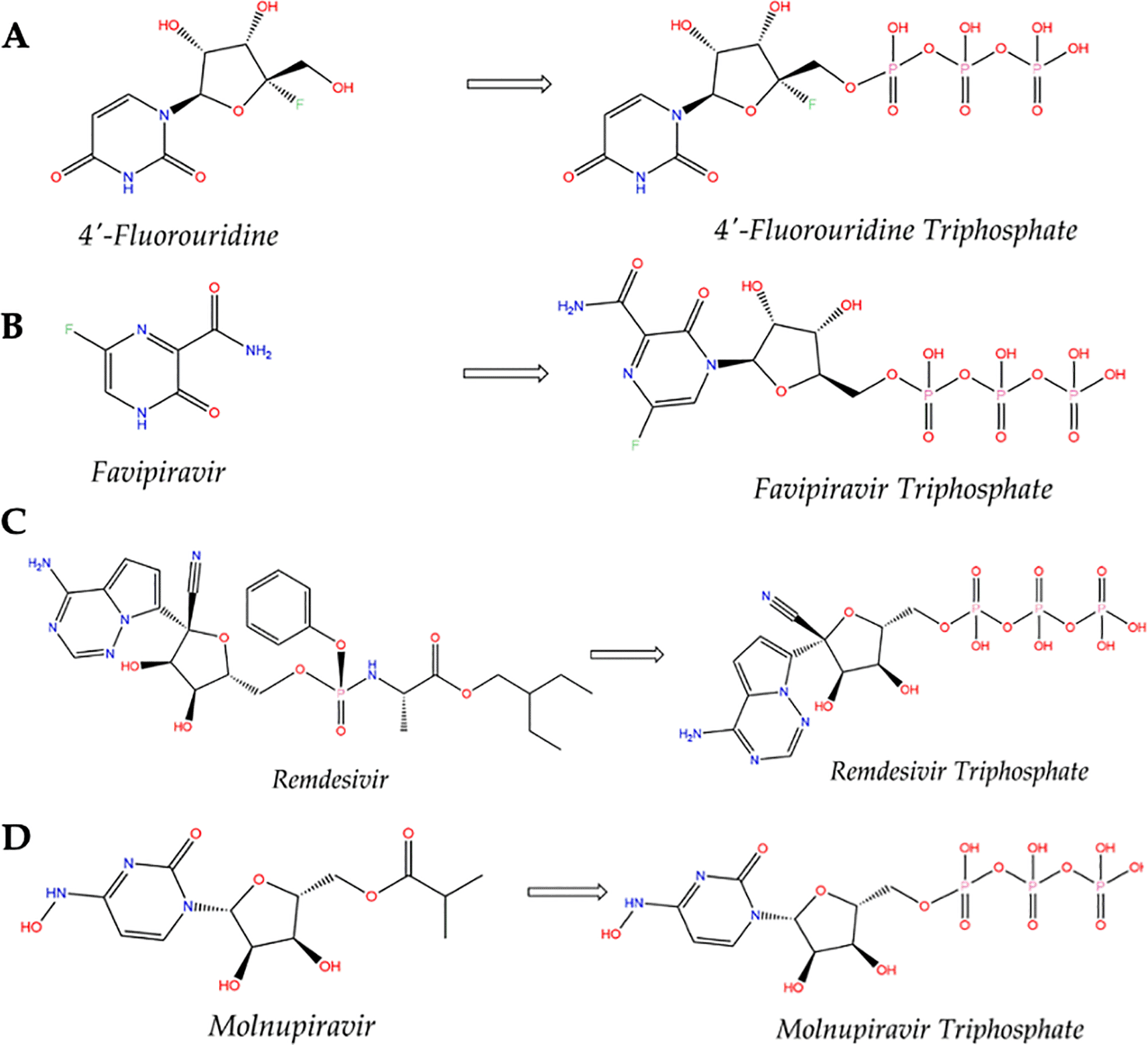
Fluorouridine has been shown to be effective at inhibiting biological activity, particularly cell division, in Tetrahymena pyriformis.71 After ingestion, compound 4′-FIU is further phosphorylated in the cell to form the active metabolite 4′-FIU (Figure 3A).
A previous study21 described the pharmacokinetics of 4′-FlU in ferrets, which were used as a model to test the efficacy of the compound against SARS-CoV-2. The study determined the pharmacokinetic profiles of 4′-FlU after single oral dose of 15 or 50 mg/kg in ferrets. The results showed that the peak plasma concentrations (Cmax) of 4′-FlU were 34.8 and 63.3 μM, respectively, and the overall exposure was 154 ± 27.6 and 413.1 ± 78.1 hours×nmol/ml, respectively. The pharmacokinetics of 4′-FlU showed good oral dose-proportionality. Based on these pharmacokinetic parameters, the study selected a once-daily oral dose of 20 mg/kg for efficacy tests against SARS-CoV-2 in ferrets. The study found that therapeutic treatment with 4′-FlU initiated 12 hours after infection reduced virus burden in nasal lavages by approximately three orders of magnitude for the SARS-CoV-2 isolates tested. All three variants of SARS-CoV-2 (alpha, gamma, and delta) were highly sensitive to 4′-FlU, remaining below the level of detection 36 to 48 hours after the onset of oral treatment. The shedding of infectious particles ceased completely in all animals after 2.5 days of treatment (3 days post-infection). Summary of pharmacokinetic parameters of 4′-FIU in animal study is presented in Table 1.
While the 4′-FlU demonstrates a potent dose-dependent activity against several viruses including SARS-CoV-2, the toxicity of the compound cannot be ignored. A study reported that global metabolic activity of established human and animal cell lines remained unaltered after exposure to 4′-FlU, indicating that the antiviral effect is not due to cytotoxicity.21 However, when tested on disease-relevant primary human airway epithelial cells, 4′-FlU showed low cytotoxicity levels with a CC50 of 169 μM.21 Therefore, further research is needed to determine its safety and efficacy before considering it for clinical use.
Patent WO2019173602A1 demonstrated that 4′-FIU was extremely well tolerated in an in vivo study in mice. The compound was shown to reduce RSV load in the lungs, with the most effective dose being 5 mg/kg body weight. After day 5 post-infection, animals treated with 4′-FIU demonstrated a significant decrease in bioluminescence intensity in the lungs.21 Currently, there are no clinical trials of 4′-FIU available in international repositories including Clinicaltrials.gov, EU Clinical Trials Registry, WHO-International Clinical Trials Registry Platform and others.
Drug repositioning is the only feasible option for addressing the COVID-19 global challenge in the short term. A few small-molecule antiviral drugs, such as and bemnifosbuvir, favipiravir, remdesivir, molnupiravir and paxlovid, have shown promising results against COVID-19.17,71–75 Bemnifosbuvir is a is an orally viral replication inhibitor and some of the clinical trials have been conducted or are ongoing with no available results. Favipiravir (T-705 or 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is a guanine analog that was synthesized by modifying a pyrazine analog. It is an antiviral agent that selectively and potently inhibits the RdRp of RNA viruses.76 The compound is phosphorylated intracellularly to produce the active form, favipiravir-RTP (favipiravir ribo-furanosyl-5-triphosphate) (Figure 3B), which is recognized as a substrate by RdRp and inhibits the activity of RNA polymerase.77 The mechanism of action of favipiravir involves lethal mutagenesis.78 Favipiravir demonstrated protection against RNA virus influenza in a mouse model and Crimean-Congo hemorrhagic fever virus (CCHFV) in macaques.79 However, several cell lines showed reduced or missing selectivity against SARS-CoV-2.80 It differs from 4′-FIU, which shows promising results both in vitro and in vivo.21,60 Nevertheless, further studies are warranted to confirm this finding, in particular by employing cells that have different metabolic profiles. Additionally, favipiravir has been linked to an increased risk of teratogenicity and embryotoxicity81 and a poor pharmacokinetic (PK) profile.82
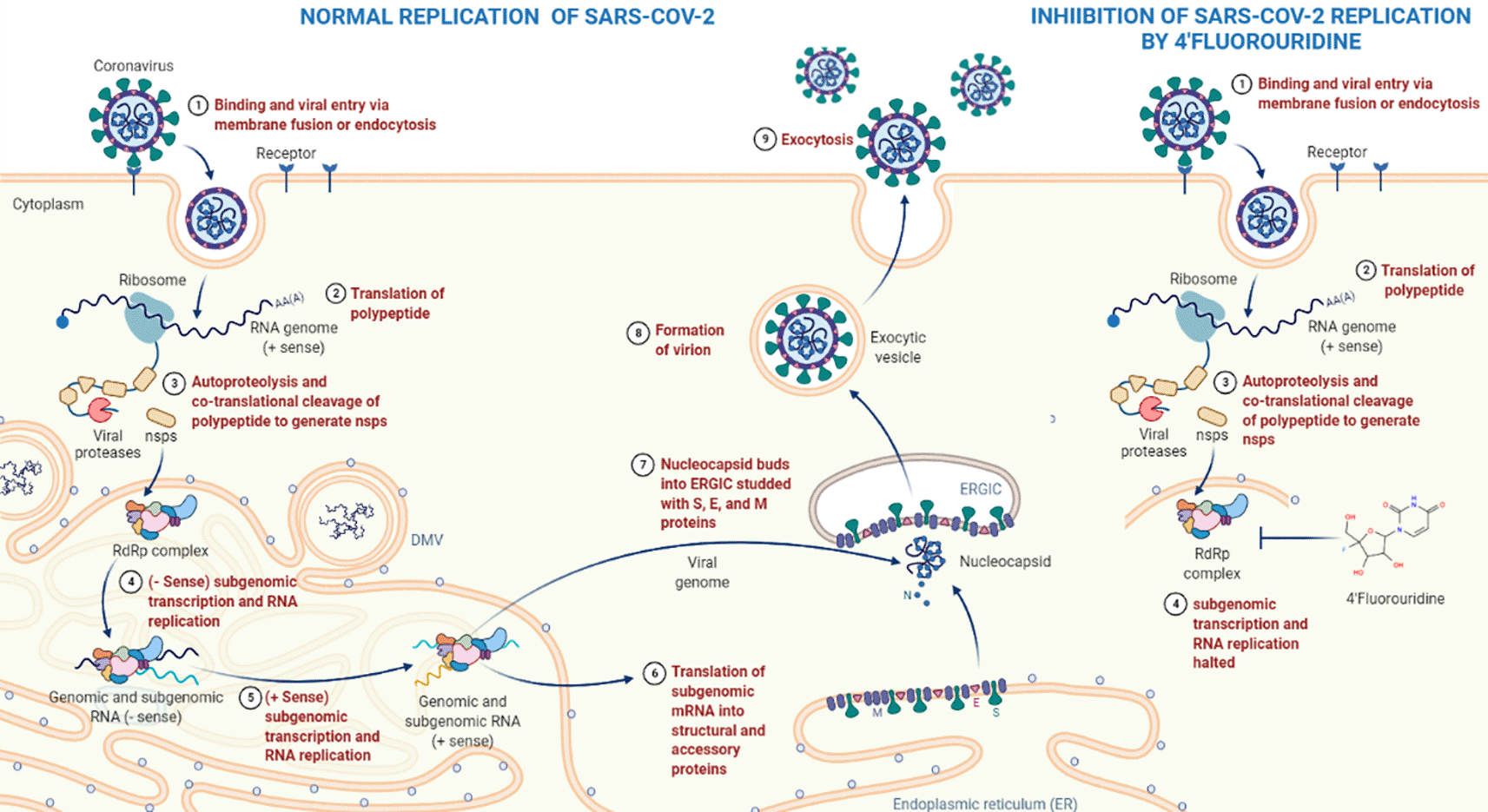
Remdesivir is a promising antiviral agent for tackling SARS-CoV-2.56 Remdesivir is an adenosine analog and a monophosphoramidate prodrug. Its activity involves the incorporation of its active form (remdesivir triphosphate, Figure 3C) into nascent viral RNA, halting RNA synthesis.59 In vitro, a study revealed that remdesivir successfully suppressed SARS-CoV-283 and has now been granted approval.58 Similar to remdesivir, 4′-FIU is also a nucleoside analog and the mechanism of action of 4′-FIU as a potential oral drug for COVID-19 is by inhibiting RdRp to prevent viral RNA replication21 (Figure 4). The advantage is that 4′-FIU can be taken orally, whereas remdesivir must be administered intravenously and frequently recommended for patients with severe COVID-19 infection,84 making 4′-FIU more convenient and an excellent choice for non-hospitalized patients and can be taken orally at home.
To provide the oral drug against COVID-19, molnupiravir, another nucleoside analog, was developed63 and has already completed its phase III trial.18 It has been approved by the US Food and Drug Administration (FDA) under Emergency Use Authorization (EUA) against SARS-CoV-2.17 Inside the body, molnupiravir is metabolized into molnupiravir-triphosphate (MTP) (Figure 3D). In contrast, molnupiravirtriphosphate prevents viral reproduction by generating more mutations than the virus can tolerate, a process known as lethal mutagenesis or catastrophe.20,85 This raises concerns that it may have an effect on human genes; thus, additional research on its genetic safety is critical.86 Since 4′-FIU does not induce mutagenesis in the same way that molnupiravir probably does, concerns about the safety of molnupiravir may be addressed with 4′-FIU. In terms of efficacy, an in vivo study demonstrated that 4′-FIU is required only as a single daily dose,21 whereas molnupiravir is required as two daily doses.73
Paxlovid is administered orally and allowing COVID-19 patients to take it at home in the early stages of infection. Despite receiving EUA approval from the FDA, this drug is not approved for the prevention of COVID-19 before or after exposure, or for initiating therapy in people requiring hospitalization for severe or critical COVID-19 infection. Paxlovid consists of two drugs, nirmatrelvir and ritonavir. Nirmatrelvir inhibits the activity of the SARS-CoV-2 main protease (Mpro), an enzyme required for SARS-CoV-2 replication. It is responsible for cleaving polyproteins at 11 cleavage sites, resulting in NSP4-9 and NSP12-15.87 Ritonavir, on the other hand, inhibits CYP3A, a liver enzyme that is involved in the metabolism of a variety of drugs, including nirmatrelvir and therefore ritonavir could extend the half-life of nirmatrelvir. Paxlovid is administered twice daily for five days, for a total of 30 tablets and it is not recommended for use for more than five consecutive days. In contrast, 4′-FIU is only required as a single daily dose21 and this make 4′-FIU may demonstrate greater efficacy. In addition, Pfizer stated that when paxlovid is used in combination with other drugs metabolized by the CYP3A enzyme, the greatest risk is that the components of ritonavir will increase the effectiveness of other potentially harmful drugs, so this antiviral may not be suitable for everyone. Because of these unpredictable drug interactions, 4′-FIU may be a viable alternative for those who are unable to take paxvloid for medical reasons.
4′-FIU inhibits the RdRp of SARS-CoV-2 and it could have the potential as oral COVID-19 treatment. This drug may be used to address the genetic concerns associated with molnupiravir because it does not cause mutagenesis. However, additional in vitro and in vivo studies are required to address 4′-FIU as a COVID-19 oral drug as well as the clinical trials to assess its efficacy to prevent severe COVID-19 and mortality.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the review discussed comprehensively in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical pharmacology
Is the topic of the review discussed comprehensively in the context of the current literature?
No
Are all factual statements correct and adequately supported by citations?
No
Is the review written in accessible language?
No
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: antiviral drug discovery
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 22 May 23 |
||
|
Version 1 12 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)