Keywords
fine needle aspiration, biopsy, lung, CT-guided, pulmonary lesions, malignancy, pneumothorax, safety
This article is included in the Manipal Academy of Higher Education gateway.
fine needle aspiration, biopsy, lung, CT-guided, pulmonary lesions, malignancy, pneumothorax, safety
Computed tomography (CT) imaging of the thorax has become the cornerstone of initial imaging in lung cancer and can provide accurate anatomic localization of the tumour, along with valuable diagnostic clues about tumour histology.1 However, tissue sampling remains the gold standard for the diagnosis and subtyping of malignant lung pathologies and in the era of personalised medicine, obtaining adequate tumour tissue is essential for molecular profiling and targeted therapy.2 In this regard, minimally invasive procedures such as fine needle aspiration (FNA) and core biopsy (CB) performed under CT guidance have become increasingly common. There is controversy regarding which technique is ideal.3 Recent surveys have shown that in practice the core needle biopsy alone is preferred over FNA alone as a sampling technique in the United States (42% vs. 15%) and the United Kingdom (88.7% vs 1.3%).4,5 Forty three percent of respondents in the United States and only ten percent in the United Kingdom reported using both FNA and CB, despite evidence that a combination of the two techniques is superior to either alone in terms of yield and diagnostic accuracy.6–8 This study was conducted to evaluate the efficacy and safety of CT guided FNA and core needle biopsy for lung pathologies. This study will add to the existing literature on the safety and efficacy of these procedures, and also serve as an audit of our institutional practice.
This retrospective study was conducted in the Department of Radiodiagnosis of Kasturba Medical College Mangalore, a tertiary care hospital, from January 2013 to December 2020 in patients with intrathoracic lesions suspicious of lung malignancies. Ethical clearance was obtained from the Institutional Ethics Committee, Kasturba Medical College Mangalore, Manipal Academy of Higher Education (approval number: IEC KMC MLR 08-16/181), prior to the start of the study and written informed consent was obtained from all the subjects. Relevant investigations (bleeding time, clotting time, prothrombin time/INR and platelet count) were done to exclude coagulopathy. Anticoagulants were withheld five days before the procedure if the patient was receiving these medications.
The inclusion criteria were patients of all ages undergoing percutaneous transthoracic lung biopsy and/or fine needle aspiration under computed tomography guidance. Exclusion criteria included uncooperative patients, patients with contraindications to the procedure (haemodynamic instability, coagulopathy, etc), those with significant paraseptal emphysema surrounding the lesion and patients who did not consent to the procedure and/or study.
Demographic and relevant clinical data were collected from the records of eligible patients. Contrast enhanced computed tomography of the chest was performed using a multidetector 16-slice CT scanner (G E Brivo and G E Bright Speed Elite General Electric, Milwaukee, United States). Contrast enhancement of the lesions was undertaken using 80 ml of non-ionic contrast given intravenously. Venous phase images were acquired 50–60 seconds after administration of contrast. After localization of the lesion, FNA and/or CB were performed. Only FNA was performed in lesions less than 10 mm in size and lesions within 2 cm of the proximal bronchial tree, heart, great vessels, trachea, or other mediastinal structures. The final decision was made by the radiologist performing the procedure.
After a detailed explanation of the procedure, the patient was appropriately positioned in the CT gantry based on pre-procedure imaging. A plain CT scan of the suspected lesion was performed (Figure 1A). A radiopaque grid was placed on the skin at the expected needle entry site (Figure 1B). A CT of the targeted area was performed to determine the skin site for needle entry and needle trajectory. Subsequently, the grid was removed. For the FNAC, a 22-gauge spinal needle (BD Spinal Needle Quincke Type Point, Becton Dickinson India Pvt Ltd, 22Gx3.50IN) was used. The needle was advanced in a stepwise manner while obtaining interval short-segment CT images to guide placement (Figure 1C). When the needle was confirmed to be inside the target tissue by CT, the stylet was removed. A 10 ml suction syringe was attached to the hub of the needle and the plunger was gently withdrawn 6–8 times. The suction pressure was then released, and the needle was withdrawn. Smears were prepared from the aspirated material on glass slides, half of which were inserted into a jar of methanol fixative (for Papanicolaou staining) and the other half were air dried.
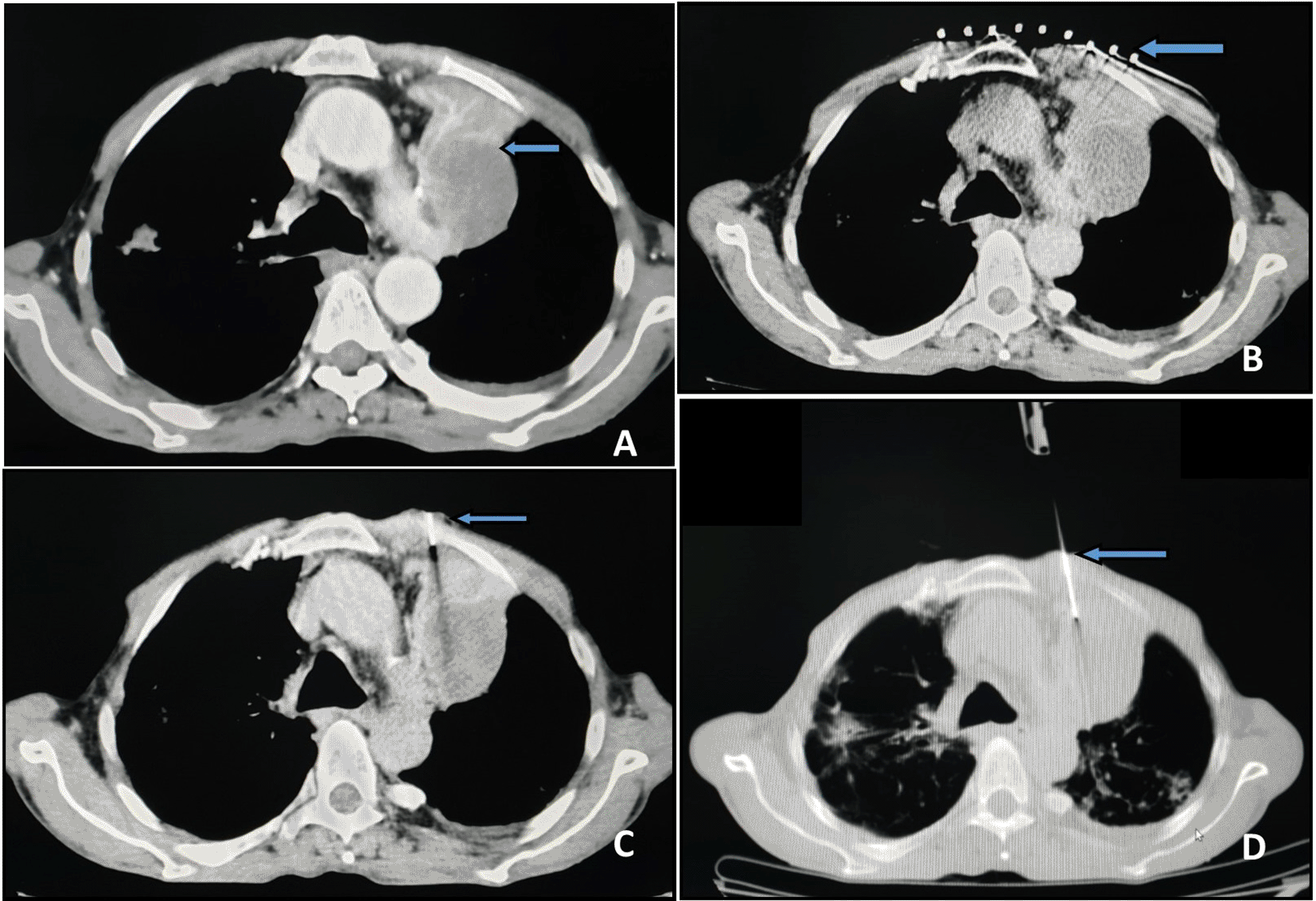
For the core biopsy, an 18-gauge spring loaded cutting needle automatic biopsy gun (Max Core Disposable Core Biopsy Instrument, CR Bard Inc) with a 22 mm throw side cutting needle was used. For deeper intraparenchymal lesions the needle was advanced till the pleura in a stepwise manner and inserted into the lesion by assessing the depth from the skin (Figure 1D). In cases of multiple lesions, the largest lesion with the safest access in proximity to the pleura was chosen. Pleural separation with saline was attempted to obtain a wider window for passage of the biopsy gun. The specimen obtained was transferred to a container with formalin for histopathological examination. A single pass was performed routinely. Repeat passes were performed only if the first pass was unsuccessful or the specimen was fragmented on visual inspection. A cytopathologist was not available on site, and specimens were transported to the laboratory after the procedure.
Adequate compression and sterile dressing were applied immediately after the procedure. A check CT was performed within 30 minutes of the procedure to evaluate for complications including pneumothorax, haemorrhage, and soft tissue hematoma.
Data were analysed using SPSS version 25 (IBM SPSS Statistics, RRID:SCR_019096). Categorical data were represented in the form of frequencies and proportions. Continuous data were represented as mean and standard deviation. The Chi-square test was used as test of significance for qualitative data. The Independent t test was used as test of significance to identify the mean difference between two quantitative variables.
A total of 375 patients met the inclusion criteria and were included in the study, of which 326 underwent concurrent FNA and CB and 49 underwent FNA alone. The mean age of the study population was 60.19 ± 12.09 years. The demographic characteristics of the study population are presented in Table 1.
The mean size of histologically benign lesions on CT was 4.3 cm ± 2 cm, and the mean size of histologically malignant lesions was 4.6 cm ± 1.7 cm. There was no significant difference between the size of benign and malignant lesions (p value 0.116). Out of 375 sampled lesions, 301 (80.3%) were abutting the pleura, and 74 (19.7%) were located at some distance from the pleura. The mean distance from the pleura in these lesions was 9.67 cm ± 7.686 cm.
The FNA smears and biopsy samples obtained were categorised as benign, malignant, atypical/suspicious or inadequate. Those reported to be benign included 20.3% (76/375) of FNA samples and 26.1% (84/326) of core biopsy samples. Malignancy was detected in 58.9% (221/375) of FNA and 69% (225/326) of biopsy samples. Atypical or suspicious for malignancy were reported in 3.7% (14/375) and 0.6% (2/326) of FNA and biopsy samples respectively. Only 4.6% (15/326) of biopsy samples were inadequate, as were 17% (64/375) of FNA smears.
In cases where both FNA and CB were performed, the result of the CB was considered to be the final diagnosis. Of the CB samples 309 out of 326 were classified as either benign or malignant (15 cores were inadequate and two were atypical). Forty-nine patients underwent FNA alone, of which 37 smears were classified as either benign or malignant (12 smears were inadequate). The histologic diagnoses reported from these specimens are presented in Table 2. Non-small cell lung cancer (NSCLC) was the commonest diagnosis (n=220). The benign specific diagnoses included tuberculosis (n=10), cryptogenic organising pneumonia (n=9), interstitial pneumonia (n=5), neurofibroma (n=1), actinomycosis (n=1), aspergillosis (n=2), lipoid pneumonia (n=1) and lipoma (n=1). A total of seven cases were reported as positive for malignancy without further classification of tumour type.
Considering biopsy as the gold standard, the sensitivity and specificity of FNA for malignancy was found to be 95.19% and 80% respectively. The positive predictive value and negative predictive value were 93.19 and 85.25, respectively. Overall, FNA had a diagnostic accuracy of 91.27%. Kappa agreement between the two methods was found to be substantial (0.767).
Thirty one out of 375 patients (8.3%) developed pneumothorax following the procedure, of which 30 were small pneumothoraces (less than 2 cm) and only one was a large pneumothorax that required chest tube drainage. No other complications apart from pneumothorax were observed.
Patients who developed pneumothorax had significantly smaller lesions and significantly larger mean distance of the lesion from the pleura. Significantly higher occurrence of pneumothorax was also observed in patients who had pre-existing lung disease versus those who did not (Tables 3 and 4).
| Pneumothorax | p value | ||
|---|---|---|---|
| Present (n=31) | Absent (n=344) | ||
| Distance from pleura (mean ± standard deviation) | 12.9 ± 8.6 cm | 1.1 ± 3.6 cm | <0.0001 |
| Size of lesion (mean ± standard deviation) | 3.6 ± 1.2 cm | 4.5 ± 1.9 cm | 0.009 |
Bronchogenic cancer is the leading cause of cancer related death worldwide, causing more deaths than colorectal, breast, brain, and prostate cancer put together.9,10 The treatment of primary lung cancer hinges on distinguishing non-small cell lung cancer from small cell lung cancer, and recent advances have made it possible to identify genetic mutations in tumour tissue which can guide targeted therapy.11 The lung is also one of the commonest sites of metastasis from other primary tumours and adequate tissue sampling of these lesions is essential for identification of the primary malignancy and treatment.12 All of this necessitates finding safe and efficient techniques to obtain adequate amounts of tissue from lung lesions. Percutaneous CT-guided fine needle aspiration (FNA) and core biopsy (CB) are minimally invasive procedures that are commonly performed for the diagnosis of intrathoracic lesions, including lung lesions. Both techniques have their advantages and disadvantages and controversy exists over which technique can independently provide samples that are adequate for diagnosis and ancillary studies.
FNA is an easy to perform and cost-effective method of sampling, with established accuracy for diagnosing malignancy. Several studies have reported that the diagnostic accuracy of FNA for malignant lesions ranges from 80–95%, and sensitivities > 90% have been reported.6,13–17 The sensitivity and diagnostic accuracy of percutaneous CT guided FNA for malignant lesions in our study were comparable to the existing literature (95.19% and 92.17%, respectively). However, the adequacy of FNA sampling in our study was lower than that of CB (82.9% versus 95.7%, respectively). This is in contrast to the findings of Poulou et al, who reported that performing CB alone decreased the diagnostic yield, especially with lesions ≤ 4 cm in size.18 The authors have hypothesized that this may be due to the inability of CB needles to obtain samples within the margin of smaller lesions, a drawback that may be overcome with FNA. Guidelines from the British Thoracic Society (BTS) regarding percutaneous transthoracic FNA and CB of the lung recommend that an adequacy of >90% be achieved for samples.19 The adequacy and diagnostic accuracy of FNA samples in our study could have been improved by Rapid On-Site Evaluation (ROSE) of the aspirate by a cytopathologist, which is not available at our institution. Previous studies have shown that with ROSE, the diagnostic accuracy of FNA was similar to or even higher than CB for malignancy.20,21 Coley et al. reported that performing FNA alone with ROSE provided adequate samples even for immunohistochemical (IHC) and molecular analysis. The authors compared three modalities – FNA, CB and FNA plus CB – with regard to providing sufficient tissue for subtyping malignancy (with IHC if necessary) and for molecular analysis when required. They reported that no significant statistical difference was found between the three for adequacy of samples.2 These studies might suggest that FNA with ROSE could obviate the need for performing a more invasive core biopsy. However, performing both procedures is beneficial, especially in patients who may have benign lesions or non-epithelial malignancies, where FNA has been shown to be inferior to CB.6,16 Combined FNA and CB could also aid in risk stratification of patients who have atypical cytology – the highest risk of developing malignancy being in patients who had atypical cells on both FNA and CB – followed by those with atypical cytology on CB but negative FNA, and the lowest risk in those with atypical FNA/negative CB.7
The incidence of complications in our study was 8.3%. Apart from pneumothorax, no other complications such as haemoptysis or pulmonary haemorrhage were observed. Pneumothorax is the commonest complication following percutaneous lung biopsy and has a reported incidence of up to 61% for FNA and 26–54% for cutting needle biopsies.19 Of these, up to 18% of patients in the former group and 3.3–15% of patients in the latter group may require drainage.19 Our study demonstrated an acceptable complication rate and only one patient required chest tube drainage for pneumothorax. Increased depth of the lesion from the pleura, smaller size of the lesion and the presence of pre-existing parenchymal disease such as emphysema were found to be risk factors for the development of pneumothorax in our study. This agrees with the findings of Dennie et al, who reported that deeper lesions, smaller lesions and the presence of chronic obstructive pulmonary disease (COPD) were more likely to be associated with development of a pneumothorax.22 Between 40–70 % of patients with lung cancer may also have underlying COPD, which could be attributed to the presence of common risk factors such as smoking.23 COPD has also been found to be an independent risk factor for the development of lung cancer, irrespective of smoking status.24 The high prevalence of COPD in lung cancer can create challenges with regard to selecting the modality for tissue diagnosis when the risk of pneumothorax is considered. A study evaluating the use of ultra-thin 25 G needles for FNA lung in patients with functional lung impairment reported an overall pneumothorax rate of 20.6%, with chest tube insertion frequency of 8.7%, in a study population where nearly half the subjects had moderate to severe functional lung impairment.17
This study had certain limitations. Firstly, due to its retrospective nature, we could not randomize patients to undergo either one or both of these procedures, which could have provided a better comparison of the two techniques. We also considered the biopsy diagnosis as the final diagnosis due to a lack of follow-up data. Lastly, since we do not routinely request cell blocks for fine needle aspirates, we could not compare the adequacy of the two techniques for IHC and/or molecular studies.
In conclusion, we report that CT guided FNA is a safe and reliable method for diagnosis of malignant lung lesions. The complication rate in the present study was minimal. In the absence of an on-site cytopathologist, we recommend a combined approach of FNA and core biopsy in all cases.
Dryad: Underlying data for ‘Efficacy and safety of CT-guided percutaneous fine needle aspiration and biopsy for malignant pulmonary lesions’. https://doi.org/10.5061/dryad.5mkkwh76w
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Written informed consent for publication of the patients’ details and their images was obtained from the patients.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chest radiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Oncology Imaging
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 12 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)