Keywords
bacterial diversity, nudibranch-associated bacteria, antibacterial activity, antifungal activity, phylogenetic analyses, Java Sea
This article is included in the Bioinformatics gateway.
This article is included in the Pathogens gateway.
bacterial diversity, nudibranch-associated bacteria, antibacterial activity, antifungal activity, phylogenetic analyses, Java Sea
In this new version, we add the information regarding Non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) in the last paragraph of Introduction as the Reviewer suggested.
See the authors' detailed response to the review by Yi Li
The number of cases of skin disease that continue to appear shows the urgency of treatment for this problem. Hay (2020) estimates that skin diseases affect nearly a billion people globally each year. However, they are still a neglected topic by public health authorities even though most casualties from skin diseases are avoidable (Alfonso et al., 2017; Bongomin et al., 2017). One of the most commonly used treatment methods for skin diseases is antibiotic products. Microbial skin infections such as mycoses, including dermatophytosis, candida, and Malassezia, dominate in hot climates such as Indonesia. Cutibacterium acnes, Staphylococcus aureus, and Candida albicans are causative agents of skin pathogens. Furthermore, these pathogenic microbes also have a role in cases of infection associated with biofilms, which are often chronic, nosocomial, and persistent bacterial infections that exhibit resistance towards broad-spectrum antibiotics.
These pathogenic bacteria live abundantly, are widely distributed, and live permanently on human skin. The bacteria in the skin can move to other people or medical devices via contact during insertion of a catheter or other medical devices onto the human body.
Although skin diseases rarely develop into deadly diseases, they are complicated to treat and pose a serious burden to the public health system. Skin infections are common in Indonesia, especially in rural areas where bacteria can spread through the bloodstream and infect distant organs. Karimkhani et al. (2017) reported that skin diseases, including dermatitis, acne vulgaris, urticaria, psoriasis, fungal skin diseases, scabies, decubitus ulcer and alopecia areata, contributed 1.79% to the global burden of disease. Hence, due to the high number of skin infections and the limited choices in antibiotics to treat these infections, the exploration of new drugs is urgently needed.
Marine nudibranchs are a highly diverse group of gastropod molluscs that are soft-bodied and shell-less and are found worldwide in various ecosystems, including coral reefs (Thompson and Brown, 1984; Hoeksema, 2007). Scientists are interested in their ecology, natural product chemistry, color pattern evolution and natural history because of their beauty and morphological diversity (Korshunova et al., 2020, 2021). However, with a total of approximately 10,000 species, only about 4% have been chemically analyzed, therefore, a high number of compounds remain to be discovered (Avila and Angulo-Preckler, 2020). Having colorful bodies and lacking the physical protection of a shell, the ability of nudibranch species to defend themselves against predators has drawn attention to the chemical defense of its secondary metabolites. Data on nudibranch species with antipredator compounds, such as Doriopsilla albopunctata, D. areolata, D. janaina, and D. pharpa, have been reported (Fontana et al., 2003; Long and Hay, 2006; Gaspar et al., 2008). Several previous studies showed that secondary metabolites from nudibranchs also have antiviral, antibacterial, anticancer, and anti-inflammatory bioactivity (Zhukova, 2014; Avila and Angulo-Preckler, 2020; Hertzer et al., 2020). Besides, some active compounds from nudibranchs that are still in the preclinical and clinical pipeline include the anticancer active compound jorumycin, isolated from the skin and the mucus of the Pacific nudibranch Jorunna funebris (Fontana et al., 2003), bursatellanins isolated from Bursatella leachii (Mollo et al., 2005), and aplyronines and dolastatins isolated from the Dolabella auricularia (Mudianta et al., 2015; White et al., 2016).
It is well known that the development of drugs from nudibranch derivatives is not easy since synthesizing complex compounds in large quantities is not profitable because it requires a large supply of raw materials, which is a threat to its sustainability. So, it is rare to find research compounds of nudibranch-derived drugs up to the stage of clinical trials for further marketing (Chen et al., 2018). Microorganisms as raw materials, on the other hand, are feasible due to their sustainable and economical supply and their ability to provide bioactive compounds (Rajasabapathy et al., 2020). Symbiotic associations between marine invertebrates from different phyla, such as the arthropods (van As and van As, 2019), bivalvia (Zannella et al., 2017), or porifera (Anteneh et al., 2021), are known to host such symbiotic associations. Böhringer et al. (2017) found antibacterial activity in 71% (35 of 49 isolates) of bacteria associated with the nudibranch genus Chromodoris sp., Phyllidiella sp., Hexabranchus sp., and Doriprismatica sp. Some bioactive compounds may be produced by their bacterial symbionts (Blockley et al., 2017; Riyanti et al., 2020). Current studies show that nudibranch-associated bacteria may act as a chemical defense for nudibranchs via antimicrobial production (Kristiana et al., 2019; Avila et al., 2020).
The production of several biologically active secondary metabolites from microorganisms needs to be activated by specific gene clusters. Non-ribosomal peptide synthetases (NRPS) and polyketide synthases (PKS) are examples of gene cluster forms (Adamek et al., 2018). These gene clusters produce antibiotic compounds such as tetracycline and erythromycin (El Samak et al., 2018; Verma et al., 2011). Therefore, the detection of polyketide synthase (PKS) and non-ribosomal peptide synthetase (NRPS) genes in the association of nudibranchs with bacteria could further explain their essential role in chemical defense in the nudibranchs. The purification of these nudibranch-associated bacterial genes has been conducted (Riyanti et al., 2009; Böhringer et al., 2017; Kristiana et al., 2020; Abdelrahman et al., 2021). The most recent study by Abdelrahman et al. (2021) reported that nudibranch-associated bacteria have antibacterial activity and possess natural product gene clusters. In the present study, nudibranch-associated bacteria with potential bioactivity found in the Jepara coastal waters of the Java Sea were molecularly identified to determine their diversity, antimicrobial activity, and the presence of PKS and NRPS gene fragments.
Nudibranch samples (J. funebris, G. rubropapulosa and G. atromarginata) were collected by scuba diving at depths of 5 to 8 m in Jepara coastal waters, i.e., Awur Bay (110°38’53.14" E, 6°36’36.82" S), Kartini Coast (110°38’15.12" E, 6°35’30.13" S), Panjang Island (110°37’24.01" E, 6°34’23,43" S) and Bandengan Coast (110°38’55.32" E, 6°32’59.28" S) in July 2020 (Figures 1 and 2). Three individual samples were taken for each nudibranch species. To prevent contamination with air before being brought to the surface, the specimens were placed in plastic bags. Then, the samples were stored separately in a plastic bag containing natural seawater until they were processed. Each sample was then placed in an ice cool-box and brought to the Marine Science Laboratory at Diponegoro University (Semarang, Indonesia) for bacterial isolation.
The indicator assay used three causative bacterial agents of tropical skin diseases, S. aureus, C. acne, M. furfur, and the fungal pathogen C. albicans, obtained from the Laboratory of Clinical Microbiology at Kariadi Hospital (Semarang, Indonesia). The agar plug diffusion method was used to examine the antimicrobial activity of isolates (Balouiri et al., 2016). The bacterial isolates were grown in marine agar for four days. Then, the bacteria growing on agar were cut cylindrically and placed on Nutrient Agar (NA), previously inoculated with skin pathogens at a density of 0.5 McFarland. After incubation at 37°C for 1×24 hours, the inhibition zone that formed indicated the presence of active compounds against skin disease pathogens.
Selected bacteria with antipathogenic potential were cultured on a marine agar medium. Bacterial colonies were removed from the plates and put into an Eppendorf tube containing 2.0 mL of sterile seawater. The McFarland standard was used to adjust the degree of turbidity to 6.0 and then centrifuged at 6000 rpm for 5 minutes. Following the manufacturer's instructions, the Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research Corporation, Catalog Number D6005) was used to extract the genomic DNA of all potent strains. To amplify the 16S rRNA gene of strains, bacterial primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) were used (Radjasa et al., 2007).
The PCR reactions were performed in 25 μL reaction volumes on a T100 Thermal Cycler (BIO-RAD). The PCR mixture consisted of 10 μL Master Mix 16S Basic (Zymo Research Corporation, Catalog Number D6005), 0.2 μM of each primer, 12 μL DNA-free water (Promega Corporation, REF. P119A), and 2 μL extracted DNA. The PCR thermal profile included an initial three min denaturation step at 95°C followed by 34 cycles of one min at 95°C, annealing; one min at 53.4°C and extension at 72°C for one min, finally the post cycling extension at 72°C for seven min.
DNA sequencing was performed at PT. Genetika Science Indonesia. The National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST server) (BLAST Similarity Search, RRID: SCR_008419) analysis was used to find the similarity values between the bacterial sequences of the prospective isolates and closely allied species. The Phylogenetic Analysis Using Parsimony (PAUP, RRID: SCR_014931) v.05 (Swofford, 1998) and CLUSTAL_X (RRID: SCR_017055) (Thompson et al., 1997) neighbor-joining algorithms were used to infer phylogenetic trees.
A series of primers listed in Table 1 were used to screen PKS-I, PKS-II, and NRPS gene clusters. The PCR condition was set to follow the procedure of Sibero et al. (2018). The products were assessed by gel electrophoresis for the presence of size amplicons.
The Bray-Curtis Dissimilarity Index was used to analyze the degree of difference between bacterial community composition of each nudibranch (Chao et al., 2006).
The accession numbers of the 16S rRNA sequences of the prospective strains were deposited in GenBank (GenBank, RRID:SCR_002760) including MZ504794, MZ596188, MZ596189, MZ508308, MZ596190, MZ596191, MZ596192, MZ596193, MZ596194, MZ596195, MZ596196, MZ596197, MZ596198, MZ596199, MZ576857, MZ604365, MZ604366, MZ604367, MZ604368, MZ604369, MZ576858, MZ604370, MZ604371 and MZ604372 for the isolates JF2.5, JF2.6, JF2.7, JF2.8, JF4.5, GR1.4, GR1.8, GR1.11, GR1.12, GA4.12, GA9.3, GA9.5, GA9.8, GA9.10, GA2.2, GA2.4, GA2.6, GA2.8, GA2.10, GA2.17, GA2.22, GA2.25, GA2.26 and GA2.27, respectively.
Out of 115 isolates, 20.87% (n = 24) showed antimicrobial activity against skin pathogens (Table 2 and Figure 3; Sabdono et al., 2022a). Among 24 active isolates, 12 isolates could inhibit the growth of more than one pathogen, whereas the remaining isolates (n = 12) inhibited only one pathogen tested. Most of the active isolates (57.7%) were capable of inhibiting the growth of M. furfur, followed by 53.8, 34.6, and 19.2% of the bacterial pathogens C. acne, C. albicans and S. aureus, respectively.
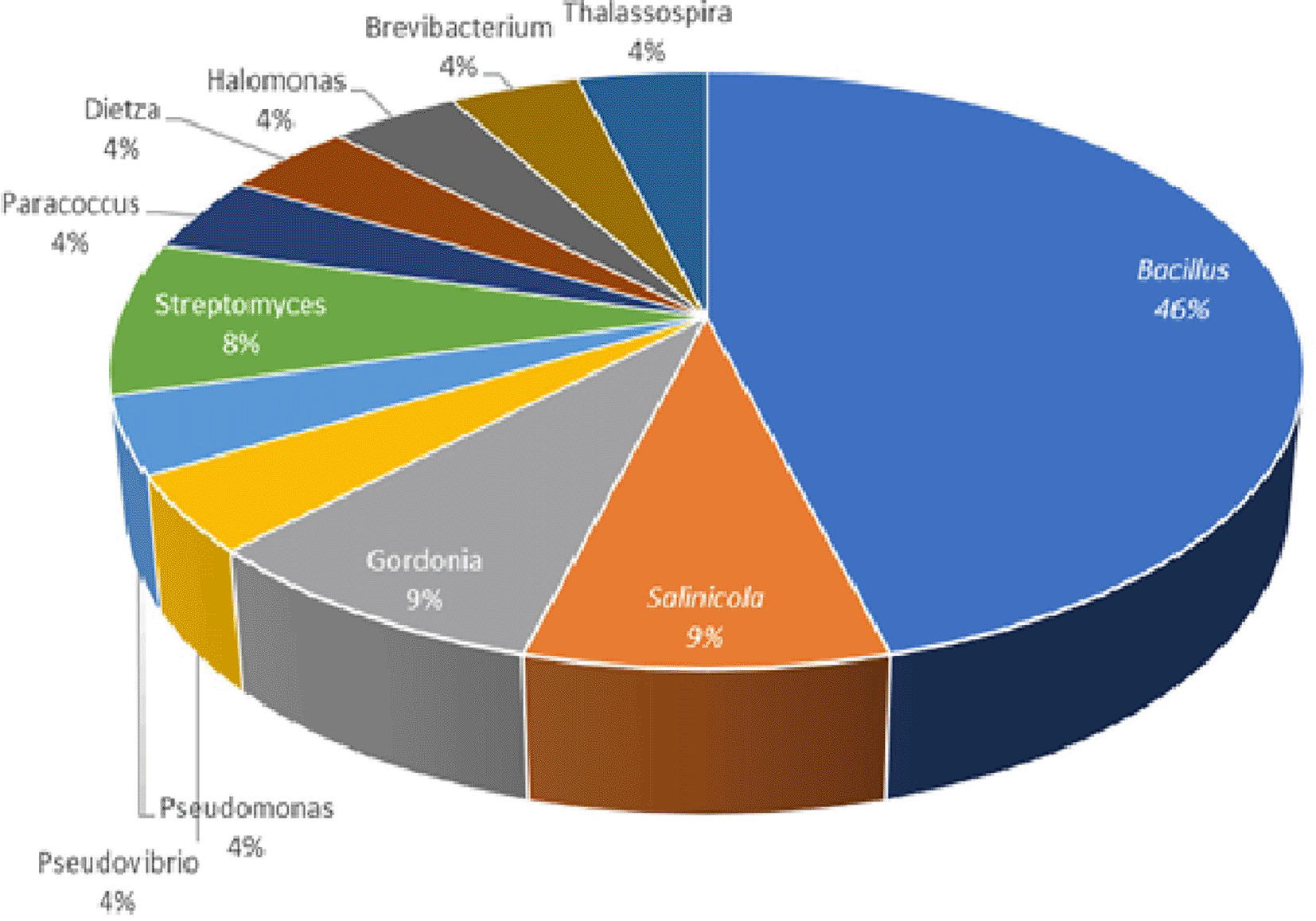
The 16S rRNA gene sequencing analyses showed that these 24 isolates could be assigned to 11 different genera within the three phyla: Proteobacteria (genera Salinicola, Thalassospira, Halomonas, Paracoccus, Pseudovibrio, Pseudoalteromonas, Pseudomonas), Firmicutes (genus Bacillus), and Actinobacteria (genera Brevibacterium, Gordonia, Dietzia). A total of 11 of the 24 isolates (46%) were members of the genus Bacillus. The remaining 13 of the 24 isolates (54%) were members of the genera Salinicola (two isolates), Gordonia (two isolates), Streptomyces (two isolates), Thalassospira (one isolate), Halomonas (one isolate), Dietzia (one isolate), Brevibacterium (one isolate), Paracoccus (one isolate), Pseudovibrio (one isolate), Pseudoalteromonas (one isolate) and Pseudomonas (one isolate) (Table 3 and Figure 4). Identification of pairwise 16S rRNA gene sequence similarities was analyzed by using the NCBI-BLAST homology. The Phylogenetic Analysis Using Parsimony (PAUP) v.05 (Swofford, 1998) and CLUSTAL_X (Thompson et al., 1997) neighbor-joining algorithms were used to infer phylogenetic trees (Figure 5).
| No. | Strains | Closely related | % Identity | Accession no |
|---|---|---|---|---|
| 1. | JF2.5 | Salinicola zeshunii strain N4 | 86.28 | NR_132717.1 |
| 2. | JF2.6 | Gordonia hongkongensis strain HKU50 | 96.03 | NR_152023.1 |
| 3. | JF2.7 | Gordonia terrae strain CL6 | 96.24 | JN862637 |
| 4. | JF2.8 | Salinicola salarius strain M27 | 85.98 | NR_042490.1 |
| 5. | JF4.5 | Pseudovibrio denitrificans strain NBRC 100825 | 99.06 | NR_113946 |
| 6. | GR1.4 | Pseudomonas stutzeri strain CCUG 11256 | 98.92 | NR_118798 |
| 7. | GR1.8 | Bacillus altitudinis strain UAC-24 | 99.45 | JX475123 |
| 8. | GR1.11 | Bacillus altitudinis strain 41KF2b | 97.94 | NR_042337.1 |
| 9. | GR1.12 | Streptomyces ambofaciens strain NBRC 12836 | 98.85 | NR_041079.1 |
| 10. | GA4.12 | Streptomyces ferralitis strain SFOp68 | 97.16 | NR_029087 |
| 11. | GA9.3 | Paracoccus beibuensis strain JLT1284 | 98.66 | NR_116400.1 |
| 12. | GA9.5 | Dietzia maris strain AUCM A-593 | 98.99 | NR_037025.1 |
| 13. | GA9.8 | Bacillus aerius strain 24K | 97.51 | NR_118439.1 |
| 14. | GA9.10 | Halomonas meridiana strain DSM 5425 | 98.95 | NR_042066 |
| 15. | GA2.2 | Brevibacterium avium strain XJB-YJ9-1 | 96.07 | KM186614 |
| 16. | GA2.4 | Thalassospira povalilytica strain Zumi 95 | 99.35 | NR_125450 |
| 17. | GA2.6 | Bacillus altitudinis strain IARI-JR-46 | 99.93 | KF054998 |
| 18. | GA2.8 | Bacillus altitudinis strain XjGEB-71 | 98.14 | JQ320096 |
| 19. | GA2.10 | Bacillus stratosphericus strain NIBSM_OsR1 | 99.52 | KY911276 |
| 20. | GA2.17 | Bacillus safensis strain EGI287 | 100 | MN704552 |
| 21. | GA2.22 | Bacillus aerius strain UB02 | 99.72 | MK696417 |
| 22. | GA2.25 | Bacillus altitudinis strain JYM50 | 99.65 | MN511801 |
| 23. | GA2.26 | Bacillus altitudinis strain BM-Y1 | 96.01 | FJ426274 |
| 24. | GA2.27 | Bacillus altitudinis strain L9 | 99.72 | MN134574 |
The PKS and NRPS genes in nudibranch-associated bacteria were detected. However, none of the 24 antimicrobial bacterial strains had PKS-I, one possessed PKS-II, and five possessed NRPS genes (Table 4 and Figure 6; Sabdono et al., 2022b).
The distribution of antimicrobial isolates is demonstrated in Figure 7. Overall, in the present study, 24 antimicrobial strains were obtained (five bacterial isolates from J. funebris, four isolates from G. rubropapulosa and 15 isolates from G. atromarginata). The nudibranch G. atromarginata harbored not only the majority of bacterial isolates but also the highest number of bacterial genera (seven of the 11 genera), followed by G. rubropapulosa (three genera) and J. funebris (three genera). Among the 11 genera of bacterial isolates, Bacillus was the major genus isolated from the nudibranchs G. atromarginata and G. rubropapulosa. The Bray-Curtis analysis was used to compare antibacterial communities in the three nudibranch species. The result showed a 100% dissimilarity of bacterial communities between J. funebris vs. G. rubropapulosa and J. funebris vs. D. atromarginata, while the dissimilarity between G. rubropapulosa vs. D. atromarginata was 68.4% (Table 5). These results showed that the antimicrobial communities in different nudibranch species varied significantly.

Marine nudibranchs such as J. funebris, G. rubropapulosa, and D. atromarginata are common in Jepara coastal waters, particularly in Awur Bay, Kartini Coast, Panjang Island, and the Bandengan coast of the North Java Sea (Sabdono et al., 2021). The natural products of these species exhibit variability in their bioactivity that has potential applications in the pharmaceutical and medical fields. He et al. (2014) highlighted the antitumor and antimicrobial activity of renieramycin-type bistetrahydroisoquinolinequinone alkaloids, which were extracted from the skin of the nudibranch J. funebris. Furthermore, Li et al. (2019) reported the discovery of a novel spongian type diterpene anticancer and antibiotic produced by the nudibranch G. atromarginata from Weizhou Island in the South China Sea. Recently, some studies have shown that several species of nudibranchs have the potential to produce biological activities. Reddy et al. (2015) reported the antimicrobial potential of the nudibranchs Phyllidia varicosa, Plakobranchus ocellatus, Phyllidiella rosans, and Halgerda stricklandi against bacterial and fungal pathogens collected from the South Andaman Islands, India. Avila and Angulo-Preckler (2020) demonstrated that more than hundreds of marine nudibranchs possess ecological and pharmacological activities. The ecological activities consist of predator avoidance, toxicity, antimicrobials, and antifouling, while the pharmacological activities include cytotoxicity and antitumoral activity, antibiotic, antiparasitic, antiviral, and anti-inflammatory activity; and activity against neurodegenerative diseases.
Recent discoveries have shown that the actual producers of some of the bioactive compounds are marine invertebrates with microbial symbionts (Raimundo et al., 2018). This implies the considerable potential for finding nudibranch bacterial symbionts for treating newly developing diseases in humans. The diversity and functional roles of nudibranch-associated bacterial communities have been characterized and determined (Kristiana et al., 2020; Böhringer et al., 2017; Riyanti et al., 2009). In this study, isolation and screening of bacteria with antimicrobial activity associated with the three nudibranchs revealed that 24 out of 115 isolates demonstrated antimicrobial activity against skin pathogens (Table 2 and Figure 3). Analyses of 16S rRNA sequencing discovered that 24 isolates were closely related to 11 genera, including Bacillus as a dominant genus, followed by Streptomyces, Gordonia, Salinicola, Thalassospira, Halomonas, Dietzia, Brevibacterium, Paracoccus, Pseudomonas, and Pseudomonas (Table 3 and Figure 4). Members of the genus Bacillus are known as the largest source of bioactive natural products, which exhibit a wide range of antibiotic activities (Stoica et al., 2019). Bacilli genera have produced hundreds of antibiotics (Awais et al., 2010).
Our phylogenetic analysis (Figure 5) shows that these bacterial genera belong to the phyla Firmicutes, Proteobacteria, and Actinobacteria. Previous studies on nudibranch-associated bacteria support the results of our study. Riyanti et al. (2009) reported that Streptomyces sp. isolated from nudibranch Jorunna sp. and Chromodoris sp. displayed antimicrobial activity against multi-drug resistant (MDR) bacterial strains. Böhringer et al. (2017) discovered that the diversity of nudibranch-associated bacteria with antimicrobial activity was dominated by the genus Pseudoalteromonas and Vibrio. Besides, Kristiana et al. (2019) found that antibacterial strains Virgibacillus marismortui, V. dokdonensis, Bacillus kochii, Vibrio algynoliticus and Pseudoalteromonas piscicida were revealed from the nudibranch genera Chromodoris and Phyllidiopsis. It was also reported that nudibranch-associated bacteria, Pseudomonas rubra, and Virgibacillus salarius, produce anti-MRSA, cytotoxicity, and anti-herpes simplex virus-1 bioactive compounds (Kristiana et al., 2020).
The discovery of PKS and NRPS genes in nudibranch bacteria could explain their pivotal functions in the chemical defense process of the nudibranch. This study showed that six isolates (23%) were positive for one PKS-II or NRPS (Table 4 and Figure 6), including Bacillus, Gordonia, Salinicola, Pseudomonas, and Streptomyces genera. From these two genes, several new polyketide and peptide compounds were discovered in marine microbes with different biological activities and ecological functions (Baker et al., 2009). Stoica et al. (2019) reported that low molecular weight polypeptides are produced by Bacillus species via ribosomal or non-ribosomal mechanisms. However, very little is known about the distribution of these biosynthetic systems in other nudibranch-associated bacteria. So far, only one study has demonstrated that most of the nudibranch-associated bacteria with biological activities contain PKS and NRPS genes (Abdelrahman et al., 2021), but it is still not understood if these isolates produce compounds that contribute to nudibranch defenses. The result of our study was only positive for one class of biosynthetic genes; nevertheless, it suggested that these bacterial isolates confine genes for biosynthesis.
Figure 7 and Table 5 show that in this study, the number and genera of bacterial strains of the three nudibranch species were significantly different. Each nudibranch species had different numbers and types of bacterial groups. G. atromarginata nudibranch harbored not only the most abundant bacterial isolate but also the highest number of bacterial genera. The understanding of how the diversity of culturable bacteria varies among nudibranch species is limited. To the best of our knowledge, no previous research has investigated the diversity of antimicrobial activity in microbes associated with nudibranchs.
In conclusion, this study revealed the significant potential of nudibranch-associated bacteria from the North Java Sea as a source of anti-skin pathogens. However, further studies need to be conducted on the diversity of the culturable bacteria associated with the same nudibranch species from different geographic regions. Furthermore, there is still minimal knowledge about how the diversity of cultured bacteria differs in nudibranch species. Hence, it is also important to conduct research on the diversity of bacteria that can be cultured associated with the same nudibranch species at different locations.
GenBank: 16S rRNA sequences of the prospective strains. Accession numbers:
• MZ504794 (https://www.ncbi.nlm.nih.gov/nuccore/MZ504794)
• MZ596188 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596188)
• MZ596189 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596189)
• MZ508308 (https://www.ncbi.nlm.nih.gov/nuccore/MZ508308)
• MZ596190 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596190)
• MZ596191 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596191)
• MZ596192 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596192)
• MZ596193 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596193)
• MZ596194 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596194)
• MZ596195 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596195)
• MZ596196 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596196)
• MZ596197 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596197)
• MZ596198 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596198)
• MZ596199 (https://www.ncbi.nlm.nih.gov/nuccore/MZ596199)
• MZ576857 (https://www.ncbi.nlm.nih.gov/nuccore/MZ576857)
• MZ604365 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604365)
• MZ604366 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604366)
• MZ604367 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604367)
• MZ604368 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604368)
• MZ604369 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604369)
• MZ576858 (https://www.ncbi.nlm.nih.gov/nuccore/MZ576858)
• MZ604370 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604370)
• MZ604371 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604371)
• MZ604372 (https://www.ncbi.nlm.nih.gov/nuccore/MZ604372)
Figshare: Diversity and antimicrobial activity of marine nudibranch associated bacteria against tropical human skin pathogens, https://doi.org/10.6084/m9.figshare.19375211 (Sabdono et al., 2022a).
Figshare: Diversity and antimicrobial activity of marine nudibranch associated bacteria PKS-NRPS, https://doi.org/10.6084/m9.figshare.19375583 (Sabdono et al., 2022b).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We thank reviewers and editors for their constructive comments that greatly improved our manuscript. We also thank Aldi Pratama Wijaya and Zola Nurbayani for helping to collect the samples, isolation, and purification in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell Biology, fish immunity, Physiology.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Microbiology, metabolic engineering
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 06 Sep 22 |
read | |
|
Version 1 13 Apr 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)