Keywords
Cytotoxicity, Viability, Propolis, Guava
This article is included in the Cell & Molecular Biology gateway.
Cytotoxicity, Viability, Propolis, Guava
In version 2, some criteria for the selection of cell lines, as well as the concentrations used in the study, have been detailed.
See the authors' detailed response to the review by Orlando Torres Garcia
Since time immemorial, man has tried to mitigate his ailments and prolong his life. This fact has been observed since there have been historical records, from civilization to civilization, until today.1 Even so, man in the 21st century has not been able to avoid death by limiting himself to mitigating symptoms of diseases and avoiding the development of others.1,2
In times when man only had at his disposal the resources that the planet gave him, he sought in these the tools to reduce physical pain and avoid death. Among the resources most exploited by different cultures throughout history are mineral, animal and vegetable resources. Until the middle of the 20th century, these were the therapeutic resources par excellence.2,3
Among the kingdoms of nature that contribute, to this day, to reducing symptoms and preventing diseases, the plant kingdom stands out.4 Plants, thanks to their marvelous and complex metabolism, constitute a true chemical arsenal. Of which only a third is currently known, considering the variety of existing species worldwide, without considering those species already extinct.3,4
Each region of the world developed its own way of healing from medicinal plants, which is unique and characteristic since species endemic to regions were used.4 Over time, these local characteristic therapies came to form the so-called traditional medicine and, when being preserved by the native peoples, is sometimes called aboriginal or autochthonous medicine, as well as traditional or autochthonous recipes4 that group together uses, forms of preparation, administration, dosage, among other modern pharmacological parameters. This is because our therapeutic reality today is governed by synthetic chemistry, but what few people know is that these successful molecules that cure are nothing more than improved copies of chemical substances that nature spontaneously created.4
One of the most studied products is propolis,5 which is composed of approximately 50% to 55% resins and balsams, 30% to 40% wax, 10% to 15% essential oils, 5% pollen and 5% minerals.5 In its components we can mention that it has phenolic compounds: Flavonoids, flavones, isoflavones and flavonones in 50%,5 which inhibit bacteria and fungi.6 The amount of flavonoids confers the antibacterial power to propolis. This quantity depends on the flora surrounding the bee hives.5 Its antibacterial action mechanism is given by the inhibition of cell division, DNA disruption, disorganization of the cytoplasmic membrane and inhibition of cell wall synthesis, causing partial bacteriolysis and inhibiting protein synthesis.5,6
Guava (Psidium guajava) is a fruit native to Central America and the Caribbean, belonging to the Myrtaceae family, distributed in the tropics and subtropics around the world. Guava fruits stand out among tropical fruits not only because of their good organoleptic characteristics (flavor and aroma) but also nutritionally, they are a source of vitamin A, B1, B3 and C, fiber, minerals such as potassium, calcium, iron and phosphorus.7 The guava also has a relevant content of lycopene, an important carotenoid with therapeutic properties, so it has been widely studied.7,8
Cytotoxic evaluation, as the main factor of biocompatibility, is determined by the cell cultures to be selected for in vitro toxicity testing.9 Continuous and real-time monitoring allows label-free assessment of cell proliferation, viability and cytotoxicity, revealing the physiological status of the cells.8 To evaluate the efficacy of natural products, it is not enough to measure their therapeutic effect, but one must be sure that they do not cause deterioration of constituent cells. The aim of this study was to evaluate the cytotoxic effect of ethanolic extracts of propolis and P. guajava on HELA cell lines, human gingival fibroblasts (HGF-1) and peripheral blood mononuclear (PBMCs) cells.
Propolis and P. guajava samples were collected by researchers in the Oxapampa valley, Pasco, Peru following the methodology described by Millones et al.5,8 and subsequently refrigerated until processed. After they were removed from refrigeration they were left for two hours to allow for them to reach room temperature. Once they had reached room temperature they were macerated Once room temperature was reached, they were macerated with a volume of 100 ml of absolute ethanol for every 10 grams of propolis sample, it was then left at room temperature for 24 hours. Then, the macerate was filtered using a 20 cm diameter glass funnel with sterile cotton; the filtered sample was collected in a glass refractory to finally be taken to an extraction hood so that the ethanol present in the extract evaporates completely and only a pasty mass remains. This step was performed two more times until the samples were observed to be discolored. Finally, they were stored in glass containers covered with aluminum foil to avoid degradation.10
The evaluation of cytotoxicity was performed considering oral cavity constitutive cell lines, human gingival fibroblasts (HGF-1); immune constitutive cells, peripheral blood mononuclear cells (PBMCs); and tumour constitutive cells, HeLa cell lines.
The HeLa cell line (ATCC, Manassas, VA, USA) (RRID:CVCL_0058) was cultured in a Petri dish with a 35 mm diameter glass bottom (MatTek Corporation, Ashland, MA, USA, CAT#: P DCF OS 30). The cell line was cultured in Eagle’s Minimum Essential Medium (Gibco) with 4% PBS (phosphate buffered saline) at 37°C in 5% CO2 and 95% air.5 The cells were incubated for 30 min in 1 mL of dye solution in Hank’s Balanced Salt Solution (HBSS, Thermo Fisher Scientific)) (100 nM Mitotracker Red FM) at 37°C, 5% CO2. After incubation, the cells were washed three times with HBSS buffer.11
Human gingival fibroblasts (HGF-1 - ATCC CRL-2014) (HGF-1) were obtained from the American Type Culture Collection (ATCC) and cultured in Dulbecco’s modified Dulbecco’s medium containing glucose (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with 4 mM L-glutamine (Sigma-Aldrich), 1% penicillin, streptomycin (Sigma-Aldrich) and 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich). Cells were incubated at 37°C in a 5% CO2 atmosphere in an incubator (Cytomat 2C450S; Thermo Fisher Scientific), and were fed every 48 hours and subcultured every 5 days at a 1:3 ratio using trypsin-EDTA (0.05%; Sigma-Aldrich) for 3 minutes at 37°C.12
PBMC cells were isolated by Ficoll density gradient centrifugation (TBD, Shanghai, China) and cultured in RPMI-1640 medium with 10% fetal bovine serum and placed in a humidified incubator (Thermo CO2 incubator, 311, USA) at 37°C, 5% CO2 and 95% humidity. The medium was changed once every 24 hours.13–16
To evaluate the cytotoxic effect, cell viability methodology was performed in microplate with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazole bromide (MTT).10 For this, confluent cell cultures of (80-100%) contained in 25 cm2 flasks were started, the medium was discarded using a 5mL serological pipette, two washes were performed to the cell layer with PBS solution (0.004% Ethylene diamine tetracetic acid), 1 mL of Trypsin-EDTA (0.05%) was immediately added and incubated at 37°C for 15 min. A 20 μL sample was taken and 20 μL of 0.4% trypan blue (Gibco, Carlsbad, CA, USA) was added to perform a cell count in a Neubauer chamber (Sigma-Aldrich, model: Bright-LineTM Hemacytometer, catalog number: Z359629) to adjust the cell concentration to 5×104 cells per reaction with Dulbecco’s Modified Eagle’s medium (d-MEM) supplemented with 10% fetal bovine serum. Then 50 μL of the cell suspension was seeded, incubated at 37° C, 5% CO2 in an incubator (Cytomat 2C450S; Thermo Fisher Scientific) until confluence was obtained (after 48 hours), then different concentrations previously evaluated in the study by Millones et al.5 (50 to 0.024 mg/mL) were then applied and the plates were incubated for 48 hours. Only d-MEM culture medium was used as a negative control and as a positive control.
The medium used was then discarded and the cell layer was washed with PBS solution (0.004% EDTA) and 50 μL of d-MEM culture medium was added.
To each well, 20 μL of MTT (5 mg/mL) was added to each culture and then the cultures were incubated for four hours. After this time, the medium was aspirated. 200 μL of dimethyl sulfoxide was added to dissolve the formazan crystals; the plate was left in agitation for 15 minutes microplate shaker (Lab-Line Instrument Inc. Melrose Park, IL) and shaken at 120 revolutions per minute to ensure complete dissolution. Finally, the plate was read at 570 nm on a Smart Spectophotometer plus reader (1705061, Bio-Rad, Hercules, CA, USA).
The experimental data were analyzed using nonlinear regression with the Gompertz model to evaluate the effect according to the concentration of the doses used, whose equation is given by:32
Where, y is the cell viability, x the administered concentration of each product (mg/mL), and α, β, and k are the parameters of the model. Comparison of the cytotoxic effect of propolis and P. guajava was performed using analysis of covariance, which in addition to the product includes the concentration administered. Graphical presentation was prioritized to highlight some analyses. The analyses were performed with Excel (Microsoft Corporation, US, 2019) (RRID:SCR_016137) and SPSS version 26 (IBM, US, 2019) (RRID:SCR_016479).
The cytotoxic effect of Peruvian propolis is shown in Figure 1, with high cell viability at concentrations of 0.24 mg/mL, reaching 1.120±0.012 HBA cells, 0.922±0.011 HGF-1 and 0.624±0.002 PBMCs cells, decreasing rapidly to 0.052±0.002, 0.051±0.001 and 0.055±0.001, respectively as concentrations increase up to 50 mg/mL. The estimated nonlinear Gompertz regression models were:
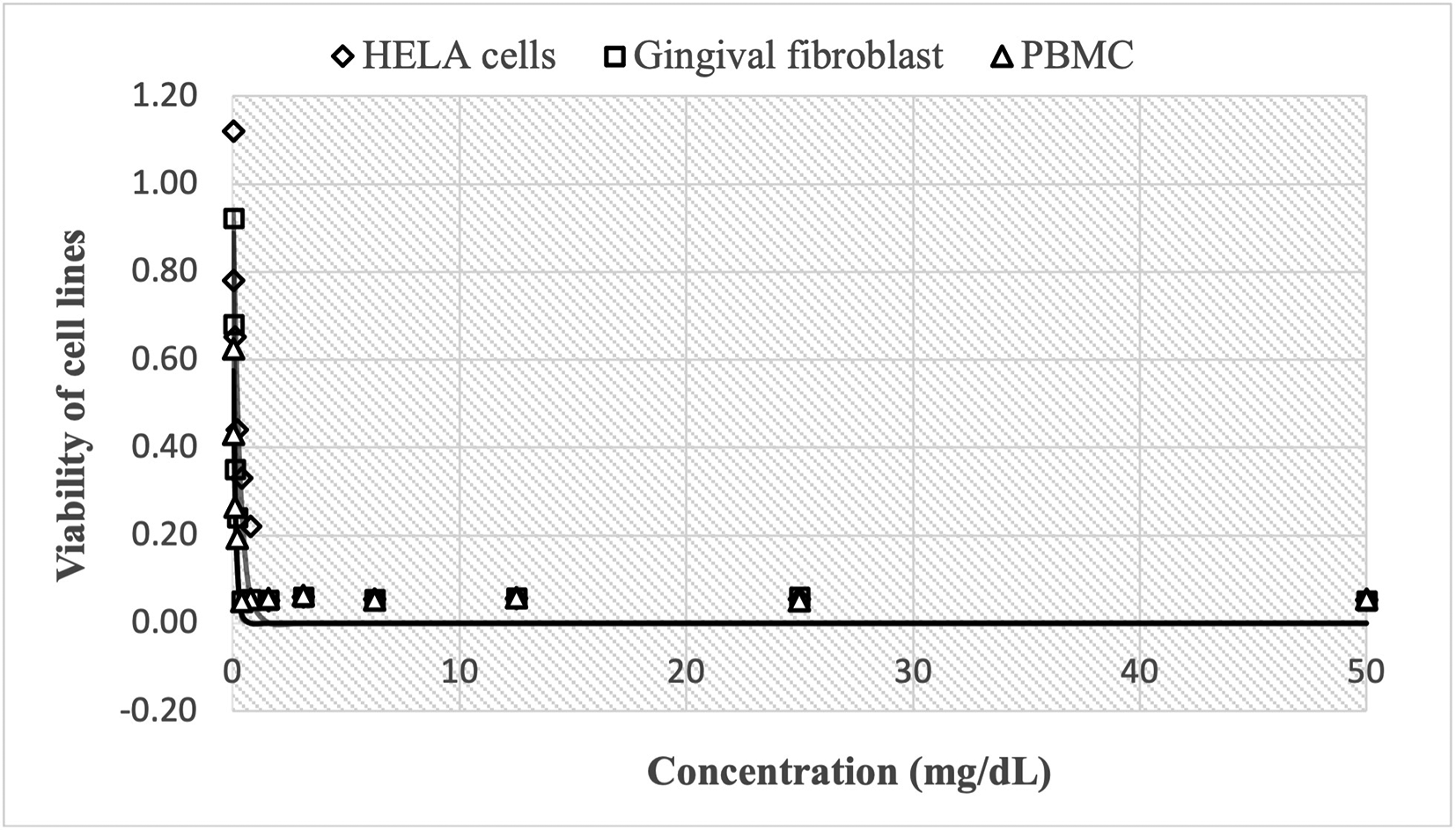
The cytotoxic effect of Peruvian propolis is shown in Figure 1, with high cell viability at concentrations of 0.24 mg/mL, reaching 1.120±0.012 Henrietta Lacks cells (HELA cells), 0.922±0.011 human gingival fibroblasts (HGF-1) and 0.624±0.002 peripheral blood mononuclear cells (PBMCs cells), decreasing rapidly to 0.052±0.002, 0.051±0.001 and 0.055±0.001, respectively as concentrations increase up to 50 mg/mL. The observed trend shows that cell growth decreases in a non-linear fashion as the dose of Peruvian propolis administered increases.
On the other hand, the cytotoxic effect of Peruvian P. guajava is shown in Figure 2, with high cell viability at concentrations of 0.24 mg/mL, reaching 1.190±0.015 HBA cells, 0.948±0.020 HGF-1 and 0.685±0.019 PBMCs cells. These decreased more slowly to 0.656±0.019, 0.165±0.020 and 0.099±0.002, respectively as concentrations increase up to 50 mg/mL. The estimated nonlinear Gompertz regression models were:
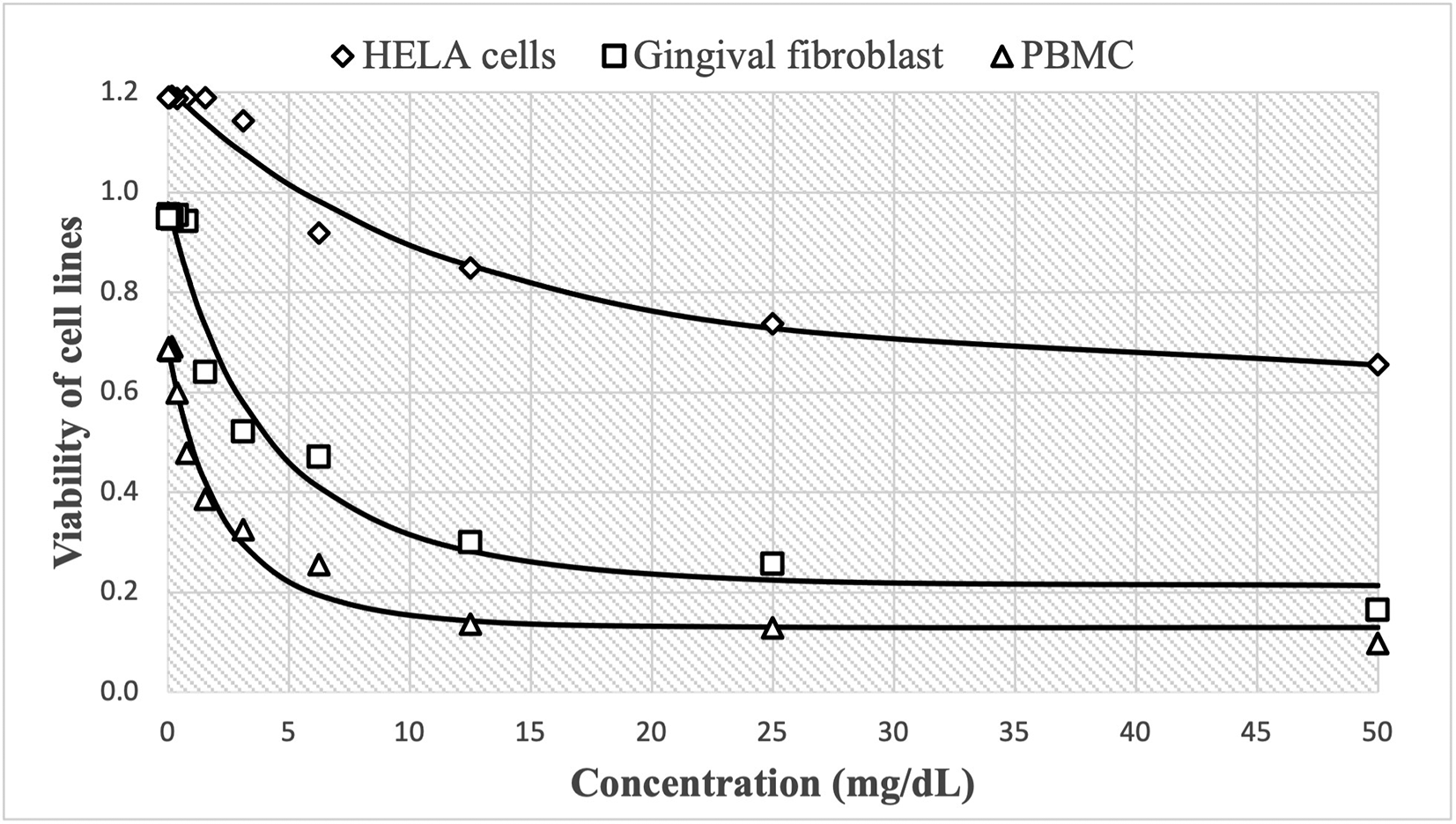
The cytotoxic effect of guajava is shown in Figure 2, with high cell viability at concentrations of 0.24 mg/mL, reaching 1.190±0.015 Henrietta Lacks cells (HELA cells), 0.948±0.020 human gingival fibroblasts (HGF-1) and 0.685±0.019 peripheral blood mononuclear cells (PBMCs cells). These decreased more slowly to 0.656±0.019, 0.165±0.020 and 0.099±0.002, respectively as concentrations increase up to 50 mg/mL. Similarly, the observed trend shows that cell growth decreases in a non-linear fashion as the dose of guajava administered increases.
The goodness of fit of the Gompertz curves to the cytotoxic effect on the cell lines of both products is shown by the coefficient of determination (R2), this value is above 90% for all curves.
The cytotoxic effect of propolis and P. guajava on the cell lines was compared using analysis of covariance, which are shown in Table 1. In each of the cell lines, differences in the cytotoxic effect between both products were observed (p<0.01 in each of the lines); likewise, the linear effect of the concentrations used for each of the products was observed (p<0.01 in each of the lines), even though the non-linear effect was verified by means of the Gompertz model.
Figures 3-5 show the cytotoxic effect of propolis and P. guajava on the cell lines as a percentage of cell viability, established from the medium controls and Triton X-100, being notorious the differences in the effect between both products, as already shown.
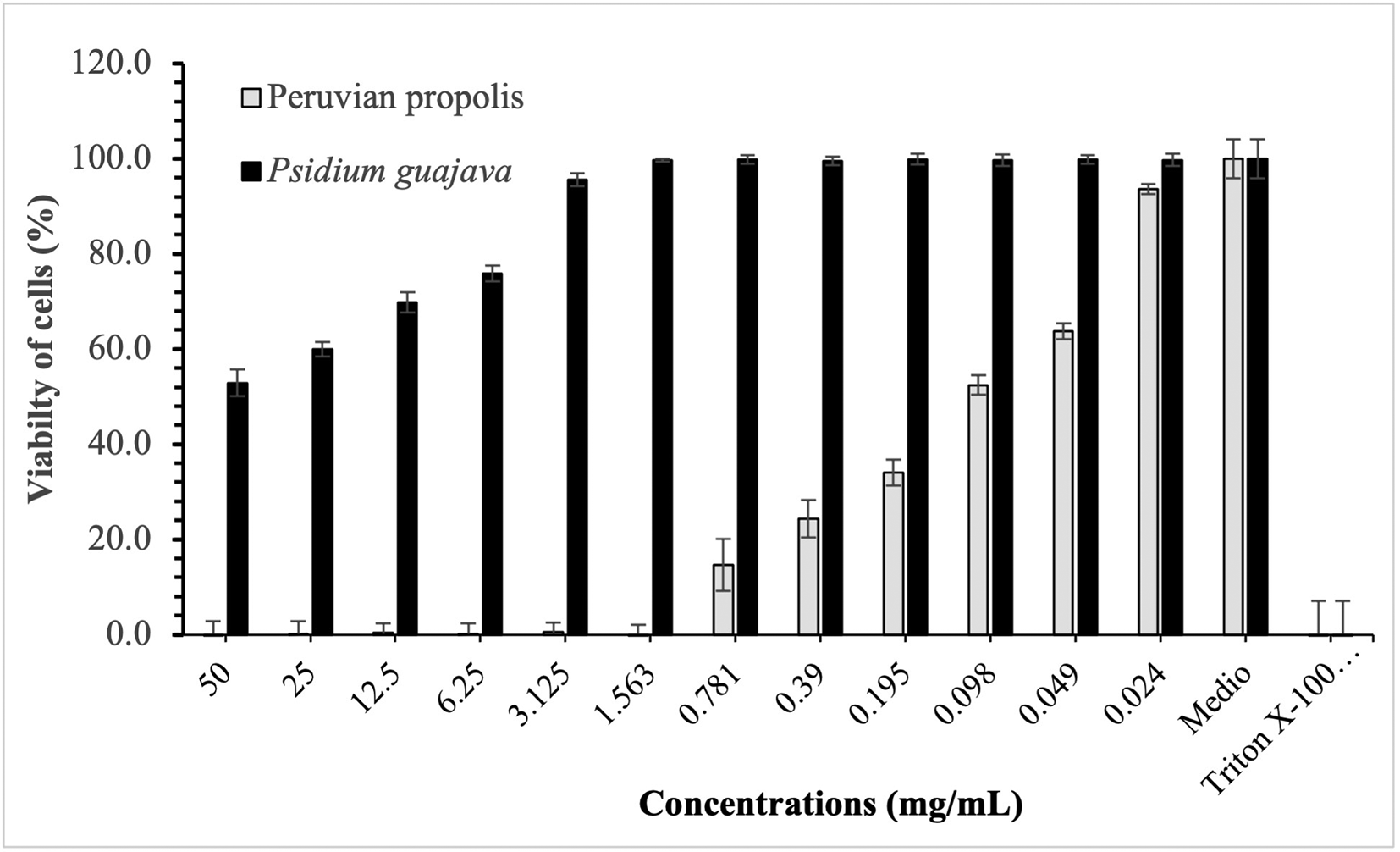
Figure 3 compares the cytotoxic effect of propolis and P. guajava concentrations and Triton X-100 on Henrietta Lacks cells (HELA cells) viability (%), showing the average and the corresponding standard deviations. With propolis, the growth of HELA cells remains in control with doses of 50-1,563 mg/mL, increasing very rapidly with smaller doses. In contrast, with guajava, cell growth is already more than 50% at doses of 50 mg/mL, growing rapidly, and reaching maximum levels at doses of 1,563 mg/mL or lower.
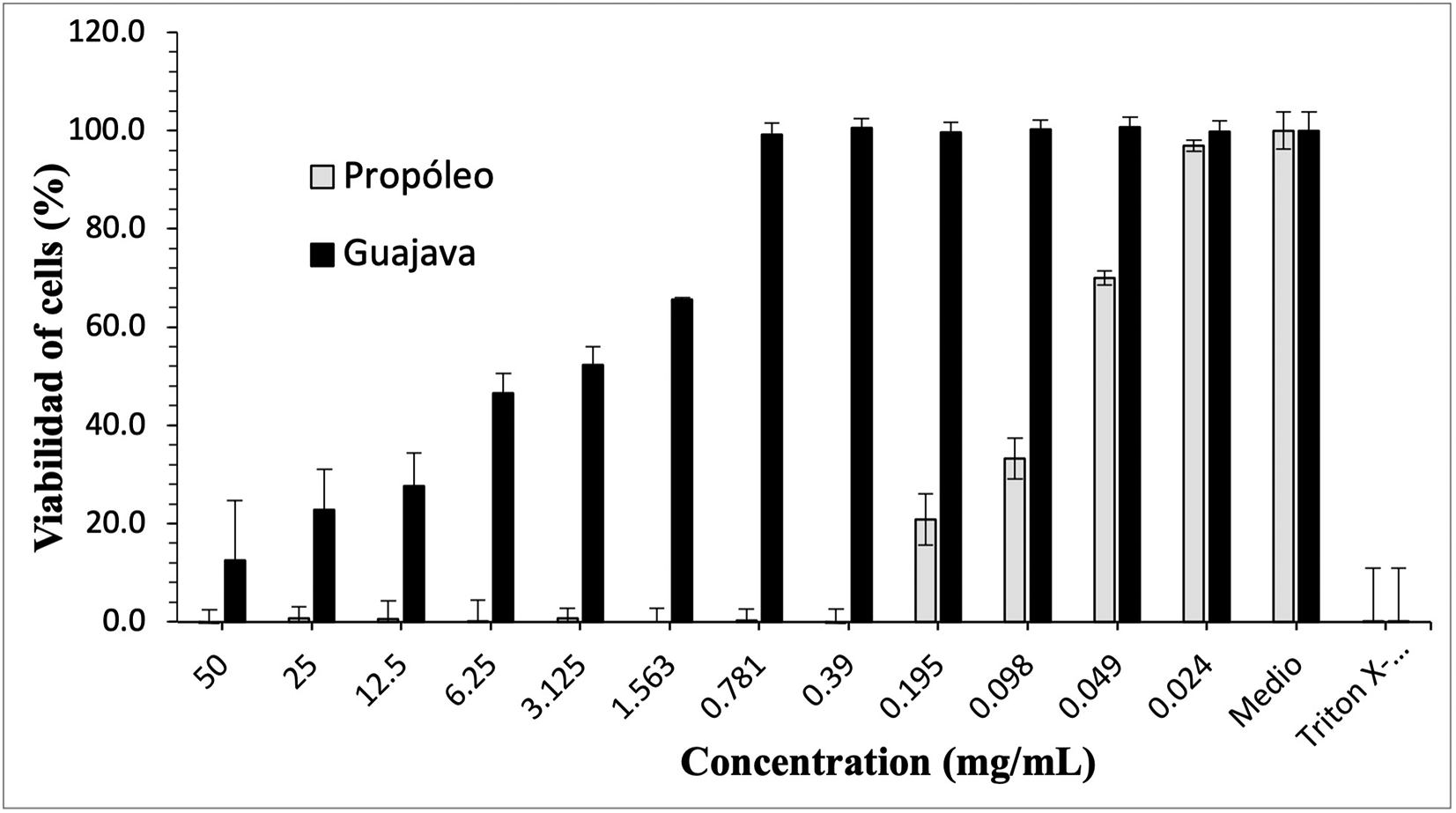
The growth of human gingival fibroblasts remains in control with propolis doses of 50-0.39 mg/mL, with rapid growth at lower doses; in contrast, with gujava, while at doses of 50 mg/mL cell growth was pc more than 10%, it begins to increase considerably up to doses of 0.781 mg/mL, at which it reaches maximum growths.
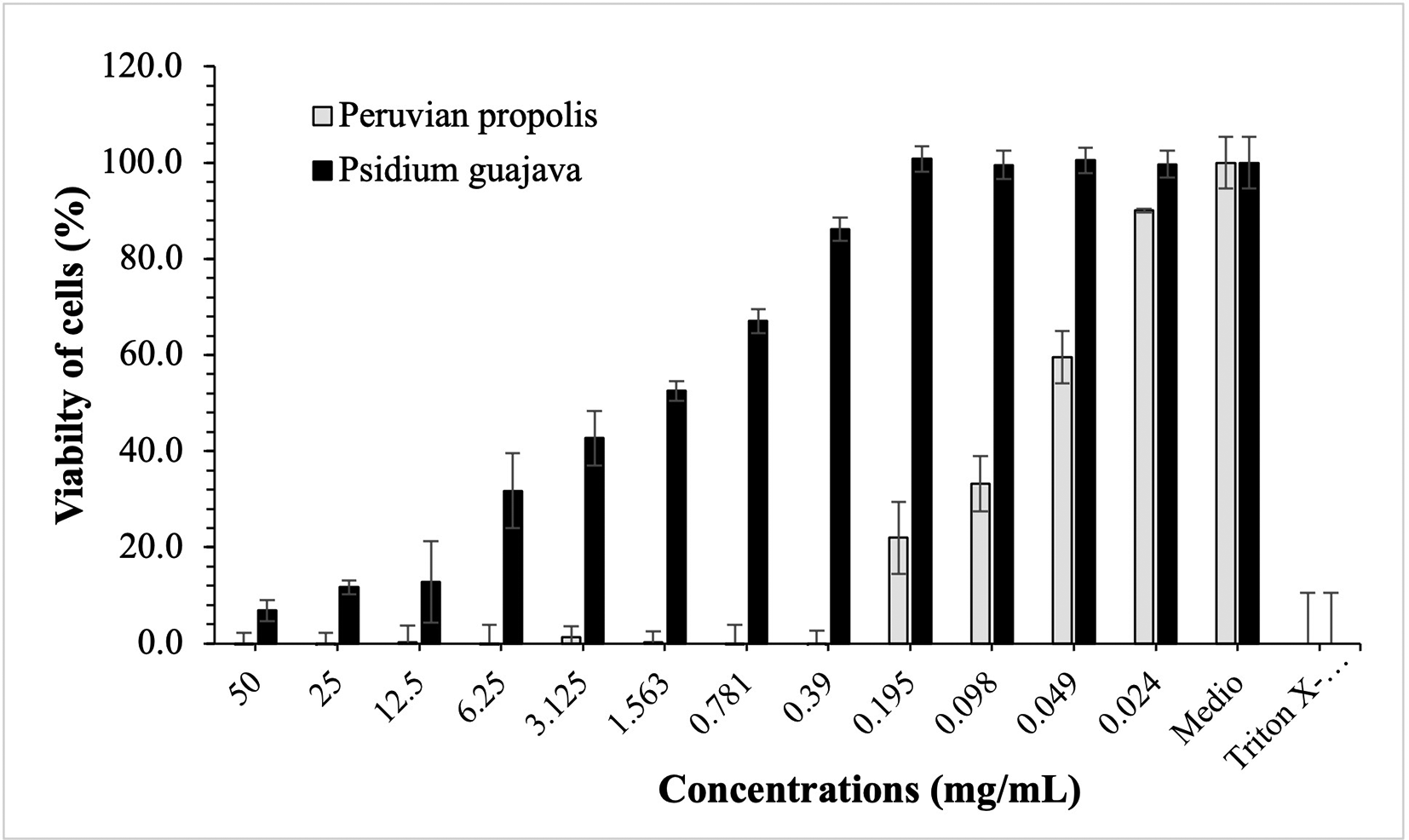
The growth of peripheral blood mononuclear cells (PBMCs cells) remains in control with propolis doses of 50-0.39 mg/mL, with rapid growth at lower doses; on the contrary, with guajava, although at a dose of 50 mg/mL cell growth was small, it begins to increase considerably up to doses of 0.195 mg/mL, at which it reaches maximum growth.
The aim of this study was to evaluate the cytotoxic effect of ethanolic extracts of propolis and P. guajava on HELA cell lines, HGF-1 and PBMCs cells. The results showed high cell viability at concentrations of 0.24 mg/mL, decreasing rapidly as concentrations increased up to 50 mg/mL.
The cytotoxicity assay revealed that Peruvian propolis and guajava extracts at lower concentrations can work safely on the fibroblast cell line. However, it is important to recognize that since this is an in vitro assay, this value may vary if other types of cell lines are used. The results obtained in the cytotoxicity assay, add to the increasingly abundant information reported by other research groups that have wanted to address this issue but focusing on alcoholic extracts,17–19 and are therefore important. The content of phenols and flavonoids obtained in the aqueous extract of propolis is lower than that reported by other research groups20 however, it should be noted that the extraction methods were different and as mentioned, these are determinant for the preparation of the extract. To determine which methodology is more efficient, the same propolis sample should be used,21–24 due to the high variability in the composition that this product may have with respect to its region of origin.24
As for P. guajava, it showed similar toxicity in the three cell lines. Some studies report a higher amount of active metabolites in the peel with respect to guava pulp, and also report a better antioxidant capacity in vitro.24,25 With the cytotoxic effect shown in HeLa cells, the world list of plants with potential for cases of cervical neoplasia published is increased, the results obtained in the study contribute to corroborate the properties traditionally attributed to these plants and highlight species of the Peruvian medicinal flora as a source of substances for the treatment of cancer.25
Despite the high cytotoxicity shown by most of the propolis samples against the cell lines studied, the samples also showed toxicity to HGF-1 culture. Ling et al.26 have also investigated the cytotoxicity of Brazilian red propolis extracts for two tumor cell lines (Hep-2 and HeLa) and for normal human embryonic epithelial kidney (Hek-293), also reporting a higher IC50 value for Hek-293 compared to the tumor cell lines.
Although both propolis and guava seem to exert a potential on PBMCs cells, only a few studies have been performed in this field of research.27–30 Despite this, our preliminary data suggest that both products could have a significant biological effect on the three cell lines tested, which opens up prospects for further research in this field.
EEP and EEG at low concentrations do not show cytotoxicity in human cell lines and their effect is dose dependent. Our findings are an advance in the preclinical evaluation of natural extracts from Peru on their safety and open a continuity to further studies for their potential applications in dentistry and medicine. Despite our positive data, further study is required to evaluate the usefulness of these extracts.
Mendeley: Cytotoxicity database. https://doi.org/10.17632/yt4h7h9cvv.131
This project contains the following underlying data:
Mendeley: Cytotoxicity database. https://doi.org/10.17632/yt4h7h9cvv.131
This project contains the following extended data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology and Immunogenetics, microbiology.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products and microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Immunology and Immunogenetics, microbiology.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products and microbiology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 Aug 22 |
read | read |
|
Version 1 19 Apr 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)