Keywords
Metformin, Type 2 Diabetes Mellites, Malignancy
This article is included in the Oncology gateway.
Metformin, Type 2 Diabetes Mellites, Malignancy
1) Epidemiology of the cancers in Saudi Arabia has been included.
2) Pathogenesis has been classified based on the possible mechanism of the effect.
3) The graphical representation of the data has been added with the related tabular presentation of the data.
4) The references have been updated.
See the authors' detailed response to the review by Deqiang Huang
Type 2 diabetes mellitus (T2DM), also known as non-insulin-dependent diabetes mellitus or adult-onset diabetes mellitus, is an epidemic that is marginally increasing in number, especially among obese patients, with global prevalence (382 million) in 2013, expected to rise to 592 million by 2035.1 In 1998, metformin became the first-line drug for managing T2DM. It was soon observed to also have a mild effect of weight reduction in obese patient.2 Metformin was found to have anti-tumor effects, which have been tested in vivo and in vitro in many studies.6–10 Some studies even suggest that metformin has a preventative effect against cancers.9 In this study, we aim to assess the beneficial effects of metformin on different cancers.
Diabetes has been an increasing problem in today’s world. T2DM prevalence has been increasing rapidly, mainly because of availability and abundance of unhealthy food, as well as an increase in sedentary lifestyle.3,4 As of 2019, it has been reported that T2DM prevalence worldwide is 9.3%,3 while in Saudi Arabia it is estimated to be 25.4% (published in 2015), with urban areas having more prevalence than rural areas.4 Cancer in Saudi Arabia has been estimated in 2020 as CRC 14.4%, breast cancer 14.2%, thyroid cancer 10.2%, NHL 6.1%, leukemia 6%, and other cancers 49.2% in both the gender.5 Among males of all ages, it is CRC 19.3%, NHL 8%, leukemia 6.7%, thyroid cancer 6.2%, lung cancer 6%, and other cancers 53.8%.5 Similarly, among females of all ages, it is breast cancer 29%, thyroid cancer 14.3%, CRC 9.2%, uterine cancer 7.5%, leukemia 5.3%, and other cancers 34.8%.5
Metformin (aka Glucophage) is an anti-hyperglycemic biguanide drug that is widely used and is well known to be the first-line of medication for T2DM. The relationship of T2DM and malignancies, that highlights its possible role in carcinogenesis, can be realized by the following diagram (Figure 1).6
There have been multiple mechanisms suggested for the anticancer effects of metformin. It acts on several biochemical pathways. They are classified as follows:
a) AMPK activation: The most notable action is lowering overall glucose levels in the blood by decreasing the hepatic gluconeogenesis and increasing the body sensitivity to insulin, consequently decreasing the plasma insulin level.7,8 One of the downstream effects of metformin is on AMPK (adenosine monophosphate-activated protein kinase); the activation of it leads to multiple metabolic alterations shown in Figure 2.9
b) AMPK-mediated TSC1/2 activation: Adenosine triphosphate (ATP) is reduced by metformin, consequently adenosine monophosphate (AMP) increases.10,11 This activates liver kinase B1 (LKB1) that initiates the AMPK signaling pathway.12–14 AMPK has a negative effect on mammalian target of rapamycin complex 1 (mTORC1) directly, and indirectly through tuberous sclerosis complex (TSC) 1/2 activation.14 Also, metformin inhibits Rag-guanosine triphosphatase (GTPase), which is an activator of mTORC1. These suppressive actions lead to inhibition of many pro-carcinogenic agents, such as hypoxia-inducible factor-1 α (HIF-1 α) and ribosomal protein S6 kinase (S6K).14 Metformin also counteracts the growth factor induced protein-kinase B (PKB) pathway through the AMPK-mediated TSC1/2 activation, as illustrated in Figure 3.6,14
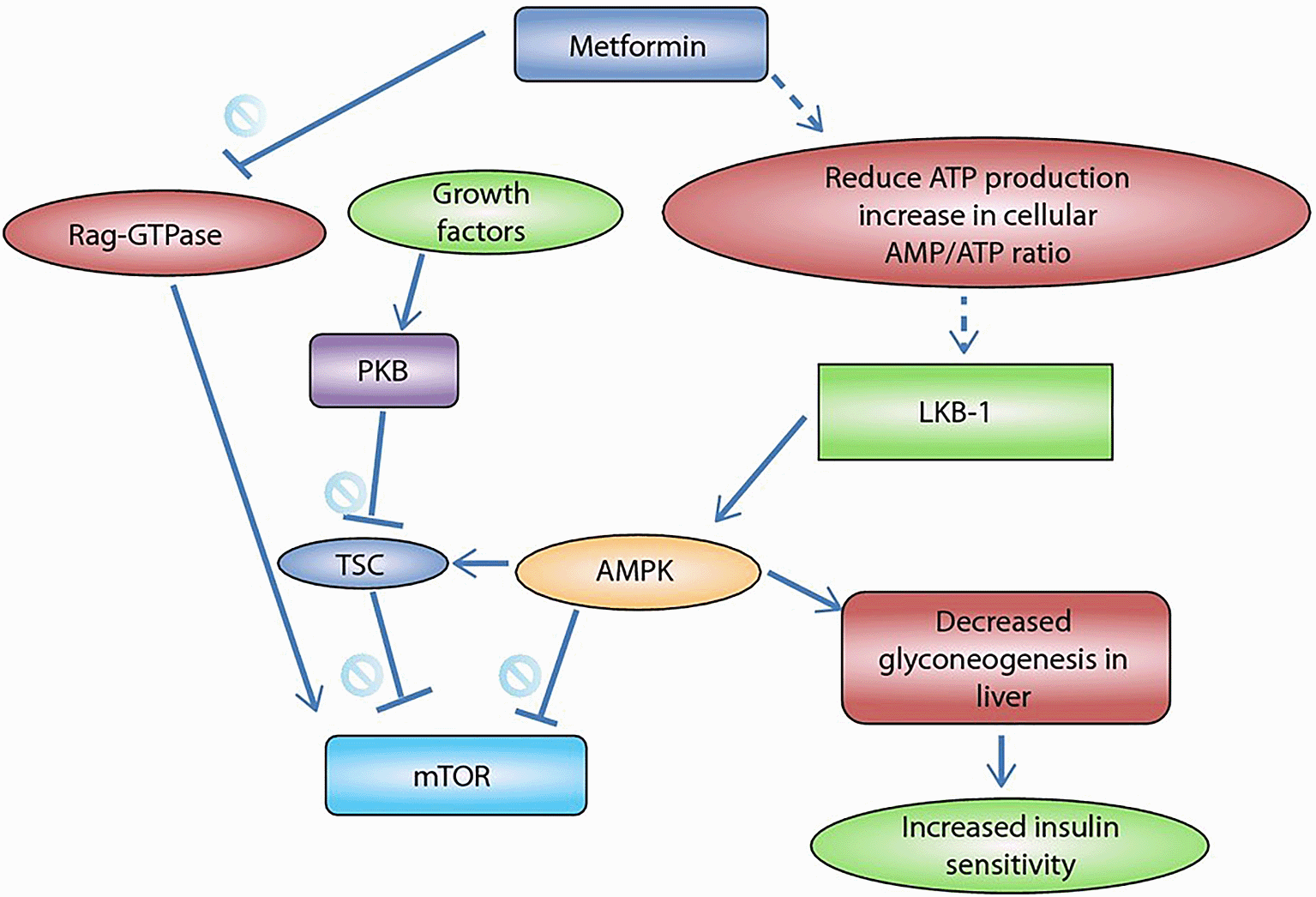
Rag-GTPase (Rag-guanosine triphosphatase), ATP (adenosine triphosphate), AMP (adenosine monophosphate), PKB (protein-kinase B), LKB-1 (liver kinase B1), TSC (tuberous sclerosis complex), AMPK (adenosine monophosphate-activated protein kinase), mTOR (mammalian target of rapamycin). This figure has been reproduced with permission from [Sen et al., 2014].6
The potential targets of anti-cancer medications can be summarized as the actions on one or more of the following main pathological features of the malignancies: overexpression of growth signals, failure of the programmed cell death (apoptosis), evasion of anti-growth signals, formation of new capillaries (angiogenesis), and invasiveness to the surrounding tissue and metastasis.15 Metformin reduces the proliferation of cancer cells by inhibiting mTOR that prevents protein synthesis and cell growth.15
c) Upregulation of miRNA34a: The anti-proliferative effect of metformin has been studied recently in two cancer cell lines, one with cervical cancer cells through the AMPK pathway,16 and the second with human renal cell carcinoma cell line by cell cycle arrest by upregulation of miRNA34a.17
It may improve the efficiency of different cancer treatment and also have a role in prevention and decreasing the mortality.18–20 Hyperinsulinemia and inflammation are cancer risk factors; metformin is thereby beneficial in decreasing various cancers.21 In addition, metformin has a significant impact on response to the radiotherapy in rectal cancer when used as neoadjuvant.6
d) PD-L1 & IGF-1R pathway: AMPK activation decreases the expression of programmed death ligand-1 (PD-L1), which facilitates cytotoxic T-lymphocyte-mediated tumor cell death. It represses the insulin-like growth factor-1 receptor (IGF-1R) pathway which is a promotor of cancer cell growth and metastasis, as shown in Figure 4.18
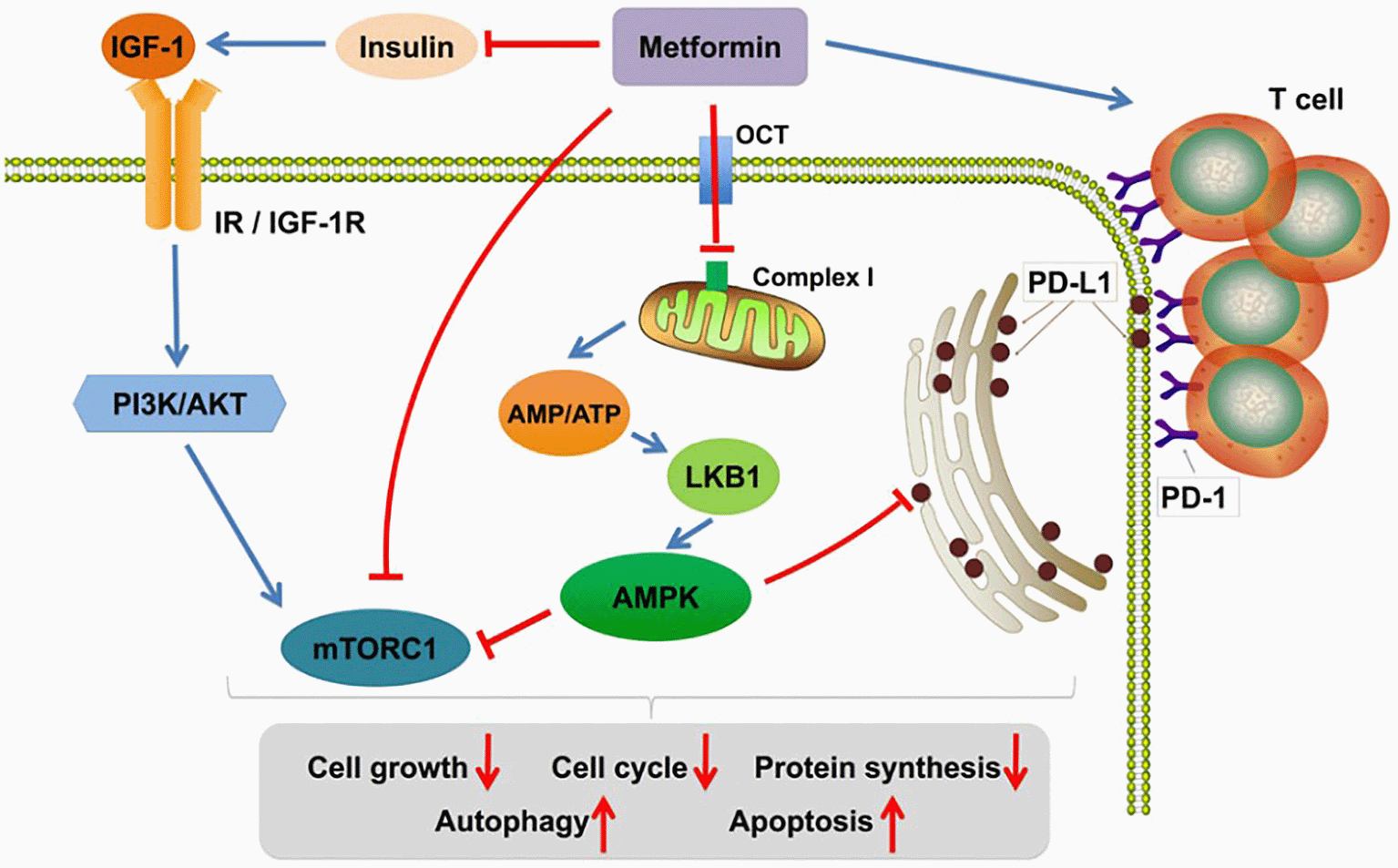
IGF-1 (insulin-like growth factor-1), IR (insulin receptor), IGF-1R (insulin-like growth factor-1 receptor), OCT (organic cation transporter), PI3K (phosphatidylinositol 3-kinase), AKT (also name as PKB-protein kinase B), AMP (adenosine monophosphate), ATP (adenosine triphosphate), PD-L1 (programmed death ligand-1), LKB1 (liver kinase B1), mTORC1 (mammalian target of rapamycin complex 1), AMPK (adenosine monophosphate-activated protein kinase). This figure has been reproduced with permission from [Chen et al., 2020].18
Metformin was found to have a direct pathway and an indirect pathway. The direct pathway includes AMPK dependent and independent effects. The direct AMPK-dependent mechanism was shown to suppress mTOR, folate level, c-MYC, NF-κB, and increase in P53 phosphorylation. In the AMPK-independent mechanism, metformin causes a decrease in cyclin D1 and reactive oxygen species (ROS) and an increase in mTORC1, apoptosis, and autophagy. Metformin also induces apoptosis in squamous cell carcinoma and inhibits a certain gene from the P53 family (DeltaNp63alpha).22
e) Inhibition of mTOR: Metformin can inhibit mTOR without the use of AMPK pathway, this is achieved mainly by inactivation of negative regulators of mTOR like Rag GTPases and regulation of REDD1 (Regulated in Development and DNA Damage Responses-1). Therefore, metformin inhibits cancer growth and proliferation by inhibition of protein translation.6,23
Research has also found that leptin and adiponectin have opposing roles in tumor growth and metastasis. Increase in serum leptin levels lead to an increase in tumor growth. While adiponectin appears to exhibit inhibitory effect on tumor growth and development. Metformin inhibits leptin production and increases adiponectin production, which further proves metformin’s anti-cancer effect.
T2DM is known to cause oxidative stress. Metformin decreases ROS and oxidative stress, which are considered to have a role in cancer in general.24 This complements metformin’s effect on cancers.
f) Notch1/Hes1 overactivation: There are multiple cancers on which metformin has exhibited effects. In colorectal cancer, there is overactivation of Notch1/Hes1, metformin relieves this overactivation, which improves the survival rates of these patients. In pancreatic cancer, metformin decreases desmoplastic reaction which in turn inhibits the growth of cancer, this effect is thought to be dose dependent. In renal cancer, metformin induces cell cycle arrest in renal cell carcinoma, as well as miRNA34a upregulation. In cervical cancer, metformin induces apoptosis and inhibits the proliferation of cancer cells. In endometrial cancer, metformin inhibits STAT3 expression; STAT3 overexpression was found to be related to high glucose concentration in the blood. In gastric cancer, metformin inhibits the sonic the hedgehog pathway, which plays a big role in the pathogenesis of gastric carcinoma. In lung cancer, metformin was observed to affect adenoma formation throughout various stages.22
In cancer cells, the mTOR signaling pathway promotes cell growth and proliferation25,26 through interplay between two rivaling pathways which are the AMPK pathway that signals for any deficiency in ATP,27 as well as the Akt pathway which signals for nutrient availability.28–30 TORC1 contains two components responsible for regulating cell growth by opposing mTOR activity, these two components are called the proline-rich Akt substrate of 40 kDa (PRAS40),31 and the raptor protein. When discussing activation of TORC1, there are generally two types of inputs responsible for such a task: over-abundance of intracellular amino acids and activated insulin or IGF1 signaling. These bio-signals initiate a protein called phosphatidylinositol-3-kinase (PI3K), which activates the Akt pathway.28–30 Consequently, activation of Akt will lead to increased TSC2 gene phosphorylation, which will stimulate TORC1 and inhibit GTP-activated protein (GAP) activity.32 Meanwhile, Akt will additionally phosphorylate PRAS40, which will antagonize inhibitions on Akt. AMPK is stimulated when there is deficiency in ATP, leading to phosphorylation of TSC2 by AMPK then switching off of the mTOR, which will then stimulate Rheb-GAP activity, a kinase thought to be involved in stimulation of mTOR phosphorylation. Biguanides, in the absence of TSC2, are also thought to activate AMPK through raptor phosphorylation. mTOR kinase activity seems to be directly responsible for this effect, and it could involve the process of partial PRAS40 protein dissociation and/or an increased 14-3-3 proteins-binding. In conclusion, the AMPK cascade exerts two main inhibitory mechanisms on mTOR via phosphorylation of TSC2 and raptor. Likewise, the Akt cascade causes two stimulatory mechanisms via phosphorylation of TSC2 and PRAS40, Figure 5.31–33
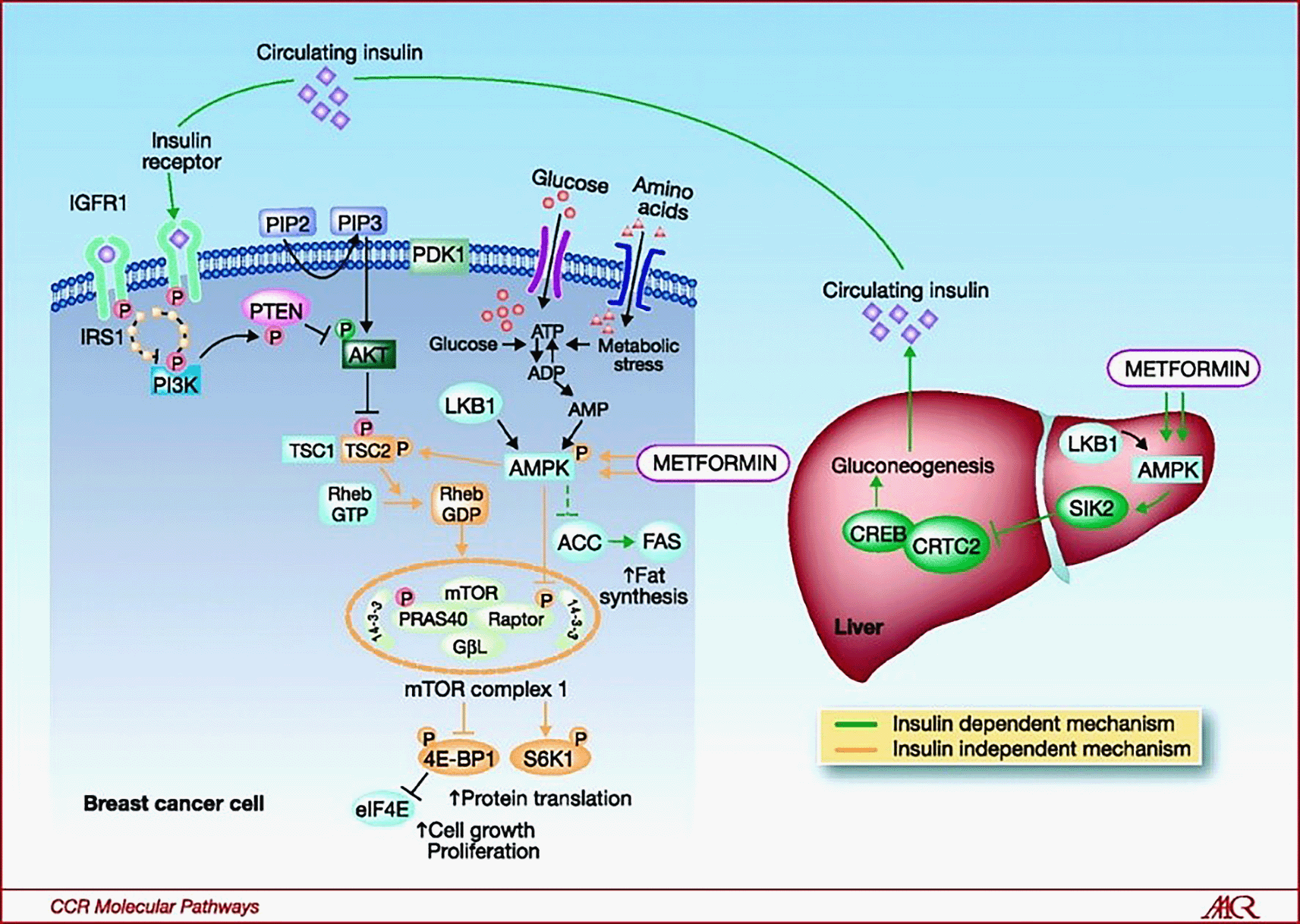
IGFR1 (insulin-like growth factor-1 receptor), PIP (Phosphatidylinositol 4,5-bisphosphate), PDK1 (3-Phosphoinositide-dependent kinase 1), IRS1 (Insulin receptor substrate 1), p (phosphorylise), PTEN (Phosphatase and TENsin), PI3K (phosphatidylinositol 3-kinase), AKT (protein kinase B), ATP (adenosine triphosphate), ADP (adenosine di-phosphate), AMP (adenosine monophosphate), LKB1 (liver kinase B1), TSC (tuberous sclerosis complex), Rheb GTP (Rheb-guanosine triphosphatase), Rheb GDP (Rheb-guanosine diphosphatase), AMPK (adenosine monophosphate-activated protein kinase), ACC (acetyl-CoA carboxylase), FAS (fatty acid synthesis), mTOR (mammalian target of rapamycin), PRAS40 (Proline-rich Akt substrate of 40 kDa), GβL (G protein β subunit-like), 4E-BP1 (Eukaryotic translation initiation factor 4E-binding protein 1), S6K1 (Ribosomal protein S6 kinase beta-1), eIF4E (Eukaryotic translation initiation factor 4E), CREB (cAMP-response element binding protein), CRCT2 (CREB regulated transcription coactivator 2), and SIK2 (salt-inducible kinase-2). This figure has been reproduced with permission from [Gonzalez-Angulo et al., 2010].33
LKB1 (Liver kinase B1) is an upstream activator which is related to the AMPK pathway and is considered as a tumor suppressor. When it mutates or loses its function, the anti-tumor effect of metformin also decreases, therefore it is an important tumor cell growth and metabolism-regulator.34
g) Overexpression of GPD1: Another study shows metformin can be combined with the overexpression of glycerol-3 phosphate dehydrogenase 1 (GPD1), which amplifies the anti-cancer effect greatly. GPD1 is an isoenzyme of human glycerol-3-phosphate dehydrogenase (GPDH), it suppresses cancer by inhibiting mitochondria and inducing cell apoptosis. This combination enhances the level of GPDH by two mechanisms: GPD1 converts DHAP to G3P, all the while metformin inhibits GPD2 activity. This combination mainly acts by suppressing cancer cells in both A549 (adenocarcinomic human alveolar basal epithelial cells) and DU145 (human prostate cell cancer line). Furthermore, metformin reduces ATP production by inhibiting mitochondrial function, this process is achieved by an increase in G3P, which in turn produce a toxic compound named methylglyoxal. This compound suppresses respiration of the mitochondria. All of this leads to an increase in ROS and consequently, cell death.35
Some studies have reported that metformin improves the effect of radiotherapy in multiple cancers, like lung cancer, prostate cancer, breast cancer, hepatocellular cancer, and esophageal cancer.36
A recent study has found that metformin enhances the direct binding capabilities of the AMPK protein to the PD-L1 protein which in turn inhibits it.37 Western blot was used to showcase the significant enhancement of AMPK phosphorylation by the treatment of metformin. Specifically, in RL95-2 cells and Ishikawa cells. T-cell activity in endometrial cells was also increased due to the AMPK enhancement by metformin.38–40 Metformin also decreases stimulation of insulin receptors in tumor cells which might explain its anti-proliferative effect. Also, metformin has been confirmed to hold an anti-tumor activity against endometrial cancer.38–40 But it still would be too quick to establish the adequacy of using metformin alone to treat endometrial cancer.38–40
The purpose of this study is to assess possible beneficial effects of metformin on various types of malignancies, to find out whether it influences decreasing cancer burden, to explore the mechanisms in which it can act as an anticancer drug, and to propose recommendations for future research addressing this topic.
Imam Abdulrahman Bin Faisal University (IAU) Institutional Review Board (IRB) approval for this study was gained (IRB#: IRB-2021-087-Med) and permission to access patient records was acquired and patient consent was waived. The IRB stated that no consent was required as no intervention was done. The existing data were only analyzed.
This was a retrospective observational study of patients with different malignancies registered in King Fahd Hospital of the University (KFHU) information system during the years 2018–2019. No potential biases were considered.
There were three variables defined. They were independent (metformin usage, metformin dosage) , dependent (cancer stage, cancer grade, outcome), and controlled (no controlled variables).
This study included 266 patients out of 300 cases of malignancy at KFHU in one year (2018–2019). Their data were collected from patient's records using their medical record numbers (MRN) from the KFHU patients information system (QuadraMed system). We collected the MRN of all diagnosed cases of malignancy from the Department of Pathology. Then further details were obtained from QuadraMed system (KFHU patients information system).
All patients with or without T2DM were included and patients with T1DM (type 1 diabetes mellitus) were excluded. Patients with malignancies were divided into diabetic and non-diabetic groups. The diabetic group was further divided into metformin users and non-metformin users. For each patient, the data was collected pertaining to the age at which their malignancy was first diagnosed, the tumor stage (e.g. TNM & other system used in KFHU), the tumor histological grade, the course of the disease along with the treatment protocols followed and the follow-ups. Patients with malignancies and without diabetes were considered as the overall control group. The record of all risk factors, including body mass index (BMI) and physical activity were collected. All relevant and available medication history and procedures are recorded.
The data was collected using the electronic QuadraMed system (KFHU patients information system) and only data that contained the patient's relevant medical information with coded ID to protect patient’s privacy was collected. Data was eventually placed on a Microsoft Excel 2019 sheet form to facilitate the calculations and assessing the findings.
The duplicate and irrelevant data were removed. The naming conventions were standardized without any typo or incorrect capitalization. The cases with missing data were excluded and unwanted outliers were removed. After this the validation was performed. The Excel sheet were coded for transformation to SPSS version 22 to be analyzed. We calculated the basic statistical values like number of cases in each category. We used chi-square test to analyze all the categorical data. Comparisons between metformin usage/dosage and cancer stage, cancer grade, and outcome were established. Finally, we analyzed the relations between cancer stage, cancer grade, and outcome.
This study included 266 patients out of 300 patients.41 The excluded patients comprised of patients with T1DM and those with missing data. The average age of patients was 51.15 years old. Age was categorized into <18 years old, 18–39 years old, 40–64 years old, and 65 years old. Of these patients, 201 were Saudi while the remaining 65 were non-Saudi and 99 of these patients were males while 167 were female. Meanwhile, 73 of these patients were diabetic and 193 were non-diabetic. The average BMI of these patients was 28.29 kg/m2. The BMI was categorized into underweight (<18.5 kg/m2), normal (18.5-24.9 kg/m2), overweight (25-29.9 kg/m2), obese class I (30-34.9 kg/m2), obese class II (35-39.9 kg/m2) and morbidly obese (40 kg/m2); 43 patients couldn’t be assessed for BMI. A total of 52 patients were using metformin while the remaining 214 were not using metformin. The average metformin dose used was 1068.26 mg/day. Details for the patients’ cancers grade can be seen in graph section (Figures 6 & 9/Tables 1 & 4). The grade of cancers of 92 patients couldn’t be assessed. Details for the patients’ cancers stage can be seen in graph section (Figures 7 & 10/Tables 2 & 5). The stage of cancers of 123 patients couldn’t be assessed. Overall, 19 patients died after diagnosis of cancer while the remaining 249 patients did not.
| Cancer grade/Metformin | I | II | III | IV |
|---|---|---|---|---|
| Metformin (37) | 8 | 22 | 7 | 0 |
| Not Metformin (137) | 29 | 47 | 59 | 2 |
| Total (174) | 37 | 69 | 66 | 2 |
| Cancer stage/Metformin | I | II | III | IV |
|---|---|---|---|---|
| Metformin (30) | 5 | 8 | 6 | 11 |
| Not Metformin (113) | 29 | 30 | 15 | 39 |
| Total (143) | 34 | 38 | 21 | 50 |
| Death/Metformin use | Met | No met |
|---|---|---|
| Died (17) | 6 | 11 |
| Not died (249) | 46 | 203 |
| Total (266) | 52 | 214 |
| Death/cancer grade | I | II | III | IV |
|---|---|---|---|---|
| Died (12) | 1 | 3 | 8 | 0 |
| Not died (162) | 36 | 66 | 58 | 2 |
| Total (174) | 37 | 69 | 66 | 2 |
| Death/cancer grade | I | II | III | IV |
|---|---|---|---|---|
| Died (12) | 1 | 0 | 2 | 9 |
| Not died (131) | 33 | 38 | 19 | 41 |
| Total (143) | 34 | 38 | 21 | 50 |
This study focused on the anti-cancer effect of metformin in patients who were diagnosed or referred to King Fahd Hospital of the University during 2018 and 2019. It included 266 patients with malignancies which were divided into three subgroups: 193 non-diabetic patients, 21 diabetic patients not on metformin, and 52 diabetic patients on metformin.
On comparing between metformin usage, dose, and the cancer grade at diagnosis time, our results showed that the effect of metformin use on the cancer grade was statistically significant (p-value 0.022), meanwhile, the effect of metformin dose on the cancer grade at diagnosis was not statistically significant (p-value 0.629). On the contrary, Damjanović et al. reported an influence of metformin dose on cancer cells.41 This could be due to the small sample size.
In analysis, comparing between metformin usage and doses on the stage of cancer at the time of diagnosis, metformin usage to cancer-stage p-value in Pearson chi-square is 0.666 (>0.05). This means that the effect of metformin on cancer-stage is statistically insignificant. The metformin dose to cancer stage p-value in Pearson chi-square is 0.598 (>0.05). This means that the anticancer effects regarding dose of metformin on the stage of cancer is also statistically insignificant. Both of these observations could be due to the same small sample size. However, the use of metformin and its dosage has impact on cancers as seen in the review.24
In analysis, studying effects of metformin usage and dose on mortality in cancer patients, metformin usage to death p-value in Pearson Chi-Square is 0.091 (>0.05), that is statistically insignificant. The metformin dose to death p-value in Pearson chi-square is 0.184 (>0.05). This means that the relation between metformin dose and survivability in cancer patients is also statistically insignificant (Figure 8/Table 3). In our opinion, the sample size and long-term follow-up data could have provided better results.
In analysis, comparing cancer-grade and patient mortality, the grade of cancer p-value in Pearson chi-square is 0.198 (>0.05), which is statistically insignificant. However, it has been shown to have an impact, as seen in the reviews.18,22,23,33 So, our finding is probably small sample size and short duration of the study.
In analysis, comparing between cancer-stage and patient mortality, the stage of cancer p-value in Pearson chi-square is 0.013 (<0.05), which is again statistically significant.
In summary, the only statistically significant finding in our data that supports metformin use as an anti-cancer medication has been regarding its beneficial effect on the grade of cancer at the time of diagnosis. This indicates that the use of metformin delays the process of transformation of early malignancy to the advanced one. Thereby it improves the prognosis of the malignancy.
According to our study and its data analysis, we found that metformin is beneficial on the grades of cancer at the time of the diagnosis. Thereby it improves the prognosis of the malignancy. Death to cancer stage was also significant, the higher the cancer stage, the higher the mortality rate. Our data is not sufficient to conclude on mortality rate. Similarly, to analyze the preventive effect of metformin on cancers, one may need large sample size with extensive long term follow up data. Our study-population includes cancer patients throughout merely two years since the time of their diagnosis.
Our findings suggest the beneficial effects of metformin on cancers thereby provide a ground for further rigorous studies so that it can be used as prophylactic and risk reduction modalities in oncological management.
This study was limited by the small number of the cases, the lack of information and availability in the QuadraMed system, as well as the increased referral rate for cases after diagnosis, which made the accuracy of the death rate inconclusive. The recommendations, in our humble opinion, that can be beneficial in the research of this area are:
• To get a bigger sample size
• Acquiring extensive long term follow-up data, even from multiple databases
• Assessing for any correlation between duration and dose of metformin with the anti-cancer beneficial effect
• Undertaking of clinical trials to investigate the usefulness of metformin as prophylaxis in high-risk cancer cases.
Dryad: Assessing the Anticancer Effects of Metformin https://doi.org/10.5061/dryad.7m0cfxpw5.42
This project contains the following underlying data:
• Data_-_Assessing_the_Anticancer_Effects_of_Metformin.xlsx (age at which their malignancy was first diagnosed, the tumor stage [e.g. TNM & other system used in KFHU], the tumor histological grade, the course of the disease along with the treatment protocols followed and the follow-ups.
• README.txt (The data includes gender, age, BMI (wherever available), age at which cancer were diagnosed including its grade and stage, diabetic status, taking or not taking metformin with doses, complications, and fate.)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors would like to acknowledge the Deanship of Scientific Research of Imam Abdulrahman Bin Faisal University and Majmaah University, Kingdom of Saudi Arabia for supporting this work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: I am a certified Medical Lecturer in Clinical Biochemistry and Toxicology with special interest in Translational Research.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer resistance, Cancer biology, Anti-cancer drug discovery, drug repurposing.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pharmacy
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Biochemistry, Cancer research
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cancer biology, pediatric cancer, metastasis.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: AMPK signal pathway
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
|
Version 2 (revision) 09 Aug 22 |
read | read | read | read | ||
|
Version 1 19 Apr 22 |
read | read | ||||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)