Keywords
Wound-healing, Keratinocyte, Natural Products
This article is included in the Cell & Molecular Biology gateway.
Wound-healing, Keratinocyte, Natural Products
The skin, including the dermis (deeper layer) and epidermis (surface layer), is the most significant barrier between the external environment and the human body.1,2 Skin protects against environmental factors such as harmful UV rays and pathogens and prevents water loss.3
The skin’s vital functions are physical, chemical, bacterial, and wound-healing barriers.4 Wound-healing in the skin is one of the mechanisms that maintain homeostasis. In general, the wound-healing process is divided into 4 phases: the coagulation and hemostasis phase, the inflammatory phase, the proliferative phase, and the remodeling phase.5 A wound-healing response begins when the skin layer (epidermis) is injured externally.6 In response to skin damage, epithelialization is referred to as faulty epidermis breakdown.7 Keratinocytes are responsible for discovering the epidermis after injury through epithelialization.8 During new tissue formation, keratinocyte proliferation and migration have essential roles in re-epithelialization and effective wound-healing.9,10
Ineffective skin wound-healing is a big problem in the health sector. Several factors, including aging, diabetes, infection, immunodeficiency, and cancer, can lead to unsuccessful wound care and ultimately lead to morbidity and mortality.7 Efficient wound-healing process using traditional medicine, which is based on plant sources.11 Secondary metabolites are highly variable in their structure, so they have great potential for managing and treating drugs.12 Growth rates are a source of many biochemicals that can support skin health and integrity and are widely used in cosmetic formulations.13
In vitro studies of human skin’s epidermis and dermis have been commonly performed using HaCaT cells.1 HaCaT cells are immortalized human keratinocytes used to study dermatological conditions such as contact dermatitis, psoriasis, or skin cancer due to their high availability and ease of cell culture.5 Therefore, this review article aims to determine the wound-healing mechanism of several plant extracts on HaCaT cells.
The results are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.14 Scoping reviews purpose to; establish evidence available, elucidate key ideas, establish how research is done and determine knowledge gaps for a certain topic.
Articles were included based on the following criteria:
1. The studies primarily focus on the molecular mechanism of wound-healing of several compounds and extracts in HaCaT cells
2. The studies using an in vitro approach
3. The studies were published from 8th October 2021 up to 31st March 2022
4. The studies were in English and Indonesian language
5. The study’s full texts were available
Articles were excluded based on the following criteria:
Articles relevant to the study topic were searched and retrieved electronically from PubMed (https://pubmed.ncbi.nlm.nih.gov/advanced/) and Google Scholar (https://scholar.google.com/) using advanced search builders. The search from the databases was lastly done on 10th December 2021.
An advanced search in three databases, PubMed and Google Scholar, was searched to identify peer-reviewed articles on wound-healing and wound-healing mechanisms using compounds and extracts against HaCaT cells. Specifically, the search queries consisted of relevant medical subject titles (MeSH) and keywords relevant to the topic. The search terms included:
Wound-healing AND HaCaT Cells AND Compounds AND Extracts
The articles obtained were further vetted using the search strategy and filters outlined in the search strategy section. Assessment of the resulting articles was done independently by all the reviewers. Disagreements between them were resolved through consensus. First, articles from the initial search were obtained. Duplicate references were removed through manual deduplication. The titles and abstracts of the retrieved articles were screened for relevance to the study topic. Full-text reports were examined for compliance with eligibility criteria.
Data chatting from the sources of evidence was first assessed independently and then discussed by the team to reach a consensus. The information abstracted was as shown in the table.
The selection of the review articles based on the molecular mechanisms of action as the primary outcome domain was guided by the following items:
1. Title of study
2. Year of publication – 2009-2021
3. Study objectives – wound-healing mechanism of several compounds and extracts in HaCaT cells
4. Study design – review
5. Results – summary of findings on molecular mechanisms
6. Discussion – detailed explanation of the results and limitations of the review
7. Conflict of interest – Authors declare no conflict of interest
Skin is the largest organ of the human body,15 about 15% of the bodyweight consisting of the epidermis, dermis, and subcutaneous (Figure 1). The epidermis is the outermost layer of the skin and maintains a vital barrier against external trauma. The main cellular content of the epidermis is keratinocytes (about 95% of the epidermis), and fibroblasts are the main cellular components of the dermis.6 The epidermis, mainly composed of keratinocytes, is classified into stratum corneum, granular layer, spinous layer, and basal layer, based on the stages of keratinocyte differentiation. Keratinocytes have an essential role in inflammation.16 The skin’s primary function is to protect the body from exogenous factors by forming a protective barrier that covers the body; therefore, any injury or damage to the skin must be repaired immediately to provide continuous protection to our body systems.1
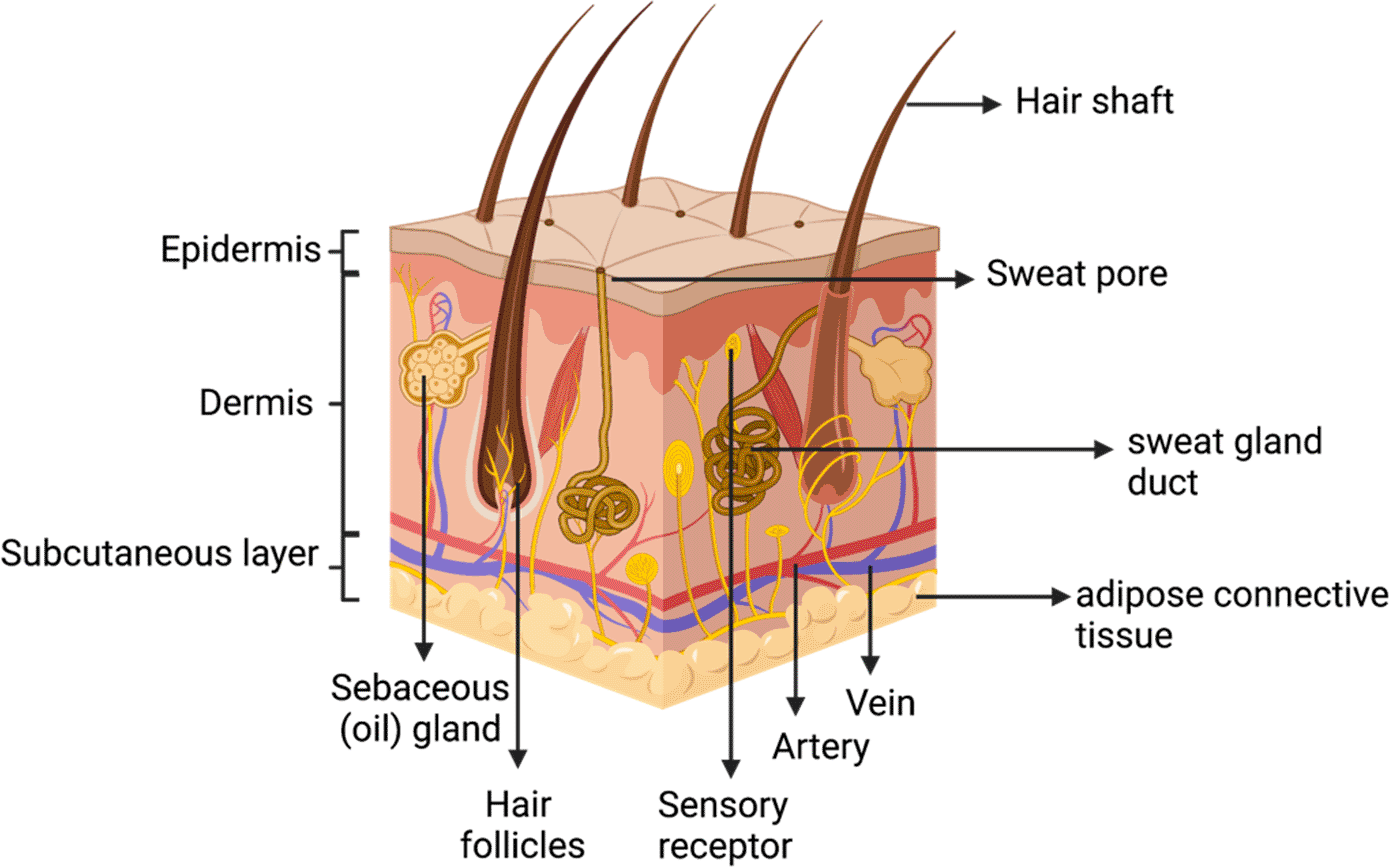
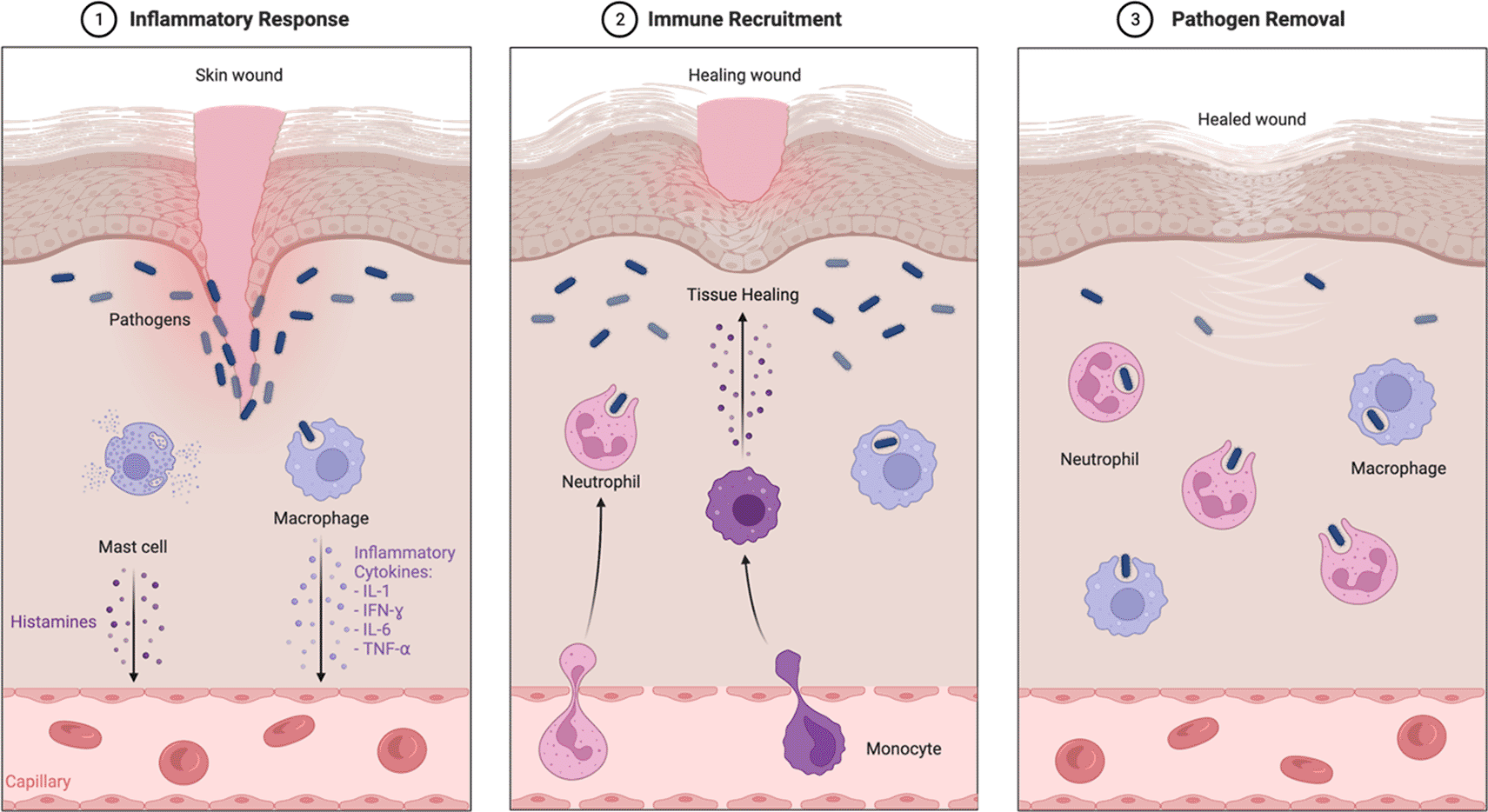
Wound-healing is a complex biological mechanism involving cellular interactions between cells, including smooth muscle cells, fibroblasts, endothelial cells, myofibroblasts, keratinocytes, and immune cells.17 Wound-healing processes restore skin integrity through four stages: hemostasis, inflammation, multiplication, and remodeling.18,19
1. Coagulation phase and formation of a platelet scab to cover the wound opening to prevent further blood loss or entry of pathogens,
2. Inflammatory phase, The flow of inflammatory cells to the wound site for protection against pathogens and activates skin cells. During this phase, neutrophils and macrophages are activated by releasing pro-inflammatory cytokines such as IL-1𝛽, IL-6, IL-8, and TNF, and growth factors such as PDGF, TGF-𝛼, TGF-𝛽, IGF-1, and FGF (Figure 2).9
3. Proliferation phase, skin cells multiply rapidly to replace lost cells. Restoring the basal keratinocyte layer in the basement membrane between the epidermis and the dermis begins to proliferate through various signaling molecules during the proliferative stage. When a certain level of repair is reached, the cytoplasmic shape of the keratinocytes is altered to move to the upper layers of the epidermis, differentiate, and transform through different cell layers to reach the final maturation stage. Thus, the proliferation and migration of keratinocytes suture the wound site during wound-healing.9
4. Remodeling Phase. In this phase, fibroblast and a vascular density decrease, old collagen fibers from the initial scar are replaced with matrix, and new collagen fibers are synthesized to form new tissue.9
Plants are the potential for providing wound-healing activities. Many studies reported the activity of plants for wound-healing and its mechanism. Thereby, this study reviews several plants that exhibit wound-healing activity below.
Aristolochia bracteolata
Aristolochia bracteolata contains aristoctam, aporphines, protobiberberines, flavonoids, alkaloids, tannins, sterols, steroids, and several other compounds used for skin treatments, as well as utilized for its anti-inflammatory properties (Figure 3).20 A. bracteolata extract selectively inhibited cell proliferation at higher concentrations (>100 g/mL) and lower concentrations (<25 g/mL). This extract showed linear and dose-dependent cell proliferation. The wound-healing study showed that wound closure was 50,38% ± 1,39 and 69,81% ± 1,89, respectively, at a 25 g/mL concentration after 24 hours and 48 hours. The extract was tested for anti-inflammatory activity by determining the inhibitory activity on lipopolysaccharide (LPS)-induced nitric oxide (NO) production in 264.7 RAW cells. The results found that A. bracteolata had a strong inhibitory effect on the production of NO and tumor necrosis factor-α/TNF-α. A. bracteolata extract inhibited the expression of the inducible nitric oxide synthase (iNOS) gene by lipopolysaccharide (LPS). A. bracteolata showed decreased pro-inflammatory cytokine mRNA expression concentration-dependent, indicating a mechanism for iNOS inhibition, gene expression analyzed by Real-Time PCR. Type III collagen levels are known to increase during the early stages of healing. During the early stages of wound-healing, type IV collagen is synthesized and replaced by type I collagen in the later stages of wound repair. It is evident from the data that this extract can stimulate collagen production from fibroblast cells, thereby increasing levels of extracellular matrix during tissue repair. The expression and secretion of type I and type IV collagen are also inherent in fibroblasts’ biological function because these proteins are essential components of the extracellular matrix.21
Boerhavia diffusa
The methanol extract (EM) of the leaves of Boerhavia diffusa significantly increased the viability and migration of human keratinocytes (HaCaT) cells. GC-MS analysis revealed the presence of caffeic acid, ferulic acid, and D-pinitol as the primary bioactive metabolites (Figure 4). The content of secondary metabolites in the extract of punarvana such as phenolics and flavonoids reduces lipid peroxidation, increases collagen fibrils’ survival by increasing collagen fibers’ strength, prevents cell damage, and accelerates DNA synthesis. Phenolics, flavonoids, and terpenoids enhance wound-healing due to their antioxidant and antimicrobial properties. Antioxidants enhance the healing process by reducing the damage caused by free oxygen radicals. D-pinitol, which is an insulinomimetic. Apply topical insulin promotes diabetic wound-healing by regulating wound inflammatory cells and improving cellular function. Bioactive insulin activates the IR/IRS/PI3K/AKT pathway involved in skin wound-healing, leading to tissue regeneration and growth, proliferation, and migration of keratinocytes and fibroblasts. In addition, caffeic and ferulic acids can promote wound-healing mainly due to their potent antioxidant and anti-inflammatory properties.22
Achyrocline satureioides (Lam.)
Achyrocline satureioides (Lam.) extracts are medicinal plants originating from Brazil, Uruguay, Argentina, and Paraguay. This plant contains quercetin, luteolin and 3-O-methylquercetin (Figure 5).23 The results showed a significant increase in the viability of HaCaT cells on ASE-loaded nanoemulsions (NEASE) (up to 5 g/mL of flavonoids). Preliminary tests showed that NEASE was able to increase cell migration at low flavonoid concentrations. ASE did not induce HaCaT cytotoxicity and tended to increase keratinocyte cell viability compared to controls after 24 h of treatment for all concentrations tested (0.625-10 g/mL).24
Calophyllum inophyllum Linn.
Anti-inflammatory and wound-healing activities have been reported of calophyllolide (CP) isolated from Calophyllum inophyllum Linn (Figure 6). The results showed that CP did not affect the viability of HaCaT cells in the concentration range. CP reduced fibrosis formation and effectively promoted wound closure in a mouse model without causing weight loss. The molecular mechanisms underlying wound repair reduce MPO activity and increase M2 macrophages. CP prevents a prolonged inflammatory process by downregulating pro-inflammatory cytokines-IL-1β, IL-6, TNF-α, but upregulating anti-inflammatory cytokines, IL-10.25
Ulmus parvifolia
The bark of Ulmus parvifolia contains phenolic compounds and steroid glucosides, used to treat edema. This plant has been isolated containing catechin-7-O-β-D-apiofuranoside (Figure 7).26 The results showed that HaCaT cells grown in the presence of U. parvifolia root bark extract showed a faster and dose-dependent growth rate than untreated cells. Collagen protein remodeling during wound-healing may be affected by proteolytic activity in the extracellular matrix by matrix metalloproteinases (MMPs). MMPs play an essential role in all stages of wound-healing during normal tissue remodeling and morphogenesis by modifying the wound matrix. Understanding the role of MMPs during infection and chronic tissue repair could pave the way in identifying potential targets for chronic wounds. In addition, MMPs also regulate cell-cell and cell-matrix signaling by releasing cytokines and growth factors sequestered in the extracellular matrix (ECM). TGF-β is a family of growth factors that play an essential role in wound-healing by regulating the inflammatory response, keratinocyte proliferation and migration, angiogenesis, collagen synthesis, and ECM remodeling.3
Aloe vera
Aloe vera extract contains mannose-6-phosphate, increasing wound contraction and collagen synthesis. Isolated polysaccharides from Aloe vera also induce matrix metallopeptidase (MMP)-3 and metallopeptidase inhibitor-2 gene expression during wound repair (Figure 8).27 The gel was tested to be non-cytotoxic against nauplii and compatible with human blood and skin cells. The Aloe vera promotes the attachment and proliferation of HaCaT and HFF1 cells. It also significantly accelerated wound closure through re-epithelialization and wound.28 Aloe vera gel exhibited significant wound-healing properties as indicated by the statistically significant increase in the percentage of wound closure and migration rate for the two highest concentrations used.29
Hibiscus syriacus
Hibiscus syriacus (HS) contains flavonoids (dihydroquercetin, herbacetin, and kaempferol) (Figure 9). HS ethanol extract accelerated wound-healing activity in epithelial formation and fibronectin production. In addition, HS enhances the expression of genes involved in skin hydration and homeostasis. HS contains compounds that can stimulate the expression of biomarkers relevant to skin regeneration and hydration, thereby counteracting the molecular pathways that cause skin damage and aging. Fibroblasts and keratinocytes are the keys to the wound-healing process in the skin. Treatment of HaCaT cells with 0,002% HS for 24 hours significantly improved the wound-healing response. Wound repair at 0,002 and 0,01% HS increased by 50 and 20%, respectively. Aquaporin 3 (Aqp3) is an integral membrane pore protein expressed more in the basal than in the upper layers of the epidermis. Specifically, the Aqp3 and filaggrin genes were increased by 20 and 58%, respectively. Aqp3 selectively conducts water molecules in and out of cells and prevents the passage of ions and other solutes. Filaggrin is a filament-associated protein that binds to keratin fibers and is responsible for the integrity and waterproofing capacity of the top layer of skin.13
Sideroxylon obtusifolium (Roem. & Schult.)
N-Methyl-(2S,4R)-trans-4-Hydroxy-L-Proline (NMP) from the leaves of Sideroxylon obtusifolium (Brazilian medicinal species) has activity as anti-inflammatory and wound-healing management (Figure 10).30 A previous study showed that the methanol fraction of Sideroxylon obtusifolium (MFSO) (50 g/mL) stimulated HaCaT cells by increasing proliferation and migration rates keratinocytes during wound-healing. MFSO demonstrated stimulation of human keratinocytes (HaCaT) cells and enhanced wound-healing through modulation of inflammation in burns.31
Alternanthera sessilis
Alternanthera sessilis contains 2,4-dihydroxy-2,5-dimethyl-3(2H)-furan-3-one (8,92%), hexadecanoic acid (7,21%), palmitate (5,65%), and L-glutamic acid (5,04 %) (Figure 11). The highest concentration of extract treatment (50 g/mL) showed a migration rate of 99%. The extract showed a strong positive result of 65%, with a difference of 14% in the migration rate between the two. The extract may act on broad signaling receptors to promote proliferation and migration in HaCat. Higher concentrations are required for epithelial barrier stimulation, while lower doses are sufficient to trigger connective tissue cellular compounds.1
Wedelia trilobata L.
Grandiflorenic acid from Wedelia trilobata leaves was assessed for its possible activity on HaCaT keratinocyte proliferation and its effects on in vitro scratch tests, collagen content, TGF-β2 levels, and nitric oxide, TNF- and IL-1β determinations using Raw 264.7 cells. Grandiflorenic acid (2,5 g/mL) resulted in a 106% percentage of HaCaT keratinocyte viability, induced a migration rate of 100% in the initial in vitro assay, and the collagen content increased to 171,2 g/mL compared to control (61,1μg/mL) with human fibroblasts. Grandiflorenic acid has potential wound-healing activity due to fibroblast stimulation and inhibition of prolongation of the inflammatory phase of wound-healing, as evidenced by a decrease in inflammatory cytokine levels from Raw 264.7 macrophage cells. Grandiflorenic acid and proteoglycans increased collagen production (Figure 12).12
Aegle marmelos L.
The active compounds isolated from Maja flower (Aegle marmelos L.) are cineol, eugenol, cuminaldehyde, aegelin, 1-hydroxy-5, 7-dimethoxy-2 naphthalene-carboxaldehyde (HDNC), and Luvangetin, which had been purified >98% (Figure 13). Treatment with Maja flowers for 24 hours drastically increases cell motility and expression of keratinocytes in specific cell lines. It enhances protein expression in loricrin, filaggrin, and involucrin (a keratinocyte differentiation marker). Keratinocyte motility is enhanced by the ERK and Akt signaling pathways.32
Eriobotryae folium
The leaves of Eriobotrya japonica contain amygdalin (laetrile and vitamin B1) which have antioxidant activity with an IC50 value of 56,59 g/mL (Figure 14).33 The ethanolic extract of Eriobotryae folium (EF) increases intracellular and extracellular PGE2 levels in HaCaT cells and inhibits 15-PGDH (ED50: 168,4 g/mL) with relatively low cytotoxicity (IC50: 250,0 g/mL). On the other study, EF extract suppressed LPS-induced nitric oxide and PGE2 production by inhibiting inducible nitric oxide synthase and COX-2 expression in lipopolysaccharides that stimulated RAW264 cells decreased MRP4 and PGT expression.34
Glycyrrhiza glabra
Glycyrrhiza glabra (GG) has a positive proliferative effect on keratinocytes. The larger the dose, the higher the rate of proliferation. GG inhibits abnormal cell proliferation and is anticarcinogenic. Although GG has been shown to increase the rate of cell proliferation and migration of keratinocytes and promote wound-healing, the underlying mechanism is unclear. However, GG helps activate proliferation and cytoskeletal rearrangement proteins and promotes wound-healing. The antioxidant effect of some GG constituents, such as glycyrrhizin and glabridin, may also help enhance the wound-healing ability of keratinocytes (Figure 15).35
Calabrian honey
BL1 (multifloral) and BL5 (orange) honey showed the best healing properties among the five kinds of honey tested. Pinocembrin revealed in honey samples BL1 and BL5, is a flavanol with known biological activities, including wound-healing. At high concentrations or after prolonged contact, polyphenols can reduce the production of pro-inflammatory cytokines and interact with metabolism and cell proliferation, thereby healing wounds. Pinocembrin in vitro modulates the production of inflammatory cytokines, such as TNF-, IL-1β, IL-6, and IL-10, by suppressing NF-κB and MAPK activation.36 Pinocembrin and its 7-linolenoyl derivative were found to be innovative wound-healing agents. Immunofluorescence and functional assays showed that GPR120 mediated the activity. Pinocembrin was able to produce wound-healing of HaCaT cells after 6 and 24 h by about +30% compared to untreated. In contrast, the 7-linolenoyl derivative increased HaCaT wound closure by about +40% compared to untreated controls. Activation of GPR120 can impair by increasing levels of TGF-β, which triggers the synthesis of components of the extracellular matrix, thereby contributing to wound-healing induced by keratinocytes. Complex signaling pathways involved in the upregulation of MMPs and the turnover of extracellular matrix components stimulated by attendants can lead to tissue damage or repair processes. In particular, MMP-9 plays an essential role in cell migration and re-epithelialization (Figure 16).37
Thymus vulgaris L.
Previous studies showed that Thyme oleoresin at 25 g/mL and 50 g/mL significantly promoted HaCaT cell migration, leading to wound closure. The upper part of the plant is reported to have significant components such as p-cymene, α -terpinene, and thymol (Figure 17). Possible mechanisms in wound-healing are its ability to maintain wound moisture, wound-healing, increase oxygenation by increasing blood supply, increase epithelial cell migration, rapid maturation of collagen and reduce inflammation, increase collagen synthesis, increase the synthesis of hyaluronic acid and dermatan sulfate in wound tissue.38
Trapa japonica
Trapa japonica contains fiber and polyphenols, such as ellagic acid, eugeniin, and gallic acid, which have antioxidant and anti-inflammatory activities (Figure 18). The results showed that the extract of T. japonica decreased the TNF-α, thus significantly decreasing MMP-1 and MMP-9 mRNA expression.39
Gracilaria lemaneiformis
Gracilaria lemaneiformis contains sulfated galactan, which is anti-inflammatory and antioxidant (Figure 19). The purified Gracilaria lemaneiformis (GLP-2) fraction promoted cell proliferation and migration of HaCat cells through activation of PI3K/aPKC signaling during wound-healing of human keratinocytes. GLP-2 significantly increased wound-healing activity when compared to control cells. The results showed that GLP treatment could increase lamellipodium formation in migrating HaCaT cells. GLP-2 positively regulates Cdc-42, Rac-1, Par-3, and aPKC in HaCaT cells. Cdc42 induces filopodial extension at the cell periphery, which aids in directional cell migration. Par-3 and aPKC are considered proteins that regulate cell polarity involved in the regulation of cell polarization. Increased Akt phosphorylation was considered as an index of activation of the PI3K signaling pathway after cells were injured. In the present study, a significant increase in Akt phosphorylation was observed in GLP-2-treated cells compared to control cells at 12 h during the wound-healing process.40
Nerium indicum
Nerium indicum (NI) contains oleandrin, flavonoids, and tannins (Figure 20).41 These plants may vary on keratinocyte activity at the wound site at specific doses. Studies have shown that the test materials used, either alone or in combination, positively affect keratinocyte proliferation and migration, an essential factor required for proper wound closure and wound-healing.35
Urtica dioica L.
Urtica dioica L (UD) extract contains saponins, flavonoids, carbohydrates, ketoses, resins, and coumarins (Figure 21). The UD extract increased the proliferation rates of HEK-293 and HaCaT cells by 39% and 30% after 24 h, respectively, compared to control cells. The extract increased the cell population in the G2/M phase by almost 10%. Moreover, the extract caused a twofold increase in the rate of cell migration of both cell lines compared to the control cells. In addition, the extract was found to have moderate anti-inflammatory and antioxidant properties that enhance the overall wound-healing potential.42
Curcuma amarissima
Curcuma amarissima (CA) contains curcumenol, curdione and curzerenone (Figure 22). The results showed that the cell viability test showed that the CA extract increased the viability of HaCaT cells. This increase in cell viability was related to the CA extract’s pharmacological activity in inducing cell proliferation. CA extract rapidly induces ERK1/2 and Akt activation. Consistently, CA extract accelerated cell migration, resulting in rapid healing of the injured human keratinocyte monolayer. In particular, MEK inhibitors (U0126) or PI3K inhibitors (LY294002) blocked CA-induced enhancement of cell monolayer wound-healing. In addition, CA extract induces the expression of Mcl-1, which is an antiapoptotic protein, supporting that the CA extract enhances the survival of human keratinocytes.7
Clausena excavata
The methanol extract of Clausena excavate contains coumarins, flavonoids, and glycosides with various biological properties. These compounds regulate inflammation through inhibition of the MAPK/NF-κB pathway. In addition, it was shown that methanol extract treatment increased TGF-β1 expression, the cytokine increased wound contraction, extracellular matrix deposition, and collagen formation in wound-healing (Figure 23).43
Angelica gigas
Angelica gigas contains Coumarin, decursin, and decursinol angelate (Figure 24). This extract improves wound-healing with HaCaT human keratinocytes. ERK1/2 phosphorylation is essential for cell survival, proliferation, and inhibition of apoptosis. The expression of genes encoding ECM remodeling proteins, inflammatory cytokines, and growth factors is an essential step in human wound-healing. The simultaneous expression of these genes can accelerate this process.44
Salvia haenkei
Salvia haenkei contains rosmarinic acid (Figure 25). Hydroalcoholic extract of S. haenkei effectively increases the wound closure rate in cultured keratinocytes with the almost total invasion of the scrapes after 48 h of treatment. Gene expression analysis showed that S. haenkei regulates the nuclear factor-κB (NF-κB) transcription factor signaling pathway positively. The results showed that the S. haenkei extract does not cause a statistically significant increase in the rate of fibroblast migration. Specifically, this study analyzed the mRNA levels of several genes involved in the early inflammatory phase of skin repairs, such as the transcriptionally active subunit of the transcription factor NF-κB (RelA), the inflammatory cytokine interleukin-6 (IL-6), and tumor necrosis factor-alpha. (TNF-α), inducible nitric oxide synthase (iNOS or NOS2), and the inducible prostaglandin synthesis enzyme cyclooxygenase-2 (COX-2). Tn with S. haenkei increases the IL-6 in fibroblasts and keratinocytes (83.6 and 19.7-fold induction, respectively), whereas TNFα levels only have a mild increasing trend.11
Crassocephalum crepidioides
Crassocephalum crepidioides (Benth.) S. Moore contains β-cubebene, α-farnesene, and α-caryophyllene, which exhibit antioxidant and anti-inflammatory activities (Figure 26). C. crepidioides (CC) extract exhibited anti-inflammatory in vitro assays on the macrophage cell line RAW 246.7. In addition, reduced inflammatory cell density in granulation tissue in 7-day-old wounds, combined with decreased TNF-α and NF-B1 mRNA expression. NF-B1 and TNF-α are essential markers for the degree of inflammation. High levels of TNF-α have been reported to inhibit wound re-epithelialization, myofibroblast formation, and smooth muscle actin (SMA-α). The results showed that CC could improve the wound-healing process through its anti-inflammatory activity. TGF-β1 mRNA was also found to be elevated in granulation tissue. TGF-β1 is involved in many essential effects on the wound-healing process. The activities of TGF-β1 include the induction of fibroblast proliferation, motivating the differentiation of fibroblasts into myofibroblasts, and increasing the synthesis, deposition, and maturation of collagen. The increase in the TGF-β1 gene may explain the increase in fibroblasts and the wound-healing effect.45
Withania somnifera
Withania somnifera contains withaferin A which has anti-inflammatory, antiangiogenic, antimetastatic, and anticancer activities (Figure 27). The results showed that ashwagandha extract (AE) significantly inhibited mRNA expression of inflammatory cytokines, including interleukin IL-8, IL-6, TNF-α, IL-1β, IL-12, and promoted mRNA expression of the anti-inflammatory cytokine TGF-β1 in HaCaT cells. In addition, AE inhibited lipopolysaccharide-induced phosphorylation of p38 and c-Jun N-terminal kinase, as well as NF-κB p65. The results showed that EA was not toxic to HaCaT cells up to a dose of 10 mg/mL. AE inhibits the MAPK/NF-κB pathway. The NF-κB and MAPK signaling pathways are strongly associated with the expression of inflammatory cytokines in HaCaT cells.16
Anemarrhena asphodeloides
Mangiferin has been isolated from the plant Anemarrhena asphodeloides (Figure 28). A. asphodeloides (AA) extract promoted inhibiting Th2-type cytokines, pro-inflammatory cytokines, and filaggrin restoration in HaCaT cells. TNF-α/IFN-γ significantly increased mRNA expression of IL-4 in HaCaT keratinocytes. However, pretreatment with AA significantly suppressed the mRNA expression of IL-4. AA pretreatment of TNF-/IFNγ-stimulated HaCaT keratinocytes reduced IL-13 mRNA expression. However, pretreatment with AA significantly suppressed the mRNA expression of IL-6 in a dose-dependent manner. These results suggest that AA has a protective effect on skin keratinocytes by inhibiting the transcription of inflammatory cytokine levels associated with skin barrier dysfunction. TNF-α/IFN-γ co-stimulation decreased filaggrin protein expression and mRNA levels, but pretreatment with AA significantly increased filaggrin protein levels, although mRNA levels increased slightly. The results showed that AA had a filaggrin-recovery effect on TNF-α/IFN-γ-stimulated HaCaT keratinocytes. Treatment with AA increased Keratinocyte HaCaT migration and inhibited the expression of iNOS protein levels. It is possible to assume that AA facilitates wound-healing in the skin barrier through the inhibition of overexpression.46
Sasa veitchii
Sasa veitchii is a traditional plant that contains lignin, polysaccharides, and chlorophyll (Figure 29). It has many pharmacological activities such as antioxidant, anti-inflammatory, antibacterial and anticancer. HaCaT cells treated with S. veitchii extract for 72 hours showed significantly higher AQP3 expression and mitogen-activated p38 phosphorylated protein kinase (MAPK) than control cells. S. veitchii extract increases AQP3 expression and provides wound-healing and healing effects. The increase in AQP3 expression elicited by the Kumazasa extract may be due to increased transcription via activation of p38 MAPK signaling. It was also found that S. veitchii extract had a proliferative effect on HaCaT cells.47
Periplaneta americana
Periplaneta americana contains polyalcohols, amino acids, pyrimidines, uracils, and proteoglycans (Figure 30). P. americana extract showed effects in wound-healing that depend on the Janus-activated kinase/signal transducer pathway and transcriptional activator 3 (JAK/STAT3) and Smad3 activity. Pretreatment with STAT3 inhibitors blocked cell proliferation and migration. This extract promotes the proliferation and migration of immortalized human keratinocyte HaCaT cells. The results showed increased keratinocyte proliferation and migration after treatment (0,3125 mg/mL) for 48 hours. After treatment, JAK/STAT3 signaling expression and Smad3 activation, NF-κB/p65, and β-catenin was significantly upregulated in HaCaT cells and wound tissue after treatment. However, NF-κB and Wnt signaling appear to be minimally activated regardless of the limited expression of NF-κB/P65 and β-catenin upregulation or cell nuclear translocation.48
Angelica tenuissima
Angelica tenuissima root contains decursin and Z-ligustilide (Figure 31). The root extract of A. tenuissima accelerates wound filling under basal conditions in the keratinocytes that make up the epidermal layer. It inhibits the mRNA expression of MMP-1 and elastase. It also increases the collagen content as indicated by the production and secretion of type I procollagen with or without UVB exposure. This extract could be beneficial in suppressing UVB-mediated wrinkling of skin formation and photoaging by increasing PIP levels and decreasing MMP-1 and elastase activity. A. tenuissima can play a role in attenuating the inflammatory response caused by UVB irradiation through upregulation of photo-protective hemeoxygease-1 and suppressing pro-inflammatory cyclooxygenase-2 expression.46
Astragali radix
Astragaloside VI (AS-VI) and cycloastragenol-6-O-beta-D-glucoside (CMG) (Figure 32) enhance skin cell proliferation and migration via activation of the EGFR/ERK signaling pathway, resulting in enhanced wound-healing in vitro. AS-VI actively promotes the proliferation of human keratinocytes (HaCaT) by activating the ERK1/2 pathway rather than the JNK and p38 pathways. This plant shows that astragaloside can activate cellular processes involved in wound-healing. It is mediated, at least in part, by EGFR/ERK1/2, which could be beneficial in wound closure.49
Mimosa tenuiflora (Willd)
Mimosa tenuiflora bark contains high amounts of saponins and polyphenols such as arabinogalactan (Figure 33). Mimosa tenuiflora (Willd) aqueous extract at concentrations of 10 g/mL and 100 g/mL indicated a loss of cell viability and proliferation of dermal fibroblasts. Isolated, ethanol-precipitated compounds (EPC) (10 g/mL) have shown strong potential to increase viability by stimulating mitochondrial activity and dermal fibroblast proliferation. Stimulation of human keratinocytes was only found at a concentration of 100 g/mL. EPC did not influence the expression of specific proliferation and differentiation-related genes. Fibroblasts in the connective tissue are the main targets of the arabinogalactan polymer compound from Mimosa tenuiflora. Intense fibroblast stimulation can be noted to initiate wound closure and production of extracellular and filling materials within the wound.50
Fitzroya cupressoides
Fitzroya cupressoides, commonly called allerce, contain fatty acids, mono and sesquiterpenes, diterpenes, lignans, and phytosterols. Diterpenes and lignans were the most active compounds, with the biomolecules matairesinol, podophyllotoxin, and ferruginol (Figure 34). Allerce extract has a significant effect on wound-healing. The results showed that the extract stimulated cell division in human skin epidermal cells in the context of wound repair. These results also indicated that allerce extract accelerated the healing process after 24 and 48 h of treatment. This effect was promoted principally by stimulating HaCaT cell division in the context of wound repair.51
Plantago australis
The hydroethanolic extract of Plantago australis contains verbascoside (Figure 35). P. australis extract and verbascoside decreased cell viability at 1000 μg/mL and 100 μg/mL, respectively. The results showed approximately 81,06% wound closure (P. australis extract 25 μg/mL) and 58,7% and 57,77% (Verbascoside 5 and 10 μg/mL). P. australis extract showed a significant reduction in TNF-α. These compounds have wound-healing activity, increase cell migration, and reverse the effects of oxidation in lipopolysaccharide-activated N9 cells. This effect may also be associated with decreased TNF-α, IL-6, IL-12p70, INF-γ, and MCP-1.52
The wound-healing mechanism includes processes that restore skin integrity through four stages: hemostasis, inflammation, multiplication, and remodelling. Antioxidant and anti-inflammatory activities play an essential role in wound-healing mechanisms. Many compounds in plants have been studied to have activity in wound-healing by various mechanisms.
Figshare. PRISMA statement checklist. DOI: https://doi.org/10.6084/m9.figshare.19736032.53
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Yes
References
1. Roy H, Rahaman SA, Kumar TV, Nandi S: Current Development on Chitosan-based Antimicrobial Drug Formulations for the Wound Healing.Curr Drug Discov Technol. 2020; 17 (4): 534-541 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Drug Design and development research
Are the rationale for, and objectives of, the Systematic Review clearly stated?
No
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
No
References
1. Chuong CM, Nickoloff BJ, Elias PM, Goldsmith LA, et al.: What is the 'true' function of skin?. Exp Dermatol. 2002; 11 (2): 159-87 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Cutaneous wound healing and tissue repair; human epithelial cell physiology; extracellular interface.
Are the rationale for, and objectives of, the Systematic Review clearly stated?
No
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
Not applicable
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Natural products, bone and joint metabolism
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 17 Nov 22 |
read | read | |
|
Version 1 16 May 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)