Keywords
extraction, phytochemical study, phenols, flavonoids, Bidens pilosa L, Croton floccosus, Asteraceae, Euphorbiaceae
This article is included in the Plant Science gateway.
extraction, phytochemical study, phenols, flavonoids, Bidens pilosa L, Croton floccosus, Asteraceae, Euphorbiaceae
The following preliminary phytochemical study of the Bidens pilosa L. and Croton floccosus plants provided important information on the content of secondary metabolites and response to the DPPH radical reported for the first time in Ecuador, which may be future use for medicinal application.
The changes that were made in relation to the previous version were updating of the bibliography in the Phytochemical screening section, and comments were also added in the Determination of total phenols and flavonoids and Vegetal material sections. Lastly, Figure 1 was added. map with the collection points of the studied species
See the authors' detailed response to the review by Yailet Albernas Carvajal
See the authors' detailed response to the review by Pawel Konieczynski
The biodiversity of Ecuador ranks sixth worldwide for the number of species.1 Many species comprise the Croton genus in this country, and 39 have been recognized.2 Of these, 13 are considered native,3 and the others have been documented in the last 15 years.4,5 Many species of this genus have been used in traditional medicine by the country’s indigenous population. Antimicrobial,6 antioxidant,7 anti-inflammatory8 and anticancer9,10 biological activity studies have been carried out on them in America, Asia and Africa.
Brian A. Smith found a new species of Croton (Euphorbiaceae) on the western slopes of the Ecuadorian Andes in 2006, Croton floccosus.11 This is described as a medium-sized tree with grayish external and internal bark with a reddish exudate, along with simple leaves (4–15 × 2.5–8 cm) and alternate elliptic-oval unisexual flowers and tricoco-type fruits with persistent styles. This plant is commonly found next to streams and disturbed sites in the provinces of Pichincha and Imbabura in Ecuador.
Some 230 to 240 species of Bidens have been described,12,13 including Bidens pilosa, which was identified in 1753 by Carl Linnaeus. This is a representative perennial herb, distributed globally in temperate and tropical regions, which has traditionally been used in food and medicine without having any obvious adverse effects. About 116 publications on this species have documented its medical use,14 and phytochemical studies show that it contains a high number of flavonoids and polyines, which have anticancer,15 anti-inflammatory,16 antibacterial17 biological activity, as well as antifungal,18 antimalarial,19 and antioxidant20,21 properties.
Bidens pilosa is an erect, strongly branched plant with a strong aromatic odor. It has opposite leaves and a long petiole, whose limbs are generally deeply divided into 3 to 5 segments giving it the appearance of a compound leaf. It also has white ray flowers and numerous yellow tubular flowers.13
Although this species has been studied to a greater extent than Croton floccosus, we have not found phytochemical studies of these plants in Ecuador, which is why it has important research potential that may allow us to transform traditional knowledge into scientific knowledge.
In the following work, a preliminary phytochemical study of the leaves of the species Bidens pilosa and Croton floccosus was carried out using the Tukey test,22 which is employed in ANOVA in order to compare the means of the values obtained. This study joins the recently published work by Ruiz-Reyes et al. on Melampodium divaricatum and Zanthoxylum sprucei plants,23 in an attempt to discover which active compounds are present and if they have the potential to be used as medicinal plants.
The fresh leaves of the Bidens pilosa L. and Croton floccosus species were collected in January 2021 from the Botanical Garden at the Technical University of Manabí (UTM), Portoviejo, in the Manabí province in western Ecuador. The leaves that present better conservation in their structure are chosen to clean them of dust and branches, then the petiole of the leaf blade is extracted. The studied leaves were collected from various plants and then mixed to create the sample for analysis. The botanical identification was carried out by the botanists in charge of the herbarium in the botanical garden at the UTM. The vouchers of the specimens were deposited with the following codes Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae). It is shown in the following map of the botanical garden. The collection points of the studied species. For croton floccosus it was two points while for bidens pilosa it was three points. The coordinates of the studied species are placed on the map Figure 1.
The chemicals and reagents employed in this study were: Folin-Ciocalteu reagent, 1.1-diphenyl-2-picryl-hydrazyl (DPPH), sodium nitrite, aluminum chloride, sodium hydroxide, sodium carbonate, catechin, quercetin, trolox, methanol, and ethanol. All the reagents and solvents were supplied by Sigma-Aldrich and are of an appropriate analytical grade for study.
The plant material was dried in a tray dryer (universal oven UF110, Memmert) at temperatures of 40°C and 50°C. A reference weight was taken every two hours until it remained constant. The sample was pulverized using a blade mill (grinder) with a sieve so that a more uniform size of the material can be obtained, in order to fractionate the plant tissue and produce a better extraction. The time used for grinding was 5 minutes. The extracts of the crude plants were obtained using the ultrasonic bath, maceration and Soxhlet extraction methods. In the case of Soxhlet extraction, 10 g of plant material were used and placed in the equipment extractor cartridge with 250 mL of ethanol. Between 8 and 10 extractions were performed at the boiling temperature of the solvent, and the solvent was eventually evaporated in order to obtain the extracts. With regard to ultrasonic bath extraction, 10 g of finely divided plant material was placed in a 250 mL beaker containing 100 mL of ethanol. The mixture was sonicated at 50 W for 1 h in an ultrasonic bath and subsequently filtered. The solvent was then evaporated in a vacuum to obtain the corresponding extracts for analysis. In the case of extraction with maceration, 10 g of finely divided plant material was placed in a 250 mL beaker with 100 mL of ethanol. The mixture was macerated for 72 hours and stirred from time to time. It was subsequently filtered and the solvent was evaporated in a vacuum to obtain the corresponding extracts for analysis.
The crude extracts were evaluated phytochemically to determine the presence of chemical constituents using standard procedures, following the same methodology described by Ruiz-Reyes23,24 for the determination of metabolites: Alkaloids: Each extract (10 mg) was dissolved in 2 mL of 5% hydrochloric acid and, after mixing and filtering, three aliquots were taken. Drops of Wagner, Mayer, Bouchardat, and Dragendorff reagents were added to each one. A red-brown precipitate (Wagner), a yellowish white precipitate (Mayer), a brown precipitate (Bouchardat), and an orange-red precipitate (Dragendorff) indicated the presence of these metabolites. Flavonoids (Shinoda test): 1 ml of absolute ethanol and three drops of concentrated hydrochloric acid were added to 10 drops of the extract diluted in isopropyl alcohol. The formation of a red color indicated the presence of aurones and chalcones while the formation of an orange, red, or magenta color formation indicated the presence of isoflavones, flavonols, and flavonoids, respectively. Saponins (with sodium bicarbonate): 1 mL of distilled water and one drop of saturated sodium bicarbonate solution were added to five drops of the extract dissolved in isopropyl alcohol (20 mg/mL) in a test tube and shaken vigorously for three minutes. The formation of honeycomb shaped foam indicated the presence of saponins, and tannins: 10 mg of each extract was dissolved in 1 mL of ethanol and extract with 3 mL of boiling distilled water for 15 minutes. Once it had been allowed to stand at room temperature, 0.2 mL of 10% sodium chloride solution was added to the mixture and filtered. In addition, four drops of 10% ferric chloride solution were added. The precipitation observed was indicative of the presence of tannins. For reducing sugars, 200 μL of the extract were dissolved in a tube, Benedict’s reagent was added until the extract turned bluish, it was then heated in a water bath at a temperature of 60°C. It was considered positive if it turned reddish brown.25
Determination of the total content of phenols in the extracts was carried out using the Follin-Ciocalteau reaction and by employing gallic acid as the reference phenolic compound. The gallic acid calibration curve was prepared by weighing 2 mg of gallic acid and making it up to a volume of 10 mL with distilled water, which is the stock solution at a concentration of 0.2 mg/mL. Aliquots of 50 μL were subsequently taken, after which 200 μL of Follin-Ciocalteau reagent and 2 mL of 7% Na2CO3 were added, and the solution was made up to 5 mL. After the absence of light for 30 minutes and at room temperature, we measured the absorbance at 725 nm using the prepared solution as a blank without the analyte. Total flavonoid content was determined using quercetin as standard. For the preparation of the Quercetin standard, 2 mg of the Quercetin standard was weighed and dissolved with 70% methanol in a 10 mL volumetric flask, which is considered the mother solution with a concentration of 0.2 mg/mL. The aluminum chloride solution was subsequently prepared by weighing 500 mg of AlCl3 and dissolving it with a solution of 25 mL 5% Acetic Acid in methanol, which led to a solution with a concentration of 20 mg/mL and aluminum chloride. In order to perform the calibration curve with the Quercetin standard, aliquots of 5, 10, 15, 20, 25, 30, 35, 40 μL of the standard solution were taken, and made up to 1 mL with methanol (70%), after which 1 mL of the AlCl3 solution was added. The same solution was used as a blank without adding the standard compound. After waiting 15 min for the reaction, the absorbance of the solution was measured at a wavelength of 430 nm in the UV-Vis spectrophotometer. The preparation of the extracts was carried out by taking 5 mg of the sample, which was dissolved in 5 mL of 70% aqueous methanol. The determination procedure for total flavonoids was carried out by taking 200 μL aliquots of different extracts of the sample in triplicate and making them up with 1 mL of methanol (70%), after which 1 mL of the AlCl3 solution was added. After waiting 15 min, the absorbance of the solution was measured at a wavelength of 430 nm in the UV-Vis spectrophotometer. Both methodologies were described by Ruiz-Reyes.23,26–28
The antioxidant activity was determined using a spectrophotometer and the DPPH molecule as a reagent to generate the free radical following the methodology described by the author himself and other authors.23,29 DPPH reagent preparation: 0.02 g of the DPPH reagent was weighed out and made up to volume with 100 mL of methanol in a flask. This was then homogenized and left to react for 24 hours in an amber bottle in the dark.
• Wavelength determination
2.5 mL of the prepared DPPH reagent was taken and 15 mL ethanol added. A scan was carried out in the spectrophotometer. A maximum absorbance of 0.750 ± 0.05 should appear at a wavelength of 517 nm.
• Blank preparation
Methanol (15 mL) was added and a scan was performed in the spectrophotometer to verify that it did not have absorbance in the working wavelength range in which the maximum of the sample is found.
• Sample preparation
We took 3.0 mL of the DPPH reagent and 100, 200, 300, 400, 500 μL of the extract added. Once the sample and the blank were prepared, the absorbance reading was performed in the Thermo Scientific Genesys 180 (UV-Visible) spectrophotometer at 517 nM every 10 minutes.
A multifactorial statistical analysis of variance (ANOVA) was carried out for the different species studied, with a maximum order of interaction of 2 for the yield values. This made it possible to determine which of the extraction factors evaluated had a statistically significant effect on the yield. Table 1 presents a summary of the multifactorial arrangement used for the processes of obtaining extracts from Bidens pilosa L. and Croton floccosus at drying temperatures of 40 and 50°C.
Table 2 shows the analysis of variance for the extraction performance in the case of Bidens pilosa L., for which it was determined that there is a statistically significant difference in the treatments. The contribution of each factor was measured by eliminating the effects of the other factors. The P-values test the statistical significance of each of the factors. Since the P-value of factor C (solvent) is less than 0.05, this factor has a statistically significant effect on performance at a confidence level of 95.0%.
Figure 2 shows the influence of the factors of the experimental design with respect to the extraction yield for the extracts obtained from Bidens pilosa L. A notable decrease in yields is observed when the extraction process is carried out using hexane. Although it is not statistically significant, it is necessary to mention that there was a better yield in the extraction processes in which leaves dried at 50°C were used. The process that obtained the best yields was the Soxhlet extraction.

For each significant factor, multiple range tests provide information regarding which means were significantly different from others. Tukey’s honestly significant difference (HSD) method was selected for this test because it allows the comparison of individual means from an analysis of variance of several samples subjected to different treatments, thus widening the intervals in order to allow multiple comparisons between all pairs of means.
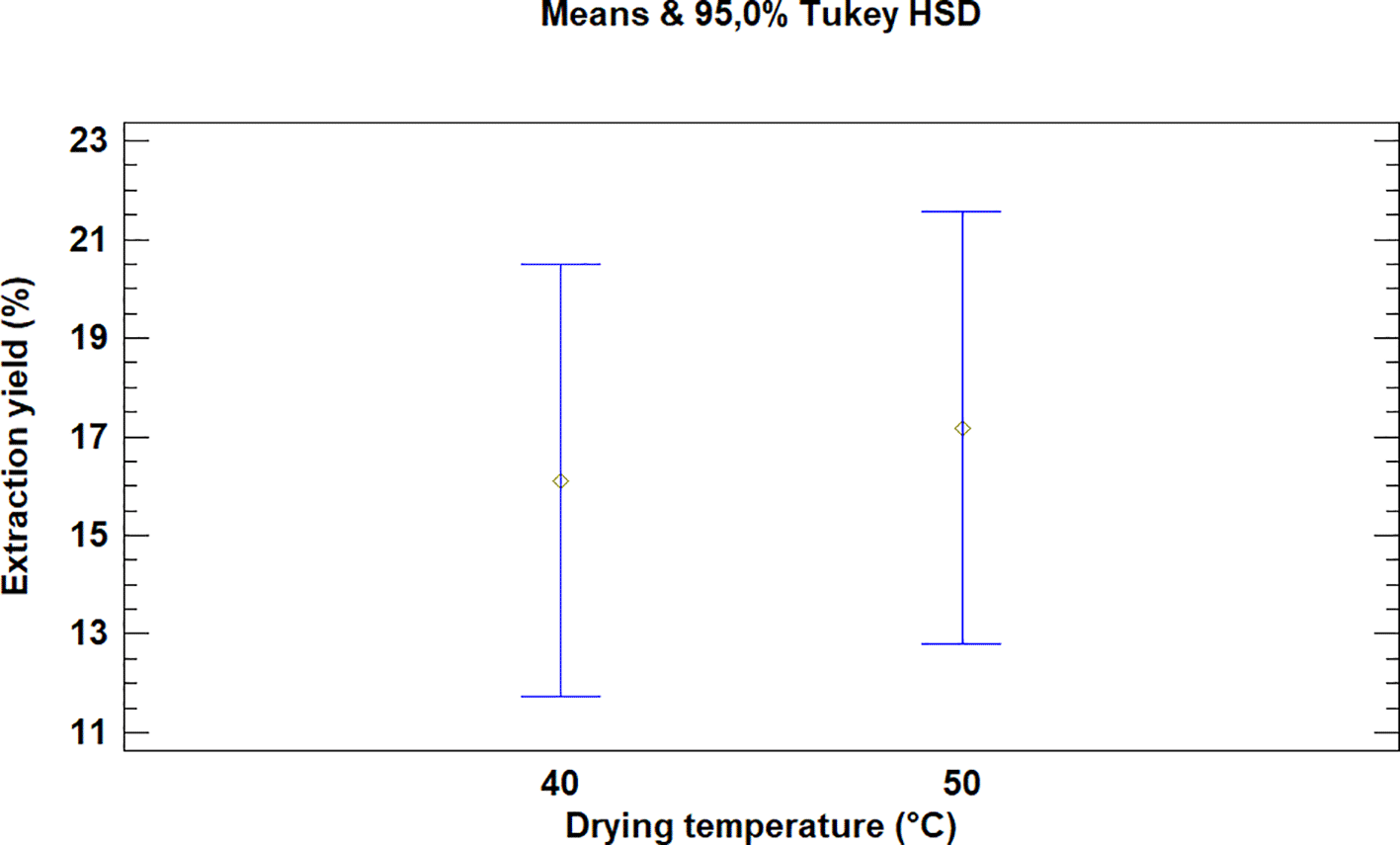
This test showed that there was no significant difference between the means for the drying temperatures of the leaves of Bidens pilosa L., as is noted in Figure 3. It is necessary to mention that although there was no statistically significant difference between the temperatures used for drying, the extraction processes during which 50°C was used led to higher yield values.

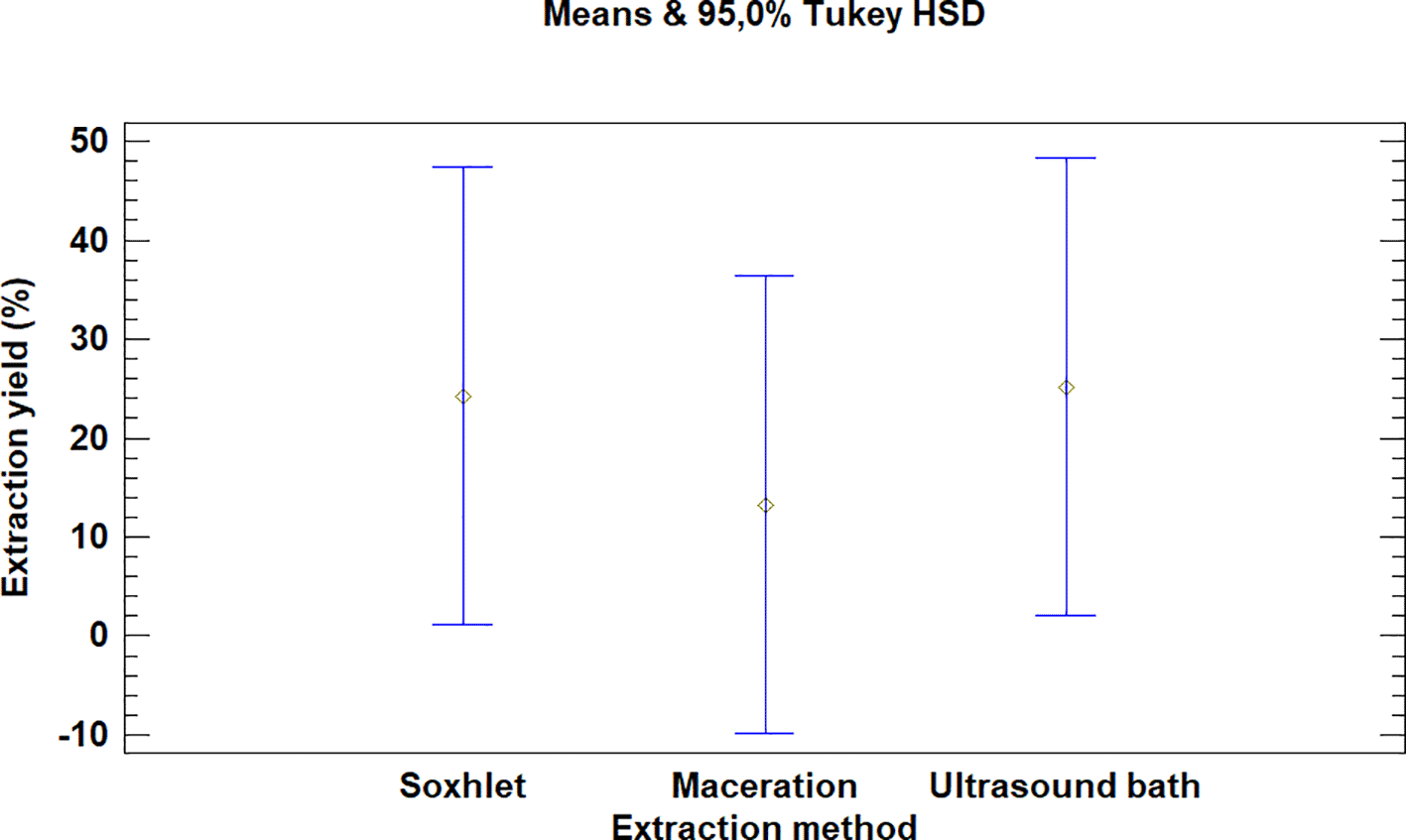
The multiple range test carried out for Bidens pilosa L. also showed that there was no difference between the extraction process methods evaluated, as is noted in Figure 4. It is, however, necessary to mention that the extraction with an ultrasonic bath and Soxhlet led to higher yields than the maceration process, despite the fact that they are not statistically different.
Furthermore, Table 3 shows the analysis of variance for the extraction performance of Croton floccosus, which determined that there is a statistically significant difference in the treatments. The contribution of each factor is measured by eliminating the effects of the other factors. The P-values test the statistical significance of each of the factors. Since the P-value of factor C (solvent) is less than 0.05, this factor has a statistically significant effect on the extraction yield for Croton floccosus at a confidence level of 95.0%. It is also necessary to highlight that the interaction between factor B (extraction process) and C (solvent) has a significant effect on the extraction process.
Figure 5 shows the influence of the factors of the experimental design on the extraction yield. A notable decrease in yields is observed when hexane is used in the extraction process. It will also be observed that there is a difference with respect to the yields obtained as regards the interaction between the extraction method and the solvent (B and C), showing that the highest yields are obtained with the Soxhlet extraction processes with ethanol at both drying temperatures.
As described above for the temperatures selected in order to dry Bidens pilosa L., there is no significant difference between the means obtained for the drying temperatures of Croton floccosus leaves (Figure 6). However, it is necessary to mention that, although there is no statistically significant difference, the extraction processes during which the drying temperature of the material was 50°C obtained better extraction yields, as occurred with the Bidens pilosa L.
It was also noted that the multiple range test carried out for Croton floccosus made it possible to identify that there was a difference between the Soxhlet and maceration process extraction methods (Figure 7). The Soxhlet extraction process method obtains higher yields than the maceration and ultrasonic bath extraction methods.
Finally, in the case of both plants, the multiple range test showed that there was a difference between the solvents used in the extraction (Figure 8). The processes in which hexane was used obtained a low yield when compared to extractions with ethanol.
Table 4 details the qualitative results of the phytochemical tests performed on the ethanolic extracts of Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae), obtained with Soxhlet extraction. There is a low presence of flavonoids and saponins, and a relatively abundant presence of phenols and tannins for both species. In addition, a relatively abundant presence of alkaloids is detected for the species Croton floccosus and no reducing sugars are detected, while a low presence of reducing sugars is detected for the species Bidens pilosa L.
| Alkaloids | Reducing sugars | Phenols | Flavonoids | Tannins | Saponins | |
|---|---|---|---|---|---|---|
| Ethanolic extract of Bidens pilosa L. obtained by Soxhlet | + | + | + + | + | + + | + |
| Ethanol extract of Croton floccosus obtained by Soxhlet | + + | - | + + | + | + + | + |
The equivalent mg of gallic acid corresponding to each gram of dry sample of Bidens pilosa L. were obtained, as shown in (Table 5). As will be noted, Tukey’s HSD test for the phenolic content of the different samples made it possible to determine that there are significant differences among them (p<0.05). Samples that share the same letter are homogeneous.
As can be seen in Figure 9, the phenolic content for the extracts has values of 27–49 mg GAE/g of dry sample for the different processes employed to obtain ethanolic extracts of Bidens pilosa L. with leaves dried at 40°C and 50°C. Those with the lowest value correspond to the extracts obtained using the maceration method and those with the highest value correspond to the Soxhlet extraction process, with a value of 48.609 ± 0.370 mg GAE/g of dry sample of the extract obtained from the material dried at 50°C. In their research, Singh et al.31 obtained methanolic extracts from leaves of Bidens pilosa L. dried at 30°C with a total phenolic content corresponding to 72 mg GAE/g of dry extract. This value is approximately 32% higher than that obtained in the present investigation, but it is necessary to mention that the extraction carried out by Singh et al. was carried out using an extraction process involving broken evaporation at a reduced pressure. In their investigation, meanwhile, Falowo et al.32 obtained phenolic content for the extracts of B. pilosa corresponding to 75.9 mg GAE/g of dry extract when an exhaustive maceration was carried out with an ethanol-water solution (7:3) with shaking at room temperature for two days.
The equivalent mg of gallic acid corresponding to each gram of dry Croton floccosus sample were obtained in the same way. These values are presented in Table 6. It was noted that Tukey’s HSD test for the phenolic content of the different samples determined that there is a significant difference among them (p <0.05) Samples that shared the same letter are homogeneous.
A range of 42–129 mg GAE/g of dry sample were obtained from the different processes employed to obtain ethanolic extracts of Croton floccosus, as can be seen in Figure 10. Those with the lowest value correspond to the extracts obtained using the ultrasonic bath method, and those with the highest value correspond to the extraction process carried out using maceration, with a value of 128.212 ± 0.601 mg GAE/g of dry sample of the extract obtained from the material dried at 50°C. This contrasts strongly with the values obtained for Bidens pilosa L., since the extracts obtained by means of maceration had the lowest total phenolic content. Taking into account that this species is considered endemic to Ecuador, Pazmiño,33 in his research, obtained ethanolic extracts from the freeze-dried “latex” of Croton floccosus, which had a total phenolic content corresponding to 271.212 ± 3.1728 mg GAE/g of dry extract. This value is approximately 53% higher than that obtained in the present investigation, and it is necessary to mention that the values presented by this author correspond to those of an extract obtained from the “latex” of this species.
The equivalent mg of quercetin corresponding to each gram of dry sample of Bidens pilosa L. were obtained, as shown in Table 7. Tukey’s HSD test for the flavonoid content of the different samples determined that there is a significant difference among them (p<0.05). Samples that shared the same letter are homogeneous.
A range of 12–18 mg QE/g of dry sample were obtained from the different processes employed to obtain ethanolic extracts of Bidens pilosa L., as can be seen in Figure 11. Those with the lowest value correspond to the extracts obtained using the maceration method, and those with the highest value correspond to the extraction process carried out with Soxhlet and the ultrasonic bath. There is statistical homogeneity between the values obtained for both processes, with a value of 17.795 ± 0.0644 and 17.665 ± 0.0042a mg QE/g of dry sample of the extract obtained from the corresponding material dried at 40°C. In their study, Cortes-Rojas et al.34 determined the flavonoid content of Bidens pilosa L. extracts by employing dynamic maceration, Soxhlet, ultrasound and microwave, obtaining corresponding values of 21.988 ± 0.127; 19.623 ± 0.100; 16.482 ± 0.180 and 11.590 ± 0.042 mg QE/g dry sample. These values coincide with those obtained in the present study, with the exception of those for dynamic maceration and microwave.
The equivalent mg of quercetin corresponding to each gram of dry Croton floccosus sample were obtained in the same way, as shown in Table 8. Tukey’s HSD test for the flavonoid content of the different samples determined that there is a significant difference among them (p <0.05). Samples that shared the same letter are homogeneous.
The established range of total flavonoids comprises values between 7–35 mg QE/g of dry samples for the different processes used to obtain ethanolic extracts of Croton floccosus (Figure 12). As can be seen, there is a great difference between the different treatments. That with the highest flavonoid content corresponds to the Soxhlet extraction process of the material dried at 40°C, with a value of 34.139 ± 0.06224 mg QE/g, while that with the lowest value corresponds to the extracts obtained by employing the ultrasonic bath method at the same temperature, with a value of 7.284 ± 0.15096 mg QE/g of dry sample.
The in vitro antioxidant activity was performed by employing the 2.2-diphenyl-1-picryl-hydracil radical scavenging method (DPPH assay). All the antioxidant activity values for the ethanolic extracts of both species were obtained from the Trolox equivalent calibration curve (TEAC). The established range for the Trolox equivalent antioxidant activity (TEAC) for Bidens pilosa L. is 74–78 μmol Trolox equivalent (TE)/g of dry samples for the different processes used to obtain ethanolic extracts with leaves dried at 40°C and 50°C (Figure 13). The means of the values were compared using the Tukey test, which showed that there is no marked difference among the processes studied with regard to the TEAC. It was, therefore, established that the drying temperatures do not directly influence the TEAC values for the maceration, ultrasonic bath and Soxhlet extraction processes.
Considering that there was no marked difference among the processes, we then proceeded to establish the mean radical inhibition coefficient (IC50) of the process that attained the highest antioxidant activity value equivalent to Trolox, Soxhlet extraction of the material dried at 50°C. Table 9 shows the inhibition coefficient (IC50) determined by the DPPH method performed on the extract of Bidens pilosa L. and Trolox (standard). The difference between the extract and the standard used in the previous determinations is compared.
| Sample | IC50 (μg/mL) |
|---|---|
| Bidens pilosa L. ethanolic extract | 239.333 |
| Trolox | 2.599 |
The values reported in the present study are lower than those reported by Cortés-Rojas et al.,34 who obtained an IC50 for the DPPH radical of 35.35 μg/mL for Bidens pilosa L. extracts from flowers and leaves collected in Brazil using dynamic maceration at 45°C and with 200 rpm of agitation. Furthermore, Singh et al.31 obtained an IC50 value of 80.45 μg/mL for extracts from the leaves of the same species collected in India using maceration with methanol for 48 h. We are, therefore, of the opinion that the differences between our IC50 values and those reported are related to the time of year and the country in which the plant was collected, along with the conditions under which the plant material was prepared and the method used to obtain the extract of secondary metabolites.
The established range for the Trolox equivalent antioxidant activity (TEAC) for Croton floccosus is 22–27 μmol TE/g of dry samples for the different processes used to obtain ethanolic extracts with leaves dried at 40°C and 50°C (Figure 14). The means of the values were compared using the Tukey test. No marked difference among the processes studied was found for the TEAC, and there was statistical homogeneity among them. It was, therefore, established that, as with Bidens pilosa L., there is no marked difference in the antioxidant activity values obtained using the different extraction processes carried out in the present research.
As with the previous process, there is no marked difference among the methods, and we, therefore, proceeded to establish the mean radical inhibition coefficient (IC50) of the process that obtained the highest value of antioxidant activity equivalent to Trolox, which was the ultrasound of the material dried at 40°C.
Table 10 shows the inhibition coefficient (IC50) determined by the DPPH method performed on the extract of Croton floccosus and Trolox (standard). The difference between the extract and the standard used in the previous determinations is compared.
| Sample | IC50 (μg/mL) |
|---|---|
| Croton floccosus ethanolic extract | 644.125 |
| Trolox | 2.599 |
It is necessary to mention that, since Croton floccosus is a species that is endemic to Ecuador, there are only a few studies referring to this species. In his study, M.E. Flores35 obtained IC50 values of 91.16 μg/mL for extracts obtained from the leaves of Croton floccosus collected during the flowering period of this species using a maceration process in which ethanol was employed as a solvent. This, therefore, made it possible to establish what is described by Altamirano36 in his research work on Croton species, which states that the collection carried out to obtain extracts must take place at the beginning of flowering because it is the moment at which these plants contain the greatest amount of active substances. This may, therefore, have influenced the properties of the extract, as may the conditions under which the material was prepared, the extraction and the storage of the extracts.
The use of phytochemical tests to identify metabolites, along with their qualitative characterization show that the plants studied have a great variety of chemical compounds, which contain flavonoids, phenols, reducing sugars, saponins and tannins in the case of Bidens pilosa L., and alkaloids, phenols, flavonoids, tannins and saponins in that of Croton floccosus. It has been shown that the content of total phenols for Bidens pilosa L. had the highest yield of phenolic compounds when the Soxhlet extraction method was used with the plant material dried at 50°C, with a value corresponding to 48.609 ± 0.370 mg GAE/g of dry sample. With regard to the content of flavonoids, a maximum value of 17.795 ± 0.0644 mg QE/g of dry sample was obtained, corresponding to the Soxhlet extraction process method at 40°C. In the case of Croton floccosus, the maximum reported value of total phenols corresponds to the maceration extraction process when the material had been dried at 50°C, with a value of 128.212 ± 0.601 mg GAE/g of dry sample. With regard to the content of total flavonoids, the best results corresponded to the extraction process carried out using Soxhlet extraction with the plant material dried at 40°C, with a value of 34.139 ± 0.06224 mg QE/g dry sample. Finally, both plant species showed antioxidant potential, although it is necessary to establish that the Bidens pilosa L. species responded better to the test established, with an IC50 of 239.33 μg/mL when compared to Croton floccosus, which attained an IC50 of 644.125 μg/mL.
Open Science Framework: Underlying data for ‘Phytochemical study of the plant species Bidens pilosa L. (Asteraceae) and Croton floccosus (Euphorbiaceae)’, https://doi.org/10.17605/OSF.IO/VNPMJ.30
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors acknowledge the support of the Universidad Técnica de Manabí (UTM) in the Convocation Project approved in 2018 with the “Tamizaje fitoquímico y determinación de la actividad antioxidante y antibacterial de 8 familias de plantas medicinales que se encuentran en la provincia de Manabí”.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical chemistry
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chemical Engineering
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Chemical Engineering
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Analytical chemistry
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 17 Oct 22 |
read | read |
|
Version 1 27 Jun 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)