Keywords
Study protocol, Randomized controlled trial, Labour, Peanut ball, Maternal and neonatal outcome, Stress, Cortisol
This article is included in the Manipal Academy of Higher Education gateway.
Study protocol, Randomized controlled trial, Labour, Peanut ball, Maternal and neonatal outcome, Stress, Cortisol
APGAR: Appearance, Pulse, Grimace, Activity, and Respiration
CS: Cesarean section
QACE: questionnaire on assessing the childbirth experiences
WHO: World Health Organization
Even after the strong recommendations of the World Health Organization regarding mobility and upright positions to prevent delay in the first stage of labour, increase in induction during labour, and continuous fetal monitoring (Calik, Karabulutlu, & Yavuz, 2018) made position changes challenging during labour. Even if a mother is constrained to bed, frequent changes of maternal positions that facilitate pelvic movements should be given during labour (Lawrence, Lewis, Hofmeyr, & Styles, 2013). The absence of changes in the position of the mother during labour can lead to prolonged labour and increases the chance of cesarean birth due to failure to progress or descend (Zwelling, 2010; Akyıldız, Coban, Gör Uslu, & Taşpınar, 2021). The peanut ball is considered an effective intervention to overcome this obstacle and promote the different positions during labour.
The peanut ball is an alternative to the conventional birth ball. The birthing ball is preferably used during the pregnancy period (Ghasab, Kohan, Firoozehchian, & Ebrahimi, 2019; Mirzakhani, Hejazinia, Golmakani, Sardar, & SHakeri, 2015), whereas the effectiveness of the peanut ball is elicited even during labour (Outland & Alvarado, 2019). The peanut ball is a curved peanut-shaped ball that has a middle indentation that allows the women to place the ball between and below the knees for allowing either the lateral, supine or sitting position, which increases pelvic dimensions, promotes progressive fetal rotation and descent during second-stage labour and ultimately promote the progress of labour (Zwelling, 2010; Roth, Dent, Parfitt, Hering, & Bay, 2016). Several studies have shown a reduction in either the length of labour (Roth, Dent, Parfitt, Hering, & Bay, 2016; Tussey CM, 2015 Jan) or a reduction in the cesarean birth (Outland & Alvarado, 2019) with use of the peanut ball in immobile women.
In 2015, Tussey et al. reported in a randomised controlled trial that effective utilisation of peanut ball during labour in the epidural woman has shortened the second stage of labour by 11 minutes and reached clinical significance in decreasing the length of time of the second stage of labour by 29 minutes. In addition, only 10% of those women who delivered with a peanut ball had a caesareans birth compared to 21% in the control group (p < .05) (Tussey CM, 2015 Jan).
Roth and his colleagues in the year 2016 demonstrated in a randomised controlled study that the first stage of labour was significantly reduced for the nulliparous women (p = .018), but not for the multiparous women after the use of peanut ball. But no statistically significant difference was found in the length of the pushing stage for either nulliparous or multiparous women (Roth, Dent, Parfitt, Hering, & Bay, 2016). These findings were supported by the quasi-experimental study done by Hickey & Savage, and they found out that peanut ball use during labour substantially (50%) decreased the rate of cesarean sections among women (164) labouring with epidurals (Hickey & Savage, 2019) However, these studies’ results are not statistically significant to prove that the peanut ball alone has decreased the labour duration (Outland & Alvarado, 2019; Roth, Dent, Parfitt, Hering, & Bay, 2016; Tussey CM, 2015 Jan; Hickey & Savage, 2019).
Furthermore, Ahmadpour and team concluded in their systematic review and meta-analysis that the 645 women who used the peanut ball during labour have no statistically significant difference between the two groups in cesarean section rate and the length of the first stage of labour. Therefore, the study recommended conducting more rigorous randomised controlled trials to evaluate the effectiveness of the peanut ball during labour (Ahmadpour, Mohammad-Alizadeh-Charandabi, Doosti, & Mirghafourvand, 2021).
Even the Grenwik, et al., study in 2019 supported the statement in their systematic review and summarised that more research is needed to prove the statistical significance in decreasing labour duration after the peanut ball use (Grenwik, et al., 2019).
In the Indian research literature, there have been no randomised studies conducted on the peanut ball interventions (Jayasudha, Lakshmi, Priscilla, Anitha, & Priya, 2021). That is why we, the researchers, through the randomised clinical trial, aimed at evaluating the effectiveness of a peanut ball device during labour on maternal and neonatal outcomes and assessing the stress response induced by labour in terms of maternal and fetal cortisol in low-risk primigravid women. If found to be effective, the proposed large-scale clinical trial, the peanut ball intervention can be implemented routinely in maternal healthcare settings for safe and comfortable delivery, which can ultimately prevent prolongation of labour and decrease the proportion of postpartum hemorrhage and stress level among the mother.
The following objectives are formulated:
1. To determine the effectiveness of the use of a peanut ball device during labour in terms of following maternal outcomes:
2. To determine the effectiveness of the use of a peanut ball device during labour in terms of following neonatal outcomes.
✓ Fetal heart rate patterns
✓ Neonatal Intensive Care Unit (NICU) admissions
✓ APGAR score (Appearance (skin colour), Pulse (heart rate), Grimace (reflex irritability), Activity (muscle tone) score and Respiration) at the time of birth at one minute and five minutes.
✓ Birth injuries
3. To identify and compare the behavioural response, stress level, childbirth experience and maternal satisfaction among low-risk primigravid women during the active stage of labour between the experimental and control group.
4. To identify and compare the stress level of neonates between the intervention and control.
The trial is designed to test the hypotheses at a 0.05 level of significance. The following hypotheses were formulated.
H1: There will be a significant difference in the maternal and neonatal outcomes during labour between the intervention and control group.
H2: There will be a significant difference in the behavioural response score between the intervention and control group.
H3: There will be a significant difference in the maternal cortisol levels between the intervention and control group.
H4: There will be a significant difference in the neonatal cortisol levels between the intervention and control group.
H5: There will be a significant difference between the childbirth experience score between the intervention and control group.
A prospective block randomised controlled trial with parallel groups is the research design adopted for the study. Block randomisation of 64 blocks with a block size of 12 each is generated using a Research randomiser, an online random number generator by a study statistician. A senior nurse who is working in the labour ward and who is not directly involved in the study will be generating the sequence. Concealment allocation will be achieved using a sequentially numbered opaque and sealed envelope (SNOSE), where participants will be assigned randomly either to receive the peanut ball intervention or standard care.
All study materials can be found as Extended data (Kamath, 2022a–f). The study will adhere to the Consolidated Standards of Reporting Trials (CONSORT) guidelines (Figure 1) (Sally Hopewell, 2008) and the protocol follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines (Kamath, 2022g).
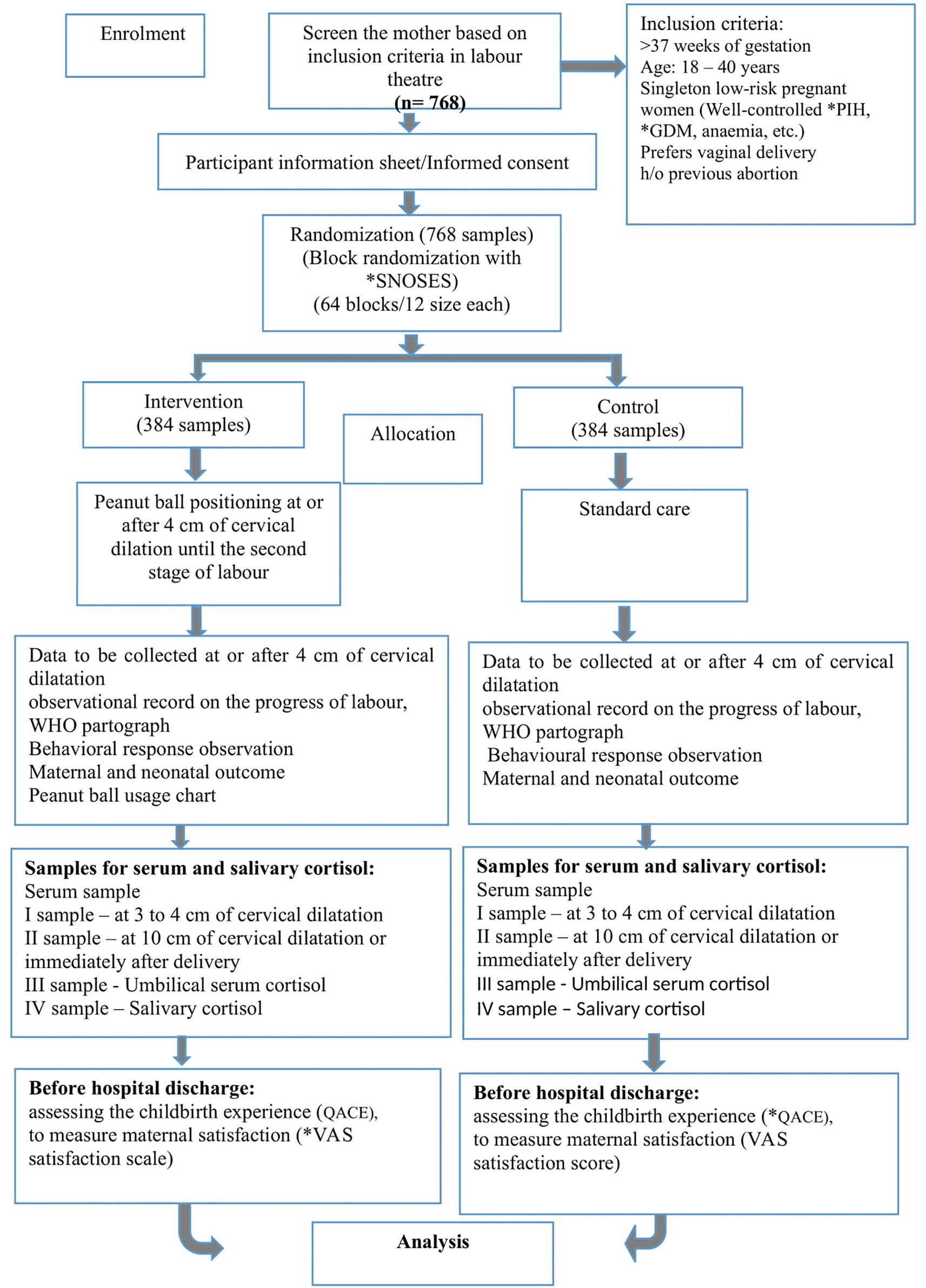
*PIH: Pregnancy Induced Hypertension, *GDM: Gestational Diabetes Mellitus, *VAS: Visual Anologue scale, WHO: World Health Organization. *SNOSEs: Sequentially Numbered Opaque and Sealed Envelopes, *QACE: Questionnaire on Assessing the Childbirth Experiences.
The study is funded by the Indian Council of Medical Research (ICMR), Government of India, (File No:5/7/58/MH/Adhoc/2019-0146/RBMCH) and is approved by Manipal Academy of Higher Education, Institutional review board (Reg no. ECR/146/Inst/KA/2013/RR-16). An estimated 768 low-risk primigravid women will be recruited from the selected tertiary care hospital of southern district of Karnataka. This research is registered under the CTRI (Clinical Trials Registry of India) (CTRI/2019/08/020802) (21/8/2019).
The eligibility criteria include low-risk primigravid women who are aged between 18 to 40 years, singleton pregnancy and in cephalic presentation, gestational age of 37.0 to 41.6 weeks, well-controlled pre-eclampsia, and gestational diabetes mellitus, history of abortions, well-controlled/moderate anaemia, history of mild intrauterine growth restriction (IUGR), willing to use the peanut ball during the first stage of labour and preferring a vaginal delivery.
The exclusion criteria include women who are known to have any major fetal anomalies and very low and late preterm, a high-risk antenatal mother with any pregnancy-related complications like severe pre-eclampsia/eclampsia, preterm labour, placenta praevia, malpresentation during the study, contraindication for vaginal delivery, multigravida, multiple pregnancies, had any sort of fracture of leg/any major problem in the past and having weight more than 90 kg.
The expected variations in the research outcome variable determines the sample size for the study; (where n is the sample size per group, = 1.96 at α = 0.05, = 0.84 (power at 80% power), σ = standard deviation of the primary outcome variable stress level of the mother as measured by serum cortisol level = 2.58 μg/dl (Stjernholm, Nyberg, Cardell, & Höybye, 2016), d = clinically significant difference = 0.55 μg/dl. The sample size, calculated with an anticipatory attrition rate of 10%, is 768.
All the low-risk primigravid women at or after 37 weeks of gestation attending the outpatient services at tertiary care hospitals for an antenatal check-up will be oriented about the implementation of peanut ball during labour by using the peanut ball positions chart prepared by premier birth tools and by showing the video on peanut ball during labour prepared by the researcher.
Thereafter, the mothers will be screened in the labour room based on the inclusion criteria. The research team will explain the research purpose and procedure to the eligible mothers and, if they are willing to participate, informed written consent will be taken.
Subsequently, the low risk primigravid women at 2–3 cm of cervical dilatation preferring a vaginal delivery will be block randomised to peanut ball intervention group or standard care control group by an external member (labour room nurse) with the help of SNOSEs. The research assistant and research nurse carrying out data collection, data entry, and data analysis will be blinded to group allocation.
The peanut ball positioning is done at or after 4 cm of dilatation by a trained nurse-midwife whenever the mother is lying or sitting on the bed. Peanut balls are available in four sizes: 40 cm, 50 cm, 60 cm, and 70 cm to fit different participants as reported by Grant & Clutter in 2014. The researcher will make sure that the suitable size of the ball is given to the woman as recommended by the premier birth tools as per the height of the mother, that is: 40 cm for women whose height is under 5′3″, 50 cm is the most common size and exclusivly used for women with 5′3″ to 5′ 6″ height, 60 cm for women 5′7″ or taller or overweight women, and 70 cm used only to sit on and straddle on the peanut ball as reported by Grant C in 2015. All the sizes of peanut balls will be available in the labour room (Figure 2) to use during labour and will be covered with clean peanut ball covers. A suitable peanut ball will be provided to the mother to give four main peanut ball positions: sitting, semi-sitting, side-lying, and tuck position until the mother is ready to push during the second stage of labour.
Adherence to intervention protocols and procedures will be done continuously by research assistants throughout the intervention by being with the patient and educating and comforting the mother.
The control group of women will receive the standard/routine care provided in the labour room of the tertiary care setting.
Participants will be recruited only after informed written consent. Participants participation in this study is voluntary and they can withdraw their participation at any time, without giving any reasons. They will still get the usual routine care during labour, and non-participation will not have any negative consequences on their subsequent medical treatment or relationship with the treating obstetrician and midwife. If the participant withdraws from the study before data collection is completed, their data collected till the withdrawal will be used in the study report.
Maternal and neonatal outcomes will be measured by the research assistants at different points of time and compared between the intervention and control groups. The maternal outcome includes measurement of the duration of the active stage of labour, duration/pushing effort in the second stage of labour, mode of delivery, the need of pain medications, perception of pain, behavioural response during the active stage of labour, serum analysis of cortisol level, the proportion of postpartum hemorrhage, and neonatal outcome includes a pattern of fetal heart rate, APGAR score (Apgar V, 1958), birth injuries, and salivary cortisol level.
During the active stage of labour blood samples will be collected twice from the mothers in both groups for serum cortisol analysis. The 1st sample will be collected when cervical dilatation is 3–4 cm and the 2nd sample will be collected after full dilatation of the cervix and these will be sent to lab for analysis. An umbilical cord blood sample will be collected from the newborn at the time of birth to check the serum cortisol level, and salivary cortisol will be collected from the newborn within one hour of birth to check the salivary cortisol level. An observational record on the progress of the labour will be done for both the groups once they reach 4 cm of cervical dilatation by using the WHO partograph Both the groups will be observed for their behavioral responses for any two hours during the first stage of the labour with the cervical dilatation ranging from 4–7 cms by using the behavioral response observational checklist (Karkada et al., 2010) Both the neonatal and maternal outcomes will be recorded by using the outcome of the labour assessment tool (Kamath, 2022f) and WHO Partograph, and during postnatal period before getting discharged from the hospital, ie., 3–4 days after the delivery, women from both of the groups will be assessed for their childbirth experience by using a questionnaire for assessing the childbirth experience (QACE) tool (Carquillat, P. et al., 2017). Maternal satisfaction regarding assistance provided during labour/delivery will be measured by using VAS (visual analogue scale) patient satisfaction score (Hayes and Patterson, 1921).
The researcher prepared a record of the outcome of labour to document maternal and neonatal outcomes. The maternal outcome consists of 18 items, such as duration of labour, nature of delivery, need of pain medications, objective assessment of pain, evaluation of maternal and fetal cortisol levels and postpartum hemorrhage etc. The neonatal tool includes details of APGAR score at birth, weight of the newborn, neonatal injuries during birth and particulars of NICU admission etc. (Kamath, 2022f).
Blood samples will be collected twice during labour to determine the stress level. The first blood sample will be collected when cervical dilatation is 4–5 cm, and the second will be collected when cervical dilatation is of 10 cm from both groups and these will be sent to the biochemistry lab for analysis. After complete coagulation, the blood sample will be centrifuged, and then the serum will be separated for analysis of cortisol concentration. A blood sample from the umbilical cord will be taken at the time of birth to check the serum cortisol level of the baby, and saliva will be collected within one hour of birth by using swab stick by keeping it in baby’s mouth for two minutes to check the salivary cortisol level. The serum will be separated by centrifuging the cord blood. Using the principles of electrochemiluminescence immunoassays, cortisol levels will be measured in serum and cord blood using the Cobas -602 e-immunoassay analyser. Assay reagents are obtained from Roche Diagnostics. The saliva samples will be immediately frozen at 20° C until analysis. The free cortisol will be determined by ELISA.
This observation checklist, prepared by Karkada EC et al., is used in this study by obtaining permission from the author to assess the behavioural responses of low-risk primigravid women during the first stage of labour. A behavioural response checklist has three areas of observation, i.e., the behavioural response in between contractions which has 20 items, facial response during a contraction, which contains eight items; behavioural responses during contractions, which includes 20 items; and lastly, physiological parameters, which have two items. Overall, the tool includes 50 items. The study encompasses negative and positive responses (Karkada, Noronha, & Dsouza, 2010).
Carquillat, P. et al., developed the QACE questionnaire, and it is self reporting tool screens for any negative experiences during child birth. It consists of six main thematic categories. These thematic categories are expectations (three items), sensory experiences (two items), perceived control (six items), relationship with caregivers and the midwife (four items), emotions (seven items), and the first moments with the baby (three items). Additional ten and the other three items are to measure causal indicators that potentially influence the childbirth experience. The response format is a 4-point Likert scale, ranging from “Totally”, “In part”, “Not so much”, and “Not at all” (Carquillat, P. et al., 2017).
The researchers modified the VAS into a patient satisfaction scale to assess the satisfaction level of low-risk primigravid women during labour. It is a 10 cm scale with smileys indicating expression of satisfaction, with the scoring: 0 – indicates no satisfaction and score ranging between 1–3 – mild level of satisfaction, 4–7 – moderate level of satisfaction, and 8–10 – high level of satisfaction (Hayes and Patterson, 1921).
The validity (item Content Validity Index (I-CVI) and Scale Content Validity Index (S-CVI/Ave) and reliability (Table 1) of the study tools were established by giving the tools, along with the criteria checklist, to a panel of nine experts from different fields, such as the women and child health department of physiotherapy, department of statistics, department of Obstetrics and Gynecological Nursing, and experts from OBG department, Kasturba Hospital Manipal to review the study tools. Content validity is established in terms of relevancy, accuracy, and appropriateness. The item Content Validity Index (I-CVI) and Scale Content Validity Index (S-CVI/Ave) of the tools were calculated based on recommendations by Polit and Beck (2012).
*I-CVI- Item - content validity index, *S-CVI- Scale level - content validity index.
The research assistant will do coding and data entry. The primary investigator will review the data entered for any discrepancies such as data entry errors, missing values etc. One of the co-authors will check the data entry errors by randomly selecting data sheets. A separate laptop will be used for the project store data and can be accessed only by the research assistant and primary investigator.
Statistical analysis will be performed using SPSS software version 16. The data analysis will be done based on the objectives and hypotheses of the study. We will follow the intention to treat analysis (ITT). The sample characteristics are measured using descriptive statistics. Two independent samples t-test/Mann-Whitney U-test (based on normality of data) will be used to determine the effectiveness of the usage of peanut ball on the maternal outcomes of labour. Associations among the variables will be computed using the Chi-square test (for categorical variables) and two independent samples t-test/Mann-Whitney U-test or one-way ANOVA/Kruskal Wallis test (for continuous variables). A comparison of behavioural response and stress level of low-risk primigravid women among experimental and control groups will be done using repeated measures of ANOVA. A comparison of childbirth experiences will be done using an independent t-test.
Data monitoring committee will be formed, which includes methodology experts, subject experts and statistician.
Approval has been obtained from Head of the Unit - Obstetrical and Gynecological department and Medical Superintendent Kasturba Hospital Manipal for this study. Ethical clearance for the study was obtained from the Kasturba Medical College and Kasturba Hospital, Institutional Ethics committee Manipal (Reg No: ECR/146/1nst/KA/2013/RR-16). The trial is registered under the Clinical Trial Registry of India (CTRI/2019/08/020802). A written participant information sheet (Kamath, 2022b) will be given regarding the details of the study, and it will be explained to all low-risk primigravid women before enrolment to the study. Their involvement benefits and harm (none) as well as whom to contact in case of doubt will be explained to the participants. Written informed consent (Kamath, 2022c) from the participants will be obtained before involving them in study.
Confidentiality of the research data collected will be maintained strictly as per the ethical standards. Only the research assistants and the researchers will have access to the participants’ data in the study. The data and results from this study may be presented at conferences and published in scientific journals without revealing the identity of the participants.
During the study period, if any medical problem arises as a direct result of the study intervention, the researchers will be responsible for ensuring proper medical care is provided to the participant. Suppose a participant suffers from any injury (physical/mental) or disease/illness as result of the correctly implemented study procedures. In that case, the researcher is responsible for that unless the injury or disease relates to the mothers negligent or reckless act.
Labour is a complex phenomenon in which the passenger (fetus) goes through the passage (pelvis) using the power (contractions) to deliver a healthy neonate (Michelle & Gayle, 2020). In order to accelerate the labour a series of interventional strategies has been adopted, and one among them is use of peanut ball during labour. Utilisation of peanut ball has proven effective in reducing cesarean section rates (Tussey CM, 2015 Jan), and even lowered the rates of instrumental births, including forceps and vacuum, and decreased the incidence of third- and fourth-degree perineal lacerations during vaginal deliveries. By widening the pelvic outlet to increase the progress of labour and thereby facilitate the descent of the fetal head.
Globally, the peanut balls are extensively utilised by midwives to deliver the women through vaginal mode, but in India it is underutilised. Jayasudha, et al. in the year 2021, did the quasi-experimental study in India, in which peanut ball use during the first stage of labour is explored (Jayasudha et al., 2021). The study lacks a controlled setting; the stakeholders consider methodologically rigorous research evidence to make a decision related to policymaking. Therefore, a clinical trial with block randomisation to investigate the effectiveness of peanut ball in improving maternal and neonatal outcomes is planned in the Indian context to make the appropriate clinical decision.
For the study the participant recruitment began in February 2020. To date, we have enrolled and randomised 550 low-risk primigravid mothers. Out of the total, 225 mothers were recruited to the intervention and 225 mothers were recruited for the control group. Mothers’ unacceptance to utilise the peanut ball during labour is a challenge faced during the initial part of the study. To encounter this, all the primigravid women at or after 37 weeks of gestation attending antenatal outpatient units were given informational material about the “Peanut ball use during labour”. This educational material has oriented and familiarised the mothers with peanut ball and its uses during labour which ultimately increased the rate of peanut ball acceptance and use during labour.
Along with that, coronavirus disease 2019 (COVID-19) imposed a lockdown, and after that, institutional restrictions to resume research activities to mitigate new cases of infection had made the study come to a halt. Recruitment for the study was stopped, and we lost participants for around five months. Due to this situation, we have fallen behind the recruitment timeline. To combat this, we utilised this duration to initiate a systematic review that will bring new evidence to implement peanut ball during labour.
Besides these challenges, there are two possible limitations in this study, first, ascertainment bias, as the investigators are aware of the intervention the participants are receiving. In this project, the investigator and participants will know the allocation group for the women, as the woman will either utilise the peanut-ball or not, so they cannot be blinded. The strategy implemented to reduce the ascertainment bias is allocation concealment using SNOSEs by the labour room senior nurse on duty. The research assistant and research nurse carrying out data collection, data entry, and data analysis will be blinded to group allocation. The second limitation is compliance with the intervention during labour. To tackle this, a trained nurse will be present throughout the labour process and encourage and motivates the mothers to use the peanut ball continuously.
Despite these possible limitations, this novel approach will be a promising intervention to promote labour progress and prevent unnecessary caesarean section and instrumental birth during the second stage of labour.
In this paper, researchers described the study design and the methodological approach adopted to evaluate the effectiveness of peanut ball during labour. Moreover, this study can be used as a guide to implement peanut ball use in the labour rooms of tertiary health care settings in the Indian context.
This project contains following extended data:
Figshare: Demographic proforma.docx. https://doi.org/10.6084/m9.figshare.19122233 (Kamath, 2022a)
Figshare: Participant Information Sheet.docx. https://doi.org/10.6084/m9.figshare.19122230 (Kamath, 2022b)
Figshare: 18 Informed Consent English.doc https://doi.org/10.6084/m9.figshare.19122242 (Kamath, 2022c)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: Screening Tool.docx. https://doi.org/10.6084/m9.figshare.19122239 (Kamath, 2022d)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Figshare: Tool III modified partograph.docx. https://doi.org/10.6084/m9.figshare.19122236 (Kamath, 2022e)
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
Figshare: Structured observational record on outcome of the labor.docx https://doi.org/10.6084/m9.figshare.19122227 (Kamath, 2022f)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Repository: SPIRIT checklist for ‘Effectiveness of a peanut ball device during labour on maternal and neonatal outcomes: protocol for a randomised controlled trial’. https://figshare.com/s/5e8db2fc8dee7a7e06e5 (Kamath, 2022g)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Is the rationale for, and objectives of, the study clearly described?
Yes
Is the study design appropriate for the research question?
Yes
Are sufficient details of the methods provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: women's Health ,Maternal Health, teaching stratergies
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 21 Nov 22 |
read | |
|
Version 1 29 Jun 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)