Keywords
cadherins, neural crest cells, placode cells, trigeminal ganglion, chick embryo
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Cell Migration collection.
cadherins, neural crest cells, placode cells, trigeminal ganglion, chick embryo
In this revised version of the manuscript, we have made various changes to the text, including minor edits throughout the manuscript (e.g., new figure numbers, updated legends, modified Materials and Methods, and other necessary text modifications in keeping with the new data we have provided); expanding on the morphology of cadherin split GFP-transfected cells in the Results and Discussion; quantifying the number of GFP-positive cells and regions (in the in vitro and in vivo GRASP assays); determining the standard error of the mean and statistical significance of the data (reported in new Tables 1 and 2); and discussing our results in light of what is currently known about cadherin interactions in the literature. We have also updated Figure 1 to only show the in vivo trigeminal ganglia lysate data in order to alleviate confusion related to cell line data that was extraneous to the manuscript. Further, we have revised the in vivo GRASP figures as per the Reviewers’s comments, leading to the generation of a new Figure 7 and 8. Finally, we have provided two new figures (Figure 9 and 10), which respectively examine N-cadherin homophilic interactions in trigeminal placode cells and trigeminal placode cell-derived neurons via a GRASP assay, and the endogenous distribution of N-cadherin and Cadherin-7 in the forming trigeminal ganglion. The results from these new figures have now been incorporated into the Discussion to provide a more comprehensive analysis of our results.
See the authors' detailed response to the review by Susan Wray
See the authors' detailed response to the review by Paolo E. Forni and Ed Zandro Taroc
See the authors' detailed response to the review by Kristin Bruk Artinger
Cranial ganglia are sensory structures of the peripheral nervous system possessing the cell bodies of the cranial nerves. These ganglia and their associated nerves function in olfaction, taste, hearing, vision, and somatosensation.1–3 The trigeminal ganglion contains three sensory branches (ophthalmic, maxillary, and mandibular) that innervate different regions of the face to mediate sensations of pain, touch, and temperature.3–5 During embryonic development, two distinct cell populations, neural crest cells and neurogenic placode cells, intermingle and aggregate to generate the trigeminal ganglion.6–10 These interactions have been studied for over 60 years and reveal that each cell type contributes distinctly to trigeminal ganglion formation, with neural crest cells acting as a scaffold for the integration of placode cell-derived neurons, while placodal neurons aid in the condensation of neural crest cells.7,10,11 Moreover, ablation of either of these cell populations leads to severe defects in trigeminal ganglion development, indicating a reciprocal relationship.7,10,12
Prior studies indicate that intercellular interactions during trigeminal ganglion formation are mediated, in part, by cadherin-based adhesion. Two cadherins, Cadherin-7 and neural cadherin (N-cadherin), are expressed in neural crest cells and placode cells, respectively, during chick trigeminal gangliogenesis. Expression of Cadherin-7, a type II classical cadherin, was discovered in migratory cranial neural crest cells in the chick embryo over 25 years ago.13 More recent studies of Cadherin-7 protein confirmed previous in situ hybridization findings and noted Cadherin-7 in chick migratory cranial neural crest cells contributing to the trigeminal ganglion.14 Both depletion and overexpression of Cadherin-7 impact the distribution of embryonic neural crest cells and placodal neurons, and as such, the overall morphology of the ganglion. N-cadherin, a type I classical cadherin, is present throughout development and has been found in derivatives of the endoderm, mesoderm, and ectoderm.15 Notably, both ectodermal placode cells and their neuronal derivatives express N-cadherin16 in the chick trigeminal ganglion. Knockdown of N-cadherin does not affect initial placode cell ingression and delamination from the ectoderm,16 but leads to increased placodal neuron dispersal during trigeminal gangliogenesis. Conversely, N-cadherin overexpression causes aberrant aggregation of placodal neurons.16 Modulation of N-cadherin levels appears to involve, in part, post-translational mechanisms linked to Slit1-Robo2 signaling in the developing chick trigeminal ganglion,16 but specific details underlying this process are not known.
While the ability of cadherins to make homophilic interactions is well understood, cadherins can also make heterophilic (i.e., non-like) connections with other cadherins, either in the same (homotypic) or different (heterotypic) cell types. Observations of heterophilic cadherin interactions have been reported during normal development of the endoderm,17 in establishing synaptic potentials within the hippocampus,18 and during Xenopus gastrulation,19 and are also noted in diseases such as cancer.20 In addition, the atypical cadherins Fat and Dachsous are capable of forming heterodimers between neighboring homotypic cells.21 Collectively, these results support the notion that heterophilic interactions can occur between different types of cadherins during development. While previous studies noted the formation of aggregates from mixtures of N-cadherin- and Cadherin-7-expressing cells in vitro,13 the potential role of heterophilic cadherin interactions between neural crest cells and placode cell-derived neurons as they assemble the trigeminal ganglion has yet to be explored.
To address this question, we performed experiments to elucidate potential heterophilic interactions between Cadherin-7 and N-cadherin during the formation of the chick trigeminal ganglion. Our in vivo and in vitro biochemistry and imaging data indicate Cadherin-7 and N-cadherin physically interact during trigeminal ganglion assembly and that this involves heterophilic interactions between Cadherin-7, expressed in neural crest cells, and N-cadherin, found in placodal neurons. These findings further clarify the reciprocal relationship observed between coalescing neural crest cells and placodal neurons during trigeminal gangliogenesis, providing an additional molecular basis for this process.
Fertilized chicken eggs (Gallus gallus) were obtained from the Department of Animal and Avian Sciences, University of Maryland, and Moyer’s Chicks, Inc. (PA), and incubated at 37°C in humidified incubators (EggCartons.com, Manchaug, MA, USA). Embryos were staged by the Hamburger-Hamilton (HH) staging method22 or by counting the number of somite pairs (somite stage, ss).
No ethical approval was required for this study for the chick embryos. The NIH Office for Protection from Research Risks has interpreted “live vertebrate animal” to apply to avians (e.g., chick embryos) only after hatching. Since our work does not utilize hatched chicks, no Institutional Animal Care and Use protocol for this work is necessary.
Four different GRASP constructs were synthesized by GenScript (RRID:SCR_002891) to allow for incorporation of split GFP moieties (subunits 1-10 or subunit 11) into the extracellular domain of Cadherin-7 and N-cadherin, with the design based on similar plasmids generated in23 and available in Addgene (m-sGFP1-10::NLG1 (Addgene plasmid #44967; RRID:Addgene_44967) and m-sGFP11::NXN were gifts from Joshua Sanes (Addgene plasmid #44968; RRID:Addgene_44968)). Briefly, each plasmid from Addgene was modified to remove the respective insert (either NLG1 or NXN), and, in its place, we inserted the Cadherin-7 or N-cadherin cDNA sequence corresponding to the mature peptide. Sequence accuracy of constructs was confirmed by GenScript and expression of each cadherin was validated through immunocytochemistry.
Chinese hamster ovary (CHO) cells (ATCC Cat# CCL-61, RRID:CVCL_0214; American Type Culture Collection) were cultured in Ham’s F12 media (10-080, Corning/Cellgro) supplemented with 10% fetal bovine serum (Genesee Scientific Cat#25-514H). Transient transfection assays were carried out using the Lipofectamine 2000 reagent (Thermo Fisher Scientific, Inc., Cat#11668019). Cells were grown to 90% confluency, and transfections were performed according to the manufacturer’s instructions and according to the protocols outlined in.24,25 The chick N-cadherin-expressing (pCIG-N-cadherin) and empty (pCIG) vectors were gifts from Dr. Marianne Bronner (California Institute of Technology).
Embryonic trigeminal ganglia were used for immunoprecipitations, with tissue harvested as described previously by.14,25,26 Briefly, forming trigeminal ganglia were dissected, pooled, pelleted, flash-frozen in liquid nitrogen, and stored at -80°C. Cultured cells were scraped into 1X Phosphate-buffered Saline (1X PBS), pelleted, flash-frozen in liquid nitrogen, and stored at -80°C. Pellets were thawed on ice and lysed in lysis buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1% IGEPAL CA-630) supplemented with cOmplete protease inhibitor cocktail tablets (Roche, Cat#04693124001) and 1 mM PMSF (Sigma Aldrich Cat#10837091001) for 30 minutes at 4°C with periodic mixing. Soluble fractions were collected following centrifugation at maximum speed for 15 minutes at 4°C (Microfuge 20R Centrifuge, Beckman Coulter, Inc., Cat#B31612), and protein concentration was quantified (BioPhotometer, Eppendorf, Cat#6131 26936) by Bradford assay (Thermo Fisher Scientific, Inc., Cat#1863028). Immunoprecipitations were carried out using protein A/G magnetic beads (Thermo Fisher Scientific, Inc., Cat#88802) according to the manufacturer’s instructions (Thermo Fisher Scientific, Inc.). Equivalent amounts of protein lysates (~120 μg) were incubated with 10 μg rabbit polyclonal N-cadherin antibody (Abcam Cat#ab12221, RRID:AB_298943) or normal rabbit IgG control (R&D Systems Cat#AB-105-C, RRID:AB_354266) overnight at 4°C with constant rotation. The following day, 0.25 mg washed protein A/G magnetic beads were incubated with the lysate/antibody mixture for one hour at room temperature with mixing. Following incubation, the samples were washed, equivalent volumes of SDS sample buffer were added, mixtures were boiled at 100°C for 10 minutes, magnetic beads were collected, and samples were loaded for immunoblotting as described below. Input amounts represent 5% (trigeminal ganglia) and 10% (cell culture) of the initial lysate amount used in the immunoprecipitation. Assays were conducted twice.
Immunoblotting after immunoprecipitation was performed according to the protocol by Refs. 14, 25, 26. Samples were processed via SDS-PAGE (10% Mini-Protean TGX gel, BioRad #456-1034) in 1X Running Buffer (25 mM Tris (Thermo Fisher Scientific, Inc., Cat#BP-152-1), 192 mM glycine (Thermo Fisher Scientific, Inc., Cat#AC120070010), 0.1% sodium dodecyl sulfate (VWR, Cat#4095-02)) and then transferred to 0.45 μm BioTrace nitrocellulose membrane (Pall, Cat#66485) via wet transfer (Biorad, Mini-PROTEAN Tetra Vertical Cell for Mini Precast gels, Cat#1658004) in 1X Transfer Buffer (Running Buffer + 10% Methanol (Thermo Fisher Scientific, Inc., Cat#A452-4)) according to the manufacturer’s guidelines. For immunoblotting, membranes were blocked in blocking buffer (1X PBS + 0.1% Tween-20 (Sigma Aldrich, Cat#P1379-500ML)) (PTW) + 5% non-fat milk (Carnation Instant Nonfat Dry Milk). Next, primary antibodies against mouse monoclonal Cadherin-7 (1:150, DSHB, Cat#ccd7-1, RRID:AB_528111) or rabbit polyclonal N-cadherin (1:1000, Abcam Cat#ab12221) were diluted as indicated in blocking buffer and incubated overnight with shaking at 4°C. Unbound primary antibodies were washed off with PTW (three times, 10 minutes each), followed by incubation at room temperature for 45 minutes with the following secondary antibodies diluted in blocking buffer (1:10,000): goat anti-mouse polyclonal IgG (H&L) antibody peroxidase conjugated (Rockland Cat# 610-1302, RRID:AB_219656) or goat anti-rabbit polyclonal IgG (H&L) secondary antibody peroxidase conjugated (Rockland Cat# 611-1302, RRID:AB_219720). After washing three times, 10 minutes each, in PTW, proteins were detected using enhanced chemiluminescent substrates mixed in a 1:1 ratio (SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific, Inc., Cat#34580) or SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific, Inc., Cat#34095)). Immunoblot images for figures were gamma-modified and processed using Adobe Photoshop (RRID:SCR_014199) CC 2019 (20.0.6 release, Adobe Systems, San Jose, CA, USA).
Embryos collected at various stages, or cultured cells in two-well chamber slides (LAB-TEK, Cat#154461), were used for immunostaining. For the former, detection of various proteins was performed on 14 μm transverse sections following 4% paraformaldehyde (PFA) fixation overnight, gelatin embedding, and cryostat sectioning, according to the protocol described previously by.14,26,27 For the latter, cells were fixed in 4% PFA for 15 minutes, followed by immunocytochemistry. Tissue or cells were permeabilized by washing two times, 10 minutes each, in 1X PBS + 0.1% Triton X-100 (Sigma Aldrich, Cat#TX1568-1) (PBSTX), followed by a one-hour blocking step of PBSTX + 10% sheep serum (Sigma Aldrich, Cat#S2263-100ML). All primary and secondary antibodies were diluted in 1X PBSTX + 5% sheep serum. The following antibodies and dilutions were used for immunostaining: mouse monoclonal anti-Cadherin-7 (1:50-1:70, DSHB Cat#ccd7-1); rat monoclonal anti-N-cadherin (1:50, DSHB Cat#MNCD2, RRID:AB_528119); mouse monoclonal anti-human natural killer-1 (HNK-1) (1:100, DSHB Cat#3H5, RRID:AB_2314644); and mouse monoclonal anti-Tubulin beta-3 chain (Tubb3) (1:500, Abcam Cat# ab78078, RRID:AB_2256751). The following secondary antibodies were used at 1:200-1:500 dilutions: goat anti-mouse polyclonal IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (Thermo Fisher Scientific Cat# A-11005, RRID:AB_2534073) and goat anti-mouse polyclonal IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 647 (Thermo Fisher Scientific Cat# A-21235, RRID:AB_2535804) (for Cadherin-7); goat anti-rat polyclonal IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 (Thermo Fisher Scientific Cat# A-11007, RRID:AB_10561522) (for N-cadherin); goat anti-mouse polyclonal IgM (Heavy Chain) Secondary Antibody (Thermo Fisher Scientific Cat# A-21238, RRID:AB_2535807) (for HNK-1); and goat anti-mouse polyclonal IgG2a Human ads-AF555 (SouthernBiotech Cat# 1080-32, RRID:AB_2794491) (for Tubb3). Sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) to mark cell nuclei using DAPI-containing mounting media (DAPI Fluoromount-G, Southern Biotech, Cat#0100-20).
For sequential electroporation of both premigratory neural crest cells and trigeminal placode cells, unilateral chick neural tube electroporation to target neural crest cells contributing to the trigeminal ganglion was first performed, as described previously by.14,27 Briefly, GRASP constructs were introduced unilaterally into premigratory midbrain neural crest cells in developing 3 to 5 somite stage (3-5ss) chick embryos at a concentration of 2.0-2.5 μg/μl, using fine glass needles to fill the chick neural tube. Platinum electrodes were placed on either side of the embryo, and two 25 V, 25 ms electric pulses were applied across the embryo. Once embryos reached HH10-11 (10-13ss), a unilateral ectodermal electroporation was carried out (on the same side of the embryo that was electroporated previously) to target trigeminal placode cells.26 Electrodes were placed vertically on top of and below the embryo and three, 9 V pulses were delivered over 50 ms at 200 ms intervals. After electroporation, eggs were re-sealed with tape and parafilm and re-incubated for the desired time period (approximately 36 hours to reach HH15-16) prior to harvesting for fixation and transverse sectioning, which was carried out according to the protocol by.14 Unilateral co-electroporation of N-cadherin split GFP constructs into trigeminal placode cells was carried out as described.26
For all experiments, images of at least five serial transverse sections through a minimum of three embryos, or eight cell culture images/replicates, were acquired with the LSM Zeiss 800 confocal microscope with Airyscan detection (Carl Zeiss Microscopy, Thornwood, NY, USA) at 20X magnification. Laser power, gain, and offset were kept consistent for the different channels during all experiments where possible. ZEN Digital Imaging for Light Microscopy (RRID:SCR_013672), version 2.3 software (Carl Zeiss Microscopy) and Adobe Photoshop CC 2019 (20.0.6 release) were used for image processing. For the in vivo GRASP experiments, images were false-colored in Adobe Photoshop to allow for better visualization and comparison of signal. Equivalent functions for image processing can be performed on Fiji (RRID:SCR_002285), which is freely available.
Images from both in vitro and in vivo experiments were analyzed using the Squassh28 plugin from the MOSAICsuite in FIJI.29 Segmentation parameters were adjusted per experiment, to minimize interfering background signal. For the in vitro assays, the amount of GFP positive and DAPI positive cells were counted and divided by each other to determine transfection efficiency. For N-cadherin in vivo control experiments, GFP positive cells and N-cadherin positive cells were counted and divided by each other to determine electroporation efficiency. For the in vivo heterophilic cadherin experiments, the number of GFP regions were counted per section, and averaged to find the average amount of GFP-positive regions. After batch analysis, the quality of image-segmentation was confirmed visually in order to ensure accurate segmentation.
Cranial neural crest cells and trigeminal placodal neurons express distinct cadherins, Cadherin-7 and N-cadherin, respectively, during early trigeminal gangliogenesis.14,16 Given these findings, we sought to determine whether these specific cadherins facilitated trigeminal ganglion assembly through heterophilic interactions. To address this, we performed co-immunoprecipitation assays on lysates prepared from dissected forming trigeminal ganglia of HH15-16 chick embryos (Figure 1).47

Lysate from HH15-16 trigeminal ganglion tissue was incubated with either an antibody against N-cadherin or with whole rabbit IgG serum as a control. Immunoprecipitated proteins were captured with protein A/G beads, separated by SDS-PAGE, followed by immunoblotting for N-cadherin (A) and Cadherin-7 (B). Lanes 1-4 are as follows: 1) Protein ladder; 2) Input, trigeminal ganglia lysate; 3) trigeminal ganglia lysate following IP with rabbit IgG; and 4) trigeminal ganglia lysate following IP with N-cadherin antibody. Arrowheads point to N-cadherin (A) or Cadherin-7 (B), respectively, while asterisks identify Cadherin-7 immunoreactive products as observed previously.14 N=2 experiments. N-cadherin, neural cadherin; IP, immunoprecipitation.
Analysis of immunoprecipitated proteins by SDS-PAGE followed by immunoblotting for N-cadherin revealed detection of N-cadherin within the forming trigeminal ganglia (Figure 1A, lane 2) and in N-cadherin immunoprecipitates with this antibody (Figure 1A, lane 4), but not with the rabbit IgG serum (Figure 1A, lane 3). These data indicate that the N-cadherin antibody can effectively immunoprecipitate N-cadherin from trigeminal ganglia tissue, providing us with a key experimental tool to identify other proteins that physically interact with N-cadherin in vivo.
To this end, we next performed immunoblotting using a validated Cadherin-7 antibody13,14 (Figure 1B). Our data again reveal a band corresponding to Cadherin-7 observed in the trigeminal ganglia lysate input sample (Figure 1B, lane 1, arrowhead), along with immunoreactive lower molecular weight bands (Figure 1B, asterisk, *) containing portion(s) of the Cadherin-7 extracellular domain, as observed in our prior work.14 Strikingly, we also observed Cadherin-7 after N-cadherin pull-down (Figure 1B, lane 4, arrowhead), but not with the control IgG serum (Figure 1B, lane 3). These findings reveal N-cadherin and Cadherin-7 physically interact in vivo. As N-cadherin is noted in trigeminal placodal neurons and cranial mesenchyme16 but only neural crest cells express Cadherin-7,14 our data suggest heterophilic interactions between Cadherin-7 in neural crest cells and N-cadherin in placodal neurons and/or the mesenchyme.
Given the results of our pull-down experiments, we hypothesized that physical interactions between Cadherin-7 in neural crest cells and N-cadherin in placodal neurons mediate, in part, the successful aggregation of these cell types during trigeminal gangliogenesis. To address this, we adapted and modified a GRASP assay to evaluate interactions specifically between these two cadherins, both in vitro and in vivo. GRASP relies upon functional complementation (i.e., GFP fluorescence) between two non-fluorescing or split GFP fragments (GFP1-10, GFP11). Reconstitution of GFP can only occur when the split GFP molecules are in close proximity to each other, as observed in other systems that defined interactions between extracellular domains of two membrane proteins.23,30–32 We designed Cadherin-7 and N-cadherin GRASP vectors (Figure 2) with GFP subunits fused in frame to the respective cadherin extracellular domain (Cadherin-7 GFP1-10, Cadherin-7 GFP11, N-cadherin GFP1-10, N-cadherin GFP11; GenScript). Constructs were based on GRASP plasmids developed by the Sanes lab (Addgene), which generate intact GFP fluorescence due to neuroligin-neurexin interactions, with no GFP noted with single constructs.23

Cartoon diagram showing GFP-cadherin fusion proteins that were constructed by joining the kappa light chain to distinct GFP subunits (1–10, or 11), followed by a linker region and then the mature cadherin peptide. GFP, green fluorescent protein; GRASP, GFP reconstitution across synaptic partners; N-cad, neural cadherin; Cad7, Cadherin-7.
We first showed that all constructs expressed their respective cadherins by transfecting CHO cells, which lack endogenous cadherins,33 with each GRASP construct, followed by immunostaining for each cadherin (Figure 3A’, B’, C’, D’, arrows). Importantly, no GFP fluorescence was noted under any condition, as expected. We next evaluated the specificity of the split GFP moieties to generate GFP by co-transfecting CHO cells with the same split GFP constructs, but fused to a different cadherin (i.e., Cadherin-7 GFP1-10 and N-cadherin GFP1-10). In these control experiments, expression of each cadherin was observed once again (Figure 4A”’, B”’, arrows), but no GFP was reconstituted, reinforcing the specificity of the assay.
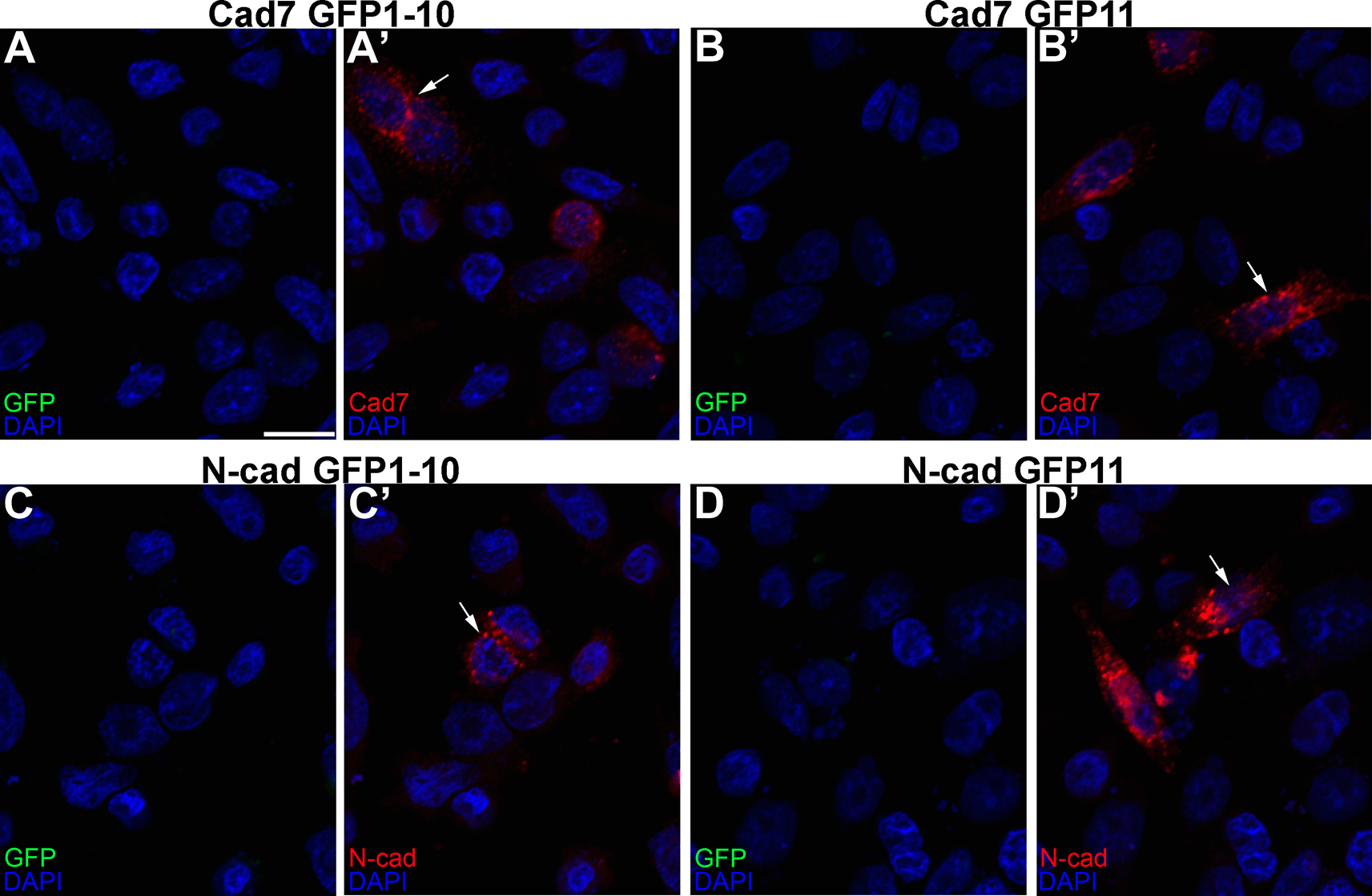
Single transfections of Cad7 GFP1-10 (A-A’), Cad7 GFP11 (B-B’), N-cad GFP1-10 (C-C’), and N-cad GFP11 (D-D’) were conducted in CHO cells, followed by immunocytochemistry for Cad7 (A’, B’, red) or N-cad (C’, D’, red). GFP fluorescence was also examined in the appropriate microscope channel (488) but not observed. Arrows point to cadherin expression in transfected cells. DAPI (blue), cell nuclei. N=3 replicates for all treatments. Scale bar in (A) is 50 μm and applies to all images. GFP, green fluorescent protein; GRASP, GFP reconstitution across synaptic partners; CHO, Chinese hamster ovary; N-cad, neural cadherin; Cad7, Cadherin-7.

Co-transfection of CHO cells with Cad7 GFP1-10 + N-cad GFP1-10 (A-A”’), or Cad7 GFP11 + N-cad GFP11 (B-B”’), was performed, followed by immunocytochemistry for Cad7 (A’, A”’, B’, B”’, purple) and N-cad (A”, A”’, B”, B”’, red). GFP fluorescence was also examined in the appropriate microscope channel (488) but not observed. Arrows point to cadherin expression in co-transfected cells. DAPI (blue), cell nuclei. Scale bar in (A) is 50 μm and applies to all images. N=8 replicates for both treatments. GFP, green fluorescent protein; GRASP, GFP reconstitution across synaptic partners; CHO, Chinese hamster ovary; N-cad, neural cadherin; Cad7, Cadherin-7.
Next, we addressed whether cis interactions between complementary split GFP constructs could generate an intact GFP molecule in vitro. To this end, we co-transfected CHO cells with complementary split GFP constructs expressing the same cadherin (Figure 5) and examined GFP fluorescence and cadherin expression by immunostaining. GFP fluorescence was detected with both Cadherin-7- (Figure 5A, A”, arrows) or N-cadherin- (Figure 5B, B”, arrows) expressing split GFP constructs, along with expression of each respective cadherin (Figure 5A’, B’), demonstrating effective GFP reconstitution via homophilic cadherin interactions. Further, these transfected cells exhibited a round morphology with few protrusions emanating from the cell surface, similar to that seen with control transfections.

CHO cells were co-transfected with complementary split GFP constructs expressing the same cadherin (Cad7 GFP1-10 and Cad7 GFP11 (A-A”); N-cad GFP1-10 and N-cad GFP11, (B-B”)), followed by immunocytochemistry for Cad7 (A’, A”, purple) or N-cad (B’, B”, red). GFP fluorescence was also examined in the appropriate microscope channel (488, A, A”, B, B”, green). Arrows point to GFP fluorescence in transfected cells, indicative of physical interactions between each split GFP-expressing cadherin. DAPI (blue), cell nuclei. Scale bar in (A) is 50 μm and applies to all images. N=8 replicates for both treatments. GFP, green fluorescent protein; GRASP, GFP reconstitution across synaptic partners; CHO, Chinese hamster ovary; N-cad, neural cadherin; Cad7, Cadherin-7.
To evaluate this in the context of the potential formation of heterophilic cadherin complexes, the same co-transfection experiment was conducted in CHO cells but this time using complementary split GFP constructs fused to a different cadherin (Figure 6). Our results revealed GFP reconstitution (Figure 6A, A”’, B, B”’, arrows) and cadherin expression (Figure 6A-A”’, B-B”’), pointing to the ability of Cadherin-7 and N-cadherin to interact in cis and form heterophilic complexes, further validating our in vivo biochemistry results in the chick trigeminal ganglion. Interestingly, these transfected cells adopted a more fibroblastic, and often spindly, morphology, particularly when compared to cells transfected with homophilic cadherin split GFP-expressing constructs, which were rounder in appearance (Figure 5).
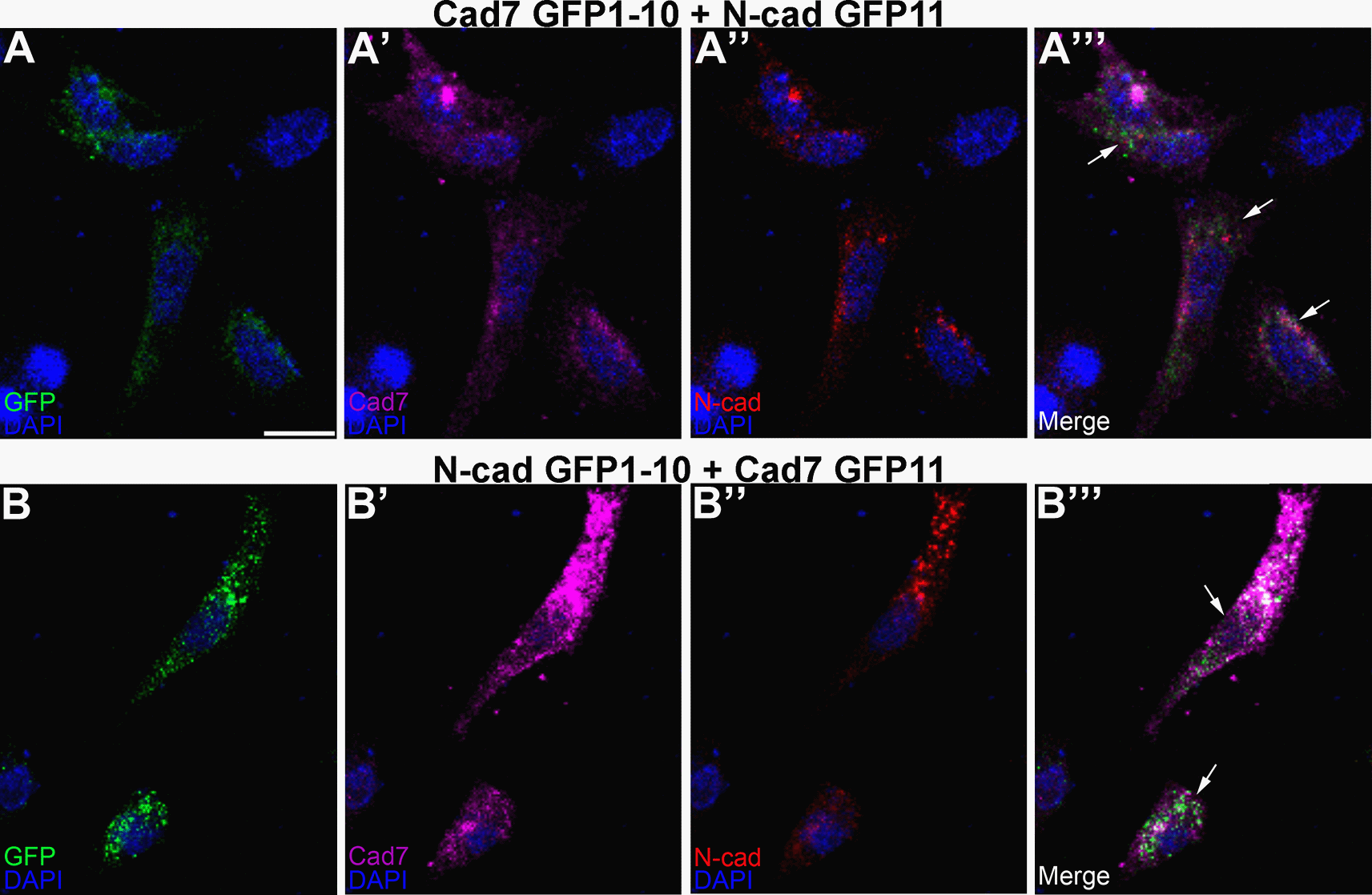
Cad7 GFP1-10 and N-cad GFP11 (A-A”’), or N-cad GFP1-10 and Cad7 GFP11 (B-B”’), were co-transfected into CHO cells, followed by immunocytochemistry for Cad7 (A’, A”’, B’, B”’, purple) and N-cad (A”, A”’, B”, B”’, red). GFP fluorescence was also examined in the appropriate microscope channel (488, A, A”’, B, B”’, green). Arrows point to GFP fluorescence in transfected cells, indicative of physical interactions between each split GFP-expressing cadherin. DAPI (blue), cell nuclei. Scale bar in (A) is 50 μm and applies to all images. N=8 replicates for both treatments. GFP, green fluorescent protein; GRASP, GFP reconstitution across synaptic partners; CHO, Chinese hamster ovary; N-cad, neural cadherin; Cad7, Cadherin-7.
Next, we calculated the percentage of GFP-positive cells in all of our transfection assays and observed a greater number of GFP-positive cells after transfection of homophilic split cadherin GFP constructs compared to that observed after transfection of heterophilic split cadherin GFP constructs (Table 1). While there is no statistically significant difference in the percentage of GFP-positive cells in N-cad GFP1-10 + N-cad GFP11- versus Cad7 GFP1-10 + Cad711-transfected cells (p=0.19), we did find a statistically significant difference in the percentage of GFP-positive cells after transfection of heterophilic cadherin split GFP constructs. Transfection of Cad7 GFP1-10 + N-cad GFP11 gave rise to a 1.5-fold increase in the percentage of GFP-positive cells compared to N-cad GFP1-10 + Cad7 GFP11 (p=0.013).
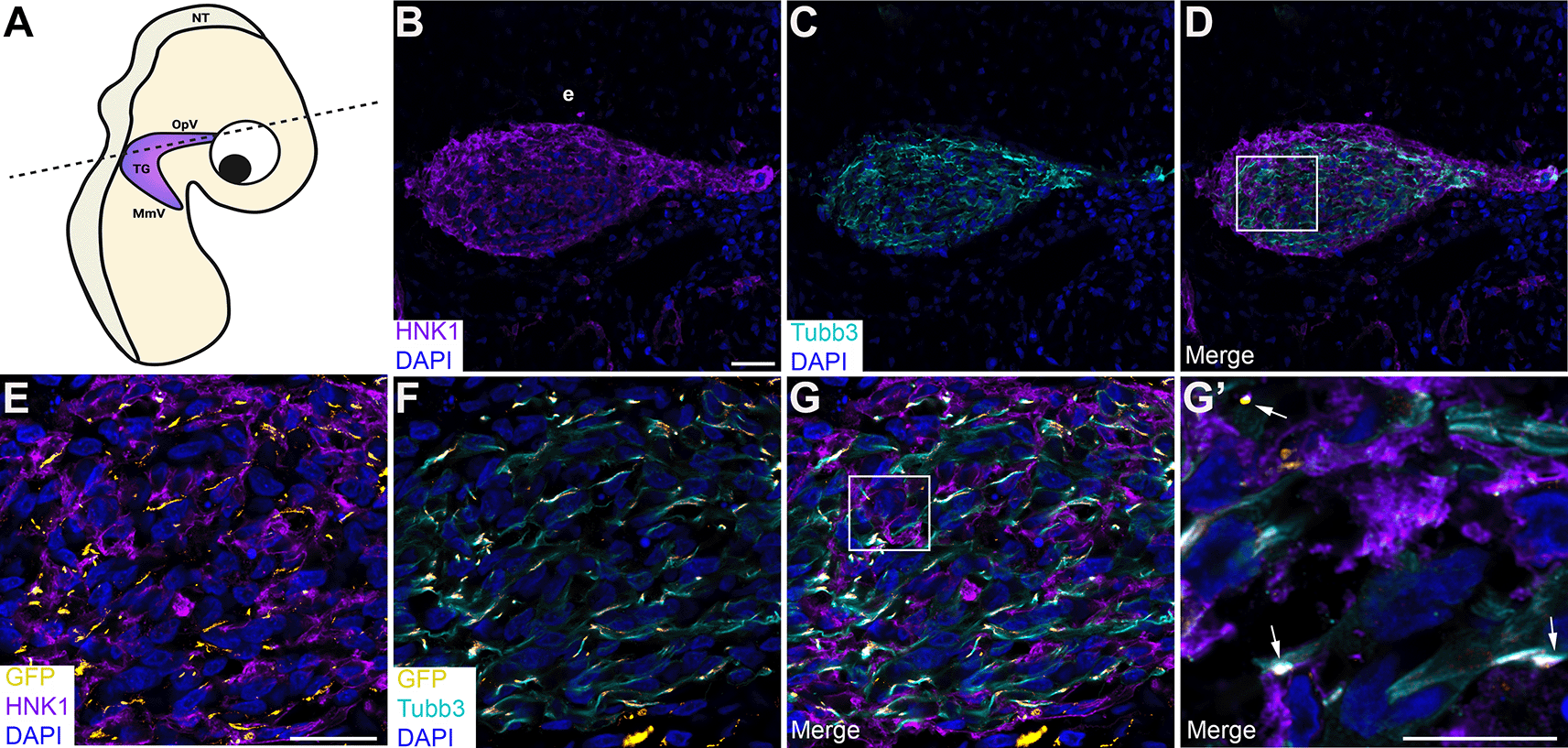
To corroborate our findings and examine cadherin intercellular interactions during trigeminal ganglion assembly in vivo, we turned to a sequential electroporation assay in which a Cadherin-7 split GFP construct was first electroporated into premigratory neural crest cells, followed by a second electroporation of a complementary N-cadherin split GFP construct to target trigeminal placode cells in the surface ectoderm (Figures 7 and 8). Transverse sections taken from electroporated embryos were processed for immunohistochemistry to identify neural crest cells and placodal neurons within the forming trigeminal ganglion. Remarkably, we observed GFP-positive regions and/or puncta (Figure 7E-G’; Figure 8E-G’; arrows) between neural crest cells (labeled by HNK-1; Figure 7B, D, E, G, G’; Figure 8B, D, E, G, G’) and placodal neurons (labeled by Tubb3; Figure 7C, D, F, G, G’; Figure 8C, D, F, G, G’) in the presence of the appropriate split GFP constructs. These data indicate Cadherin-7 and N-cadherin are in close proximity to interact in trans and permit the reconstitution of GFP in vivo, even in different cell types.

(A) Cartoon diagram of a chick embryo showing the forming trigeminal ganglion, with the dotted line indicating the axial level at which images were captured. Diagram created with BioRender.com. Sequential electroporations in the chick embryo were conducted as follows: Premigratory neural crest cells were first electroporated with Cad7 GFP1-10 followed by electroporation of trigeminal placode cells with N-cad GFP11. Section immunohistochemistry for HNK1 (purple, marks neural crest cells; B, D, E, G, G’) and Tubb3 (cyan, marks neurons which are placode cell-derived at this stage; C, D, F, G, G’) was then performed. GFP signal (yellow, arrows, E-G’) was captured in the appropriate channel (488). (B-D) Representative sections that show the morphology of the trigeminal ganglion at this stage of development. (E-G) Higher magnification images of the boxed region in (D). (G’) Higher magnification image of the boxed region in (G), with GFP identified by arrows. DAPI (blue), cell nuclei. Scale bar in (B) is 100 μm and applies to (C-D); scale bar in (E) is 100 μm and applies to (F-G); and scale bar in (G’) is 50 μm. N=6 embryos. NT, neural tube; MmV, maxillomandibular lobe; OpV, ophthalmic lobe; TG, trigeminal ganglion; e, ectoderm; N-cad, neural cadherin; Cad7, Cadherin-7; GFP, green fluorescent protein; HNK-1, human natural killer-1; Tubb3, Tubulin beta-3 chain.
(A) Cartoon diagram of a chick embryo showing the forming trigeminal ganglion, with the dotted line indicating the axial level at which images were captured. Diagram created with BioRender.com. Sequential electroporations in the chick embryo were conducted as follows: Premigratory neural crest cells were first electroporated with Cad7 GFP11 followed by electroporation of trigeminal placode cells with N-cad GFP1-10. Section immunohistochemistry for HNK1 (purple, marks neural crest cells; B, D, E, G, G’) and Tubb3 (cyan, marks neurons which are placode cell-derived at this stage; C, D, F, G, G’) was then performed. GFP signal (yellow, arrows, E-G’) was captured in the appropriate channel (488). (B-D) Representative sections that show the morphology of the trigeminal ganglion at this stage of development. (E-G) Higher magnification images of the boxed region in (D). (G’) Higher magnification image of the boxed region in (G), with GFP identified by arrows. DAPI (blue), cell nuclei. N=13 embryos. Scale bar in (B) is 100 μm and applies to (C-D); scale bar in (E) is 100 μm and applies to (F-G); and scale bar in (G’) is 50 μm. NT, neural tube; MmV, maxillomandibular lobe; OpV, ophthalmic lobe; TG, trigeminal ganglion; e, ectoderm; N-cad, neural cadherin; Cad7, Cadherin-7; GFP, green fluorescent protein; HNK-1, human natural killer-1; Tubb3, Tubulin beta-3 chain.
Next, we quantified the number of GFP-positive regions/puncta in serial sections through the forming trigeminal ganglion in each sequential electroporation experimental condition. Remarkably, this analysis yielded similar results to what we observed in vitro, namely a 1.6-fold increase in the number of GFP-positive regions/puncta after sequential electroporation of Cad7 GFP1-10 + N-cad GFP11 compared to that seen in Cad7 GFP11 + N-cad GFP1-10-electroporated embryos (p=0.014, Table 2). In contrast, co-electroporation of N-cad GFP1-10 + N-cad GFP11 into trigeminal placode cells, followed by incubation of embryos to trigeminal ganglion-forming stages, yielded robust GFP fluorescence throughout the plasma membrane of placodal precursors (Figure 9A-C) and neurons (Figure 9D’, arrows). Quantification of the number of GFP-positive cells in these experiments revealed that 53% of the N-cadherin-positive trigeminal placode cells and neurons were also GFP-positive when split GFP construct electroporations were performed in cis (Table 2). These data are consistent with those seen in vitro after transfection of the same cadherin split GFP construct combination. Together with our biochemistry data and results in cultured cells, our findings support the assertion that heterophilic interactions between Cadherin-7 in neural crest cells and N-cadherin in placodal neurons occur during trigeminal gangliogenesis.
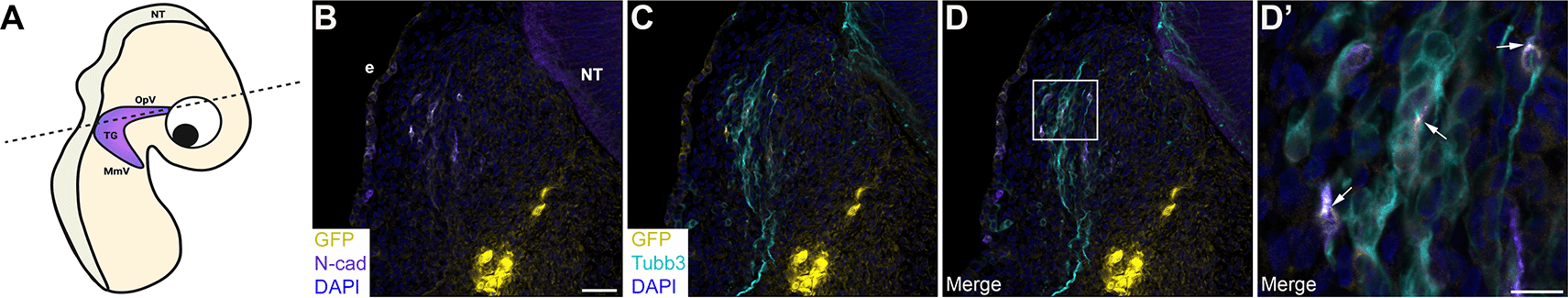
(A) Cartoon diagram of a chick embryo showing the forming trigeminal ganglion, with the dotted line indicating the axial level at which images were captured. Diagram created with BioRender.com. To target trigeminal placode cells, ectodermal electroporations were conducted in the chick embryo with both N-cad GFP1-10 and N-cad GFP11 constructs. Section immunohistochemistry for N-cad (purple; B, D, D’) and Tubb3 (cyan, marks neurons placode cell-derived at this stage; C-D’) was then performed. GFP signal (yellow, arrows, B-D’) was captured in the appropriate channel (488). (B-D) Representative sections that show the morphology of the trigeminal ganglion at this stage in development. (D’) Higher magnification image of the boxed region in (D), with GFP and N-cad double-positive cells identified by arrows. Scale bar in (B) is 100 μm and applies to (C-D), and scale bar in (D’) is 50 μm. N=3 embryos. NT, neural tube; MmV, maxillomandibular lobe; OpV, ophthalmic lobe; TG, trigeminal ganglion; e, ectoderm; N-cad, neural cadherin; GFP, green fluorescent protein; Tubb3, Tubulin beta-3 chain.
Electroporation efficiency of Cadherin split GFP plasmids.
| N-cad GFP 1-10; N-cad GFP 11 | Cad7 GFP1-10 + N-cad GFP11 | Cad7 GFP11 + N-cad GFP1-10 | |
|---|---|---|---|
| GFP-positivity | 53±0.29% | 16 regions±2.07a | 10 regions±1.42b |
Moreover, the preceding data are in keeping with the in vivo distribution of N-cadherin-expressing placodal neurons and Cadherin-7-expressing neural crest cells in the forming ganglion at this developmental stage (Figure 10). Although it is evident that neural crest cells and placodal neurons are in close proximity and can interact (Figure 10B’, arrows), the distribution of these cells also reveals that homophilic cadherin interactions are likely to occur within each cell type proper (i.e., neural crest cell-neural crest cell, placodal neuron-placodal neuron) as the ganglion begins to assemble. Collectively, our in vivo results support a role for neural crest cell-placode cell interactions during early trigeminal ganglion development.

(A) Cartoon diagram of forming TG in the developing chick embryo. Plane of section is shown by the dotted line. Diagram created with BioRender.com. (B) Representative transverse section taken through the forming TG followed by immunohistochemistry for Cad7 (green, marks neural crest cells) and N-cad (red, marks neurons which are all placode-derived at this stage). (C) Higher magnification (digital) of the TG in (B). DAPI (blue), cell nuclei. Scale bar in (B) is 100 μm and applies to (B’) but is 50 μm. N=5 embryos. TG, trigeminal ganglion; Cad7, Cadherin-7; N-cad, neural cadherin; NT, neural tube; OpV, ophthalmic branch; MmV, maxillomandibular branch; e, ectoderm.
Cranial neural crest cells and placode cells initially form in close proximity but become spatially separated as development ensues.8,34–36 While these cells give rise to distinct derivatives, they will both form sensory neurons of the trigeminal ganglion, innervating much of the head and face to relay information related to pain, touch, and temperature to the central nervous system.3–5 The cellular origin of the trigeminal ganglion has been known for decades7,10,37; however, molecular mechanisms mediating early interactions between neural crest cells and placodal neurons to build the trigeminal ganglion have not been well characterized. In the chick embryo, studies uncovered the importance of cadherin-mediated interactions, as distinct cadherins are expressed by neural crest cells (Cadherin-7)13,14 and placode cells and their neuronal derivatives (N-cadherin) during early trigeminal ganglion assembly.16 The presence of two different cadherins on these coalescing cells begs the question as to whether heterophilic interactions exist between them to allow for proper trigeminal ganglion formation, particularly since cells expressing these cadherins can form mixed aggregates in vitro.13
Our studies now address this question through the use of biochemistry and an adapted GRASP assay to examine cadherin interactions during trigeminal ganglion development. Through in vitro transfection experiments and use of embryonic trigeminal ganglia tissue, we demonstrate a physical interaction between Cadherin-7 and N-cadherin. This is the first report to reveal, biochemically, that Cadherin-7-N-cadherin complexes can form and, notably, are present while the trigeminal ganglion assembles. While we cannot rule out the presence of other protein(s) in the embryo to serve as a “bridge” to allow these cadherins to associate, these data still provide strong evidence that these interactions do exist in vivo.
To generate the trigeminal ganglion, Cadherin-7-expressing cranial neural crest cells first migrate through the embryonic mesenchyme to the trigeminal ganglionic anlage. Here, they intermingle with newly differentiated, N-cadherin-expressing trigeminal placode-derived neurons, which have delaminated from the surface ectoderm and have also migrated through the mesenchyme. Since the cranial mesenchyme expresses N-cadherin, it is possible that the Cadherin-7-N-cadherin complexes we detected through our biochemistry studies represent interactions between Cadherin-7 on neural crest cells and N-cadherin expressed in mesenchymal cells. However, based upon the abundance of neurons in relation to the mesenchyme in dissected trigeminal ganglia, we think the primary source of N-cadherin in these interactions comes from the placodal neurons. Moreover, migratory neural crest cells form “corridors” through which placodal neurons migrate, as they provide a more permissive substrate for migration than the surrounding cranial mesenchyme.38,39 As such, neural crest cells and placodal neurons are tightly juxtaposed during the assembly of the trigeminal ganglion, making it more likely that the interactions we are detecting arise from Cadherin-7 on neural crest cells and N-cadherin on placodal neurons.
To further define and directly visualize these heterophilic cadherin interactions, we conducted a GRASP assay in cell culture and in the embryo. We generated two split GFP constructs (GFP domains 1-10 or GFP domain 11) fused to both Cadherin-7 and N-cadherin and examined the ability of these cadherins to associate in cis and in trans to generate GFP. Through cell culture co-transfection experiments, we demonstrated that GFP could be reconstituted as long as the split GFP constructs were complementary, providing further evidence that Cadherin-7 and N-cadherin can interact in cis. Importantly, no GFP was generated after co-transfection of like split GFP moieties fused to different cadherins, pointing to the specificity of the GFP reconstitution.
Intriguingly, we noted that transfection of cells with like cadherins and complementary GRASP constructs gave rise to cells that often exhibit a round shape or are only somewhat fibroblastic, with few protrusions emanating from the cell (Figure 5). Conversely, transfection of cells with different cadherins and complementary GRASP constructs caused cells to adopt a much more fibroblastic, and often spindly, morphology. This could be due to the presence of both a Type I (N-cadherin) and Type II (Cadherin-7) cadherin in these cells. A parallel to this can be found in vivo with respect to the overlapping expression domains of N-cadherin and Cadherin-7 in the developing chick spinal cord.40 These neuroepithelial cells are organized in a pseudostratified manner and thus exhibit a spindly morphology as they are densely packed within the neural tube/forming spinal cord.
Quantification of GFP in our transfection experiments led to the conclusion that more GFP-positive cells were present upon transfection of complementary split GFP constructs fused to the same cadherin (homophilic interactions) compared to transfection of complementary split GFP constructs fused to different cadherins (heterophilic interactions). These findings are consistent with measurements of dissociation constants in homophilic and heterophilic cadherin cell aggregates in vitro, which revealed that homophilic cadherin interactions are stronger than heterophilic interactions.41 Moreover, we find that the number of GFP-positive cells is highest in cells transfected with split GFP constructs fused to N-cadherin compared to Cadherin-7. These results are in keeping with a report showing that N-cadherin confers a much higher degree of adhesivity compared to Cadherin-7.42
Finally, we noted a statistically significant 1.5-fold increase in GFP-positive cells upon transfection with Cad7 GFP1-10 + N-cad GFP11 compared to Cad7 GFP11 + N-cad GFP1-10 (p=0.013). This trend is also observed in vivo (see below). While we might expect these numbers in our heterophilic experiments to be similar, the difference could be explained by the inability of cells to effectively express N-cad GFP1-10, or, conversely, the capacity of cells to more readily express Cad7 GFP11. We favor the former idea, however, for the following reasons. Given that the N-cadherin coding sequence is larger than the Cadherin-7 coding sequence (~300 nucleotides difference), coupled with the greater size of the split GFP1-10 moiety, it is possible that cells do not readily express N-cad GFP1-10 (compared to N-cad GFP11). Further, since homophilic cadherin interactions are stronger and more stable than heterophilic ones,41 heterophilic interactions are, by nature, more transient. As such, the GFP we observe is a direct readout of these cadherin interactions and is correlated with their strength. Thus, we would expect homophilic interactions to yield the most GFP signal, with heterophilic interactions following next, as noted in both our in vitro and in vivo experiments. If cells do have more difficulty expressing N-cad GFP1-10, we would expect the GFP signal to be stronger in cells expressing Cad7 GFP1-10 + N-cad GFP11 versus those expressing Cad7 GFP11 + N-cad GFP1-10, which is in keeping with our results.
With these tools, we next explored the ability of Cadherin-7-expressing neural crest cells to associate with N-cadherin-expressing placodal neurons. Sequential electroporation experiments were conducted in which complementary split GFP constructs were introduced into neural crest cells (Cadherin-7 split GFP construct) followed by placode cells (N-cadherin split GFP construct). Because of the anatomy of the chick embryo at the time of electroporation and tissue of origin of neural crest cells (dorsal neural folds) and placode cells (surface ectoderm), we can precisely, and independently, target each cell type. Notably, we observed GFP fluorescence at sites where neural crest cells and placodal neurons come into contact, visualized on sections taken through the developing trigeminal ganglion. These data reveal that Cadherin-7 and N-cadherin can interact in trans in different cell populations, providing insight into the ability of different cadherin-expressing cells to associate in vivo.
Although the number of GFP-positive regions was not extraordinarily high in this in vivo assay, this is to be expected given the nature of the electroporation, in which only a small amount of each split GFP construct was electroporated into each cell type in order to avoid potential artifacts of overexpression. Quantification of the number of GFP-positive regions/puncta through serial trigeminal ganglion sections, however, revealed results that were in keeping with what we observed in vitro. Specifically, we noted a statistically significant 1.6-fold increase in the GFP fluorescence reported in Cad7 GFP1-10 + N-cad GFP11-electroporated embryos vs. Cad7 GFP11 + N-cad GFP1-10-electroporated embryos (p=0.014). These results align with what we observe in vitro, with these differences also possibly arising for similar reasons as outlined above (e.g., ability of cells to effectively express N-cad GFP1-10). Alternatively, there might be inherent differences in the ability of neural crest cells and placode cells to transcribe and translate expression constructs like these, with neural crest cells more easily expressing Cad7 GFP1-10 compared to placode cells expressing N-cad GFP1-10. Finally, the heterophilic interactions we are examining occur in trans, not in cis as in our in vitro experiments. As such, they are dependent upon a cadherin split GFP construct getting not only electroporated but also appropriately trafficked, and correctly targeted, to a region of the plasma membrane, where it will then be in close proximity to a complementary cadherin split GFP construct on the other cell type. For these reasons, fewer heterophilic interactions may ensue in a given electroporated tissue. On the other hand, electroporation of N-cad GFP1-10 + N-cad GFP11 into placode cells results in robust GFP fluorescence, as these electroporations are performed in cis.
Other pathways have been discovered to regulate cellular interactions occurring during initial chick trigeminal ganglion coalescence, including Slit1-Robo2,11,16 Wnt,43,44 Neuropilin/Semaphorin,45,46 and various growth factors47 (e.g., Platelet-Derived Growth Factor48), with many of these also identified in the developing mouse trigeminal ganglion.49,50 In chick embryos, Robo2 signaling likely modulates levels of N-cadherin post-translationally, but the mechanisms underlying this are still not well characterized. Upstream pathways regulating Cadherin-7 expression in neural crest cells also remain obscure, but it is plausible that the preceding signal transduction pathways could impact the expression of Cadherin-7 and/or N-cadherin during trigeminal gangliogenesis. Future studies aimed at addressing this question will provide important insights into the regulation of neural crest-placodal neuron migration and adhesion.
The juxtaposition of Cadherin-7-expressing neural crest cells and N-cadherin-expressing placodal neurons in the forming trigeminal ganglion hinted at the possibility that heterophilic interactions between these two cadherins could, in part, mediate this process. While the functional roles of each cadherin in trigeminal ganglion assembly have been well described, less attention was paid to the importance of their expression in building the ganglion. Cultured cells expressing either Cadherin-7 or N-cadherin can form intermingled aggregates,13 supporting the notion of heterophilic interactions and further assessed herein, but it was not evaluated in vivo until our studies. We now provide data uncovering a physical interaction between Cadherin-7 in neural crest cells and N-cadherin in placodal neurons within the trigeminal ganglion. Altogether, these findings shed light on the molecular mechanisms underscoring intercellular interactions requisite for trigeminal ganglion assembly during early chick embryonic development.
Digital Repository at the University of Maryland, Animal & Avian Sciences Research Works: Neural crest cell-placodal neuron interactions are mediated by Cadherin-7 and N-cadherin during early chick trigeminal ganglion assembly. https://doi.org/10.13016/llyh-dppy.51
This project contains the following underlying data:
• Figure 1: Raw western blot data (Original raw tiff files for the immunoblotting experiments)
• Figure 2: Plasmids.pdf (GRASP cadherin plasmid sequences)
• Figure 3: Transfection images for single split GFP cadherin constructs
• Figure 4: Transfection images for double, non-complementary, split GFP constructs with different cadherins
• Figure 5: Transfection images for double, complementary, split GFP constructs with the same cadherin
• Figure 6: Transfection images for double, complementary, split GFP constructs with different cadherins
• Figure 7: Tissue section images following electroporation of complementary split GFP constructs into neural crest cells and placode cells
• Figure 8: Tissue section images following electroporation of complementary split GFP constructs into neural crest cells and placode cells
• Figure 9: Tissue section images following electroporation of complementary split GFP constructs into placode cells
• Figure 10: Tissue section images following immunohistochemistry to label neural crest cells and placode cells
• Table 1: Excel file showing quantification of GFP fluorescence after co-transfection of complementary, split GFP constructs with the same or different cadherin in vitro
• Table 2: Excel file showing quantification of GFP fluorescence after co- or sequential electroporation of complementary, split GFP constructs with the same or different cadherin in vivo
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Developmental neurobiology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: GnRH neuroendocrine cells and olfactory placode development
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: GnRH neuroendocrine cells and olfactory placode development
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Developmental neurobiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Neural crest and craniofacial developoment
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 12 Dec 22 |
read | read | |
|
Version 1 04 Jul 22 |
read | read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)