Keywords
SARS-CoV-2, DENV co-infection, Maternal and fetal mobidity and mortality
This article is included in the Emerging Diseases and Outbreaks gateway.
SARS-CoV-2, DENV co-infection, Maternal and fetal mobidity and mortality
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has affected 239 million people causing 4.8 million of deaths worldwide by the year 2020/21. DENV (Dengue virus) is estimated to infect 3.9 billion people annually, with 96 million having severe and life-threatening illness and 70% of DENV infections are reported from Asia. Both DENV and SARS-CoV-2 lead to increased mortality and morbidity in pregnancy.1–5 Healthcare providers in Asia are facing an unprecedented challenge in managing co-infections with DENV during this COVID-19 pandemic.
Sri Lanka is a tropical country where DENV is endemic. The first DENV outbreak was in 1965 with 26 patients and six deaths. The worst out-break was experienced in 2017.6 Colombo Municipal Council (CMC) take up 25% of the case load every year. Dengue outbreak was experienced in the De Soysa maternity hospital (DMH) during the COVID-19 pandemic threatening maternal and fetal lives. We experienced four cases of co-infections from April to September 2021.
DENV and SARS-CoV-2 shares similar presenting symptoms. Hence, diagnosis could be delayed and may be missed, leading to life-threatening complications. Although DENV is endemic to south Asia, during this pandemic there is only one case of SARS-CoV-2/DENV co-infection reported in the literature.7 We were able to identify four patients with SARS-CoV-2/DENV co-infection during the period from 1st of April 2021 to 1st of September 2021 in the De Soysa Hospital for Women in Colombo, Sri Lanka. We believe that it is timely to report this case series, which may aid to raise awareness and management.
This is a retrospective analysis of the medical records of pregnant women admitted to DMH (a tertiary care maternity hospital in CMC area, Colombo, Western province) during 1st of April 2021 to 1st of September 2021 during the SARS-CoV-2 pandemic. Ethical clearance was obtained from the ethical review committee on 17th of December 2020, Faculty of medicine Colombo (Protocol number EC-20-EM17).
Patient 1
A 24-year-old female Sri Lankan housewife presented at 33 weeks of gestation with arthralgia, fever, shortness of breath, and sore throat in her third pregnancy. She was a diagnosed patient with immune thrombocytopenic purpura (ITP) and was in remission. She previously had a 1st trimester miscarriage followed by a neonatal death due to a ruptured hemangioma. On admission the patient had a positive polymerase chain reaction (PCR) result for SARS-CoV-2. She complained of itchy palms and soles. She had normal platelet counts and aspartate aminotransferase (AST) and alanine aminotransferase (ALT). She was anticoagulated with subcutaneous enoxaparin 40 mg daily subcutaneously for 10 days. On the tenth day of the hospital stay, she developed a fever with positive dengue non-structural protein 1 (NS1) antigen. The patient was monitored with full blood count (FBC) every 8 hours. She was sent to the ICU on day 13 of hospital admission when her platelet count dropped to 8000/mm3. She was started on oral Prednisolone 20 mg daily (continued for 7 days) and intravenous (IV) hydrocortisone 100 mg every 8 hours (for 3 days). An ultrasound scan done on day 13 revealed gallbladder wall oedema and bilateral pleural effusions. Critical phase in dengue hemorrhagic fever was diagnosed and managed as per guidelines. She was transfused 250 cc of red cell concentrate (RCC) due to a drop in pack cell volume (PCV). Due to steroids, she had elevated blood sugars, which was controlled with subcutaneous soluble insulin according to the sliding scale three times daily (daily dose of 24 units to 30 units). Her SARS-CoV-2 antibodies were also positive on day 13 of the hospital stay. Her platelets started to rise on day 14 of hospital admission. She had an unremarkable recovery and was transferred back to the ward on day 17 of admission. Her steroids were tailed off. She underwent an elective caesarean section at 37 weeks (birth weight 2.6 kg). The post-partum period was uneventful. Cord blood was sent for platelet count and the newborn platelet count was monitored on day 3.
Patient 2
A 31-year-old female Sri Lankan management assistant in her sixth pregnancy presented with per-vaginal bleeding at 8 weeks of period of amenorrhea (POA). She had three previous uncomplicated vaginal deliveries with a neonatal death at 4 months due to sepsis and a 1st trimester miscarriage. On admission, the patient also had dizziness, a headache and a temperature of 100 °F. Her Dengue NS1 antigen and SARS-CoV-2 PCR was positive, with PCR CT value of 29. Transvaginal ultrasound was suggestive of a complete miscarriage with empty uterine cavity. Her serum beta human chorionic gonadotrophin (β HCG) dropped from 300IU to 33IU over 48 hours. She had a platelet nadir of 101000/cm3 which started to rise from day three of the hospital admission. She was discharged on day 10 of hospital admission without further complications.
Patient 3
A 41-year-old female Sri Lankan teacher was admitted at 30 weeks of gestation for optimization of her preexisting medical conditions in her second pregnancy. She had a previous emergency caesarean section for placenta previa. She was a diagnosed patient with type 2 diabetes for two years with nephropathy and retinopathy. She also had chronic hypertension with the blood pressure of 140/90 mmhg. She was on insulin, methyldopa, nifedipine and Aspirin at the time of admission. The PCR for COVID-19, which was done prior to admission, was positive. She had uncontrolled blood sugar around 16 mmol/l at the time of admission for which insulin doses were titrated. On the fourth day from the positive PCR test she developed shortness of breath and her saturation deteriorated reaching 94% on air. Chest x-ray revealed bilateral patchy infiltrates. She was started on IV ceftriaxone (1 g two times daily for 5 day), oral clarithromycin (500 mg twice daily for 14 days), oral dexamethasone (2 g twice daily for 7 days) and subcutaneous enoxaparin 40 mg subcutaneously for 18 days. She also had low Hemoglobin (Hb) of 9.0 g/dl and 1.0 unit of RCC was transfused. The patient was oxygen dependent for four days. She had an uncomplicated recovery from COVID-19, and her antibiotics and dexamethasone were continued for seven days. Following the recovery of SARS-CoV-2, the patient had persistent nephrotic range proteinuria and was on evaluation. On the 20th day of the hospital admission, she developed a fever with body aches. The dengue NS1 antigen was positive. She had a further 2.0 units of RCC transfusion to correct anemia, which had been diagnosed as anemia of chronic disease. Her vital parameters, PCV and FBC’s were monitored regularly with focused assessment with sonography for trauma (FAST) scans to detect early critical phase. Her platelet count dropped to 109000/cm3. She did not develop dengue hemorrhagic fever and had a recovery after four days. She underwent an elective caesarean section at 37 weeks due to transverse lie. The newborn developed weight loss (birth weight was 2.9 kg) due to feeding issues and developed jaundice, which was treated with phototherapy. Both were discharged after the 11th day of post-partum with the necessary referrals.
Patient 4
A 32-year-old female Sri Lankan legal apprentice in her 1st pregnancy with an uncomplicated antenatal period was admitted with fever, arthralgia, and myalgia at 28 weeks of gestation. Her SARS-CoV-2 rapid antigen test was positive, but the NS1 was negative. FBC showed Hb of 11 g/dl with platelet of 213000/mm3. Her last fetal growth scan done at 28 weeks of gestation was normal. She was home quarantined. Her fever settled within a day, and she was asymptomatic afterwards. She developed reduced fetal movement on day seven of the illness and was admitted to the hospital on day eight. Intrauterine death was confirmed at the time of the admission.
Her FBC, liver function test, renal function test, NS1 dengue antibodies, blood picture and urine full report (UFR) was performed. On admission she had a platelet count of 74000/mm3 and elevated liver enzymes (AST-157U/L, ALT-99U/L). She was normotensive and did not have urine proteins. She had positive dengue IgM antibody and negative IgG. She was carefully observed with fluid management, and she recovered without further complications. She was induced after one week from stabilization of platelets.
She delivered a morphologically normal fetus with clear liquor and without evidence of fetal growth restriction (fetal weight was 1.1 kg). No abnormalities were detected in the placenta and cord macroscopically. The couple did not consent for the pathological post-mortem. Placental histology showed extensive placental infarctions with chorionic villi with syncytial knots. The membranes showed dense aggregates of acute inflammatory cells compatible with acute chorioamnionitis.
Approximately one third of the patients with COVID-19 are asymptomatic.8 Furthermore, 50–85% of patients infected with DENV are asymptomatic.9 Both illnesses have a spectrum of severity varies from mild to critical.10 Moreover, they share biochemical features such as leucopenia, thrombocytopenia and deranged liver enzyme level, which make the identification of the disease further demanding.11–13
Morbidity and mortality are higher during pregnancy for mother and fetus in both infections. DENV has been reported to cause fetal, neonatal and maternal death, low birth weight and preterm delivery,2–4 whereas COVID-19 can lead to preterm delivery, low birth weight, higher rate of oxygen dependency, higher ICU admissions, neonatal mortality and morbidity.14
Sensitivity and specificity of dengue NS1 test affect the diagnosis of DENV. The DENV antigen test was negative in the fourth patient of our case series, and she was discharged for home-based care. Even sore throat, a symptom usually associated with COVID-19, is being commonly reported in DENV cases.15
DENV and SARS-CoV-2 infections require different clinical interventions, and incorrect or delayed diagnosis can have serious consequences. Anticoagulation is generally used for SARS-Cov-2 in our population after assessment of other risk factors for thrombosis, severity of illness and mobility. It has been advised to avoid anticoagulation in DENV as it can increase the risk of thrombocytopenia and even trigger Reyes syndrome, a rare condition characterized by hepatitis and encephalopathy.16
Dexamethasone was used in patients with severe symptoms of COVID-19 (patient 4). However, there is no evidence for dexamethasone to alleviate the course of DENV. It is advisable to deliver the fetus in cases with COVID-19 depending on the case-based evaluation of risk factors and severity of the illness. In contrary, the delivery should be delayed in mothers with DENV as it can cause life threatening bleeding and lead to leaking. This dilemma was considered in management of case number 3 and 4. Immediate multidisciplinary meetings were conducted for case 3 and 4 for the management of labor. Fortunately, the course of COVID-19 was asymptomatic to mild in those patients.
It is paramount to highlight the fact that all four patients were unvaccinated at the time of admission. The authors believe the personal preference and delay in initiating the vaccination program led to this situation.
The outcomes of the patients with co-infection were unfavorable. Out of the four patients in our sample, one patient had a miscarriage, one had a fetal death and the other two patients had caesarean sections. This is comparable with non-pregnant counterparts with co-infection.17 We had no maternal death in our population due to co-infection. Cytokine storm was proved by laboratory investigations in one case report. However, we were unable to conduct such investigations due to unavailability of tests.7
Cross reactivity between the antibodies formed in both illnesses had been reported.18 Hence, confirmatory diagnosis via RT-PCR for DENV would be ideal. However, this facility is not available in Sri Lanka. Clinical management of dengue fever is decided primarily on signs and symptoms and NS1 assay. An NS1 assay has moderate sensitivity and high specificity.19 However, IgM ELISA or NS1 tests are often preferred as they are more available, and more affordable. During this case series, all patients admitted with symptoms were tested for both infections, however delays occurred when the DENV antigen was falsely negative. Further, during the pandemic, collaboration between healthcare professionals (physician, microbiologist, hematologist, anesthetist, and obstetrician) in providing the care has minimized the missing cases.
Placental histology was performed in patient number 4 which showed changes comparable with the placental histology DENV infection as in Figure 1A and B.20 We believe further studies on placental histology in COVID-19 and DENV might yield more information on direct inflammation of placenta due to these viral illnesses.
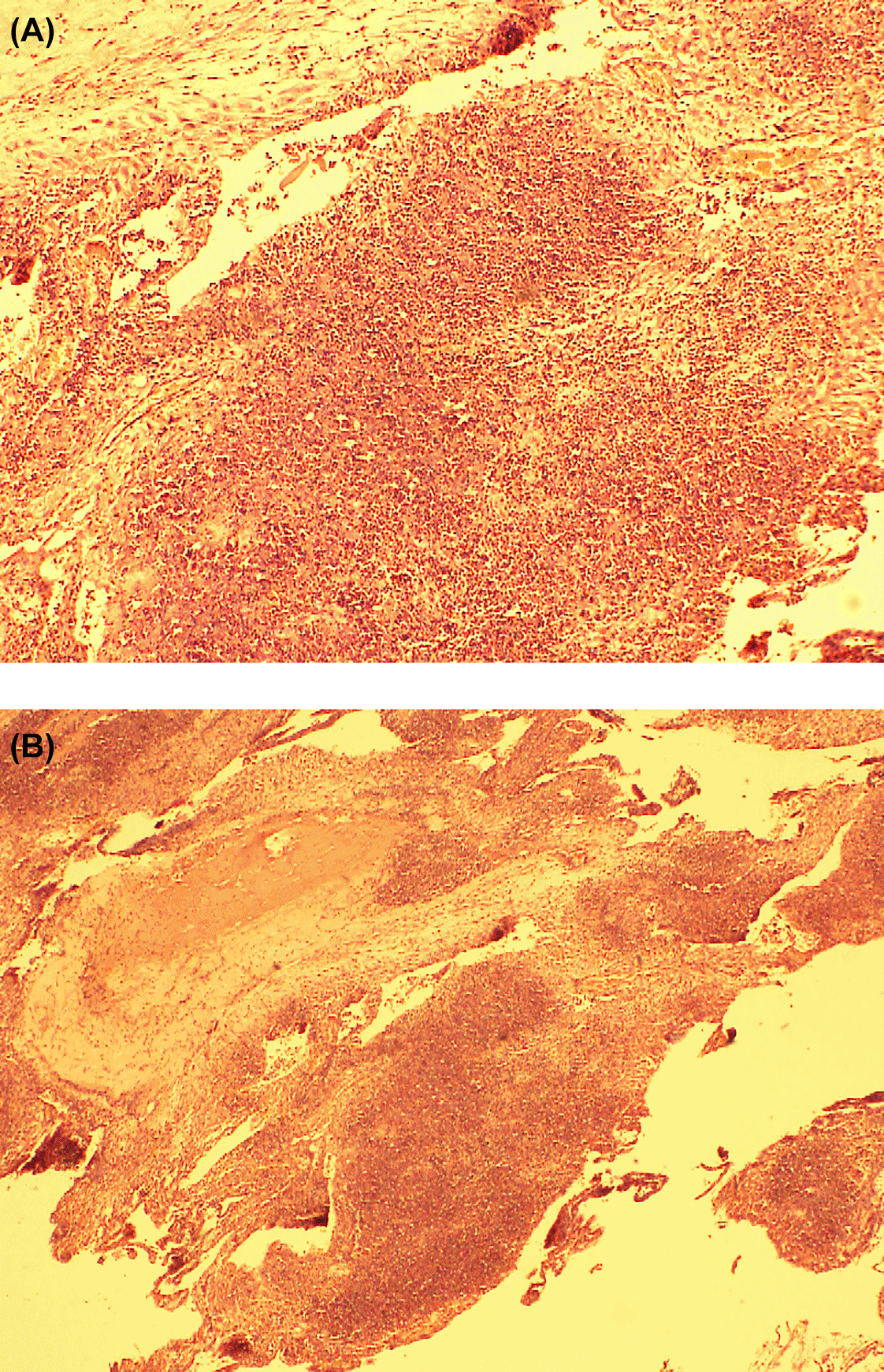
Membranes show dense aggregates of acute inflammatory cells compatible with acute chorioamnionitis.
Lack of identification of the dengue and SARS-CoV-2 serotypes is a limitation of this study as it has clinical and epidemiological significance. The authors believe that multinational collaborative studies are required to improve the quality of data and availability of data on co-infection.
Due to the SARS-CoV-2 pandemic, the DENV cases are under diagnosed and underreported. Public health care systems are overloaded with managing the COVID-19 pandemic. This study presented a few cases of co-infections in a leading maternity hospital in Sri Lanka. This is the first case series from Sri Lanka to elaborate the co-infection during pregnancy. We believe the above information will enlighten clinicians in the management of co-infection. Both diseases could be more lethal among the pregnant population. Hence, continuous collection of information and discussion about co-infections might mitigate diagnosis and clinical management.
Written informed consent for publication of their clinical details was obtained from the patients included in the article.
I thank all the patients participated for the study and all healthcare workers helped us to carry out patient care during COVID-19 pandemics. Patient number 4 is presented in 54th annual scientific sessions as an abstract. 21
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the cases’ history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the conclusion balanced and justified on the basis of the findings?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious and transmissible diseases
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 06 Jul 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)