Keywords
COVID-19, cardiac troponin-T, LDL-C to HDL-C ratio, receiver operating characteristic curve, area under the ROC curve
This article is included in the Emerging Diseases and Outbreaks gateway.
Background: People with coronavirus disease 2019 (COVID-19) are frequently at higher risk of developing cardiovascular and metabolic disorders, which are strongly related to the development of long-term illness and higher mortality. These effects may be caused by several interrelated processes, including the IL-6 driven cytokine storm or uncontrolled angiotensin II stimulation. In addition, the direct viral infection of cardiac myocytes is thought to cause cardiac injury because it increases metabolic demand, activates the immune system, and causes vasculature disruption. The objective of this study was to determine whether there is a relationship between cardiac troponin-T (cTnT) and low-density lipoprotein cholesterol (LDL-C) to high-density lipoprotein cholesterol (HDL-C) ratio values with COVID-19.
Methods: During the data collection stage, 90 participants were included, 45 healthy controls and 45 hospitalized patients diagnosed with COVID-19 using reverse transcription-quantitative PCR (RT-qPCR). Each participant provided 5 ml venous blood to begin analyzing cTnT and LDL-C:HDL-C ratio levels in their blood to see whether there is an association between the level of any of these markers and COVID-19 infection using SPSS version 23.
Results: This research reported a significant rise in the measured values of cTnT and LDL-C:HDL-C ratio in patients’ blood compared to controls, with P-values of 0.025 and 0.000, respectively, in which alpha values < 0.05, These biomarkers hold the promise in predicting COVID-19 severity, and early treatment may help reduce complications.
Conclusions: Due to the fact that cTn is a diagnostic marker of disease activity and a strong independent predictor of negative events, its usage in emergency rooms may well be advantageous. If cTn is elevated, hospitalization may be indicated. A difference in the blood LDL-C:HDL-C ratio of COVID-19 patients demonstrated an association with the illness. Because lipid studies are inexpensive and reliable to do, they may aid clinicians in identifying the severity of COVID-19.
COVID-19, cardiac troponin-T, LDL-C to HDL-C ratio, receiver operating characteristic curve, area under the ROC curve
Expression of Concern (20th October 2023): We, the Publisher and Editor of F1000Research, are issuing an Expression of Concern for the following article:
Sabah Khalid S, Mohamed Ali Z and Shareef LG. Levels of cardiac troponin-T and LDL-C to HDL-C ratio of hospitalized COVID-19 patients: A case-control study [version 1; peer review: awaiting peer review]. F1000Research 2022, 11:860 (https://doi.org/10.12688/f1000research.123619.1).
After publication of this article, questions about the integrity of the ethical approval and methodology were brought to the attention of the F1000 Editorial Team. We have reached out to the authors requesting that they respond to the concerns raised and provide additional information to support the integrity of the content. However, despite multiple attempts to contact the authors, and their institution, they have not responded to our queries within the requested timeframe. Therefore, as we continue to work through the issues raised, we advise readers to interpret the information presented in the article with due caution. The authors have been sent notification about this Expression of Concern.
Editorial Note (13th July 2023): Since publication, concerns have been raised to the Editorial Team regarding the ethical approval for this study, as well as overlap in the sample and methods to other papers by these authors. The Editorial Team requested explanations regarding the similarities on 19th May and 6th June 2023, and the institution was contacted on 21st March, 6th April and 25th April 2023 to verify the ethical approval. Neither the author nor institution have provided responses to our requests. The Editorial Team will update this Editorial Note as the situation progresses. Peer review activity has been suspended for this article until we receive an explanation from the authors/institution.
Coronavirus initially arose in 2019 in Wuhan, China, and subsequently spread around the globe. The virus belong to the family of positive single-stranded RNA (+ssRNA) with diameters in the range from (60–140 nm).1–6 Troponin is the most extensively utilized biomarker in diagnosing acute myocardial injury (AMI).7 Troponin is present both in cardiac as well as the skeletal muscles. Troponin unit is made up of three subunits: troponin T (TnT), a tropomyosin-binding subunit, troponin I (Tn-I), an inhibitory subunit, and troponin C (TnC), a calcium-binding subunit. Troponin monitors the interaction of actin and myosin through the calcium influence, which causes muscle fibers contraction and relaxation.8 Troponin is detected in the bloodstream four to six hours after an AMI and reaches a maximum after 24 h. It may persist for up to 14 days. The automated detection of cardiac troponin I (cTnI) or cardiac troponin-T (cTnT) is presently the much more sensitive and specific method for detecting AMI. Nevertheless, every scenario that causes or contributes to cardiac damage, including decompensated heart failure, pulmonary embolism, end-stage renal disease, and stroke, raises cTn.8,9
A low level of high-density lipoprotein cholesterol (HDL-C) is a powerful and reliable predictor of cardiac disease.10 Also, elevated levels of low-density lipoprotein cholesterol (LDL-C) have an essential role in the emergence and progression of atherosclerosis.11 Furthermore, it has been shown that a high LDL-C:HDL-C ratio is a stronger predictor than either HDL-C or LDL-C alone for cardiovascular events.12
The research aimed to evaluate cTnT and LDL-C:HDL-C ratio levels in the blood of severe coronavirus disease 2019 (COVID-19) infected patients and compare them to healthy controls to determine whether there is an association between these markers and COVID-19 infection severity.
The ethics committee of the College of Pharmacy at the University of Baghdad granted formal approval to the research protocol (ethics board approval code: 193220) on 10th April 2021. All research participants provided written informed consent.
Adult COVID-19 patients were compared to healthy controls in an observational, case-control study.
This research was conducted in a multi-center for COVID-19 hospitalization in Baghdad, at the following hospitals: Dar-Alslam, Alshefaa, Al-Ataa, and Dhare Alphaeadh in Iraq, from September 2021 to January 2022.
The main objective of the present research was to assess the levels of cTnT and the LDL-C to HDL-C ratio.
G*Power (RRID:SCR_013726) version 3.1.9.7 software was used to figure out the number of participants. With a 95% confidence interval, 90% power, a two-tailed alpha of 0.05, and an effect size of 0.80, the sample size had to be at least 80 patients (f). A total of 45 people in the disease group and 45 people in the control group participated in this study.
Adults (18–60 years old) with COVID-19 confirmed by positive PCR who were willing to participate and of either sex were candidates.
A history of hyperlipidemia; diabetes and hypertension; autoimmune illness; pregnancy; asthma; smoking; malignancies; and corticosteroid or immunosuppression medication as long-term therapy prior to COVID-19 infection.
During the process of selecting the study sample, selection bias may occur. This is particularly true in retrospective cohort studies when exposure and outcome have already occurred before participants are recruited. Nevertheless, since the outcome is unknown at the time of enrollment, sampling error is less probable in this case-control study. The optimal study population is well-defined, conveniently accessible, reliable, and has a high likelihood of producing the intended outcome. To avoid any source of bias, participants were recruited in a method that did not favor individuals with basic abnormally high or low levels of exposure to COVID-19. To identify volunteer bias in a sample, we asked control participants whether they feel they had been infected.
Blood samples were taken from the participants under similar circumstances, and the researcher interviewed the participants. Data were collected directly from the patients by interviewing them individually or from patients’ case files. Before enrollment, each participant signed a consent form granting us access to their case file in order to obtain the necessary information recorded on admission, such as their name, age, sex, diseases, and medication history. Each participant was asked to provide 5 ml venous blood, which was transferred into a gel tube, allowed to clot for the appropriate amount of time at room temperature, and then centrifuged for 10–15 minutes at 4,400 revolutions per minute (RPM) to extract LDL-C:HDL-C ratio analysis. Blood must be obtained at least 12 h following fasting to analyze a participant’s lipid level correctly. However, using a direct LDL-C test procedure, it is feasible to precisely anticipate such LDL-C values as in the postprandial state regardless of the time of specimen collection.13 ELISA analysis was conducted using participants’ serum to measure troponin-T (ng/ml) using specific kits. The ELISA method that was used was a sandwich method.14
This analysis employed substances of the maximum possible purity. A list of the chemical kits used in this study is provided in Table 1.
| Chemicals | Provider | Lot No. | Cat. No. | Expiry date |
|---|---|---|---|---|
| Human cardiac troponin-T ELISA detection kit | BTLAB Diagnostic (China) | 202111001 | E4509Hu | 2022/10/31 |
| Direct HDL-C | Human (Germany) | 21003 | 10018 | 2023/04/30 |
| Direct LDL-C | Human (Germany) | 21001 | 10094 | 2022/04/27 |
IBM SPSS Statistics (RRID:SCR_016479) version 27 software for Microsoft Windows was used throughout the statistical analysis process. The median is the presentation used for descriptive statistics. In order to determine whether or not the data followed a normal distribution, the Kolmogorov-Smirnov test was carried out. Descriptive statistics are presented as median, interquartile range (IQR), and mean rank T-test Mann Whitney was used to compare patient and control outcome of non-normally distributed variables. Pearson’s chi-squared test was carried out for categorical variables, while Spearman’s correlation test was utilized to analyze the relationship between parameters and outcome. The receiver operating characteristic (ROC) curve was also utilized for the purpose of measuring the area under the curve (AUC), optimal cut-off value specificity, and sensitivity. The maximum value for AUC is one, which indicates that the diagnostic analysis is accurate in the discrimination between the diseased and the non-diseased. This takes place when the ranking of analysis findings for sick and non-diseased individuals does not overlap with one another. For instance, when the AUC is half, the probability density function for that curve is on a diagonal line in the ROC space.15
This was a case-control study with 90 participants, including 45 hospitalized patients with COVD-19 who had a positive result of nucleic acid amplification testing by reverse transcription-quantitative PCR (RT-qPCR) of respiratory samples, which were nasal/oropharyngeal swabs for COVID-19 infection, and 45 healthy volunteers as a control group. Female participants made up 53% of the study population. The study showed significant increases in cTnT levels in patients compared with controls (P-value 0.025 in which alpha was < 0.05, median (IQR) was 0.0121 (0.0159) (ng/ml) while median (IQR) for control was 0.0187 (0.0227) (ng/ml)). LDL-C:HDL-C ratio also showed a significantly higher level in the COVID-19 infected group compared with controls (P-value < 0.001, median (IQR) for patients was 1.5919 (1.08) while for controls it was 0.7346 (0.67)), as shown in Table 2.
| Parameter | Group | Median | IQR | Mean rank | P-value |
|---|---|---|---|---|---|
| cTnT | Patients | 0.0121 | 0.0159 | 39.34 | *0.025 |
| Controls | 0.0187 | 0.0227 | 51.66 | ||
| LDL-C:HDL-C | Patients | 1.5919 | 1.08 | 52.00 | *0.00 |
| Controls | 0.7346 | 0.67 | 39.00 |
cTnT had an AUC of 0.363, which made it fail as a predictive marker for COVID-19 infection; it had an optimal cut-off of 0.167 (ng/ml) with sensitivity and specificity of 42.2% and 37.8%, as shown in Table 3 and Figure 1. In this study, the optimal cut-off value of LDL-C:HDL-C ratio was 1.0089 with sensitivity and specificity of 86.7% and 77.8%, respectively, with an AUC of 0.888, as shown in Table 3 and Figure 2.
| Variable | AUC | 95% CI of AUC | P-value | Optimal cut-off | SN | SP |
|---|---|---|---|---|---|---|
| cTnT ng/ml | 0.363 | 0.248–0.478 | *0.025 | 0.16750 | 0.422 | 0.378 |
| LDL-C:HDL-C ratio | 0.888 | 0.818–0.958 | *0.00 | 1.0089 | 0.867 | 0.778 |
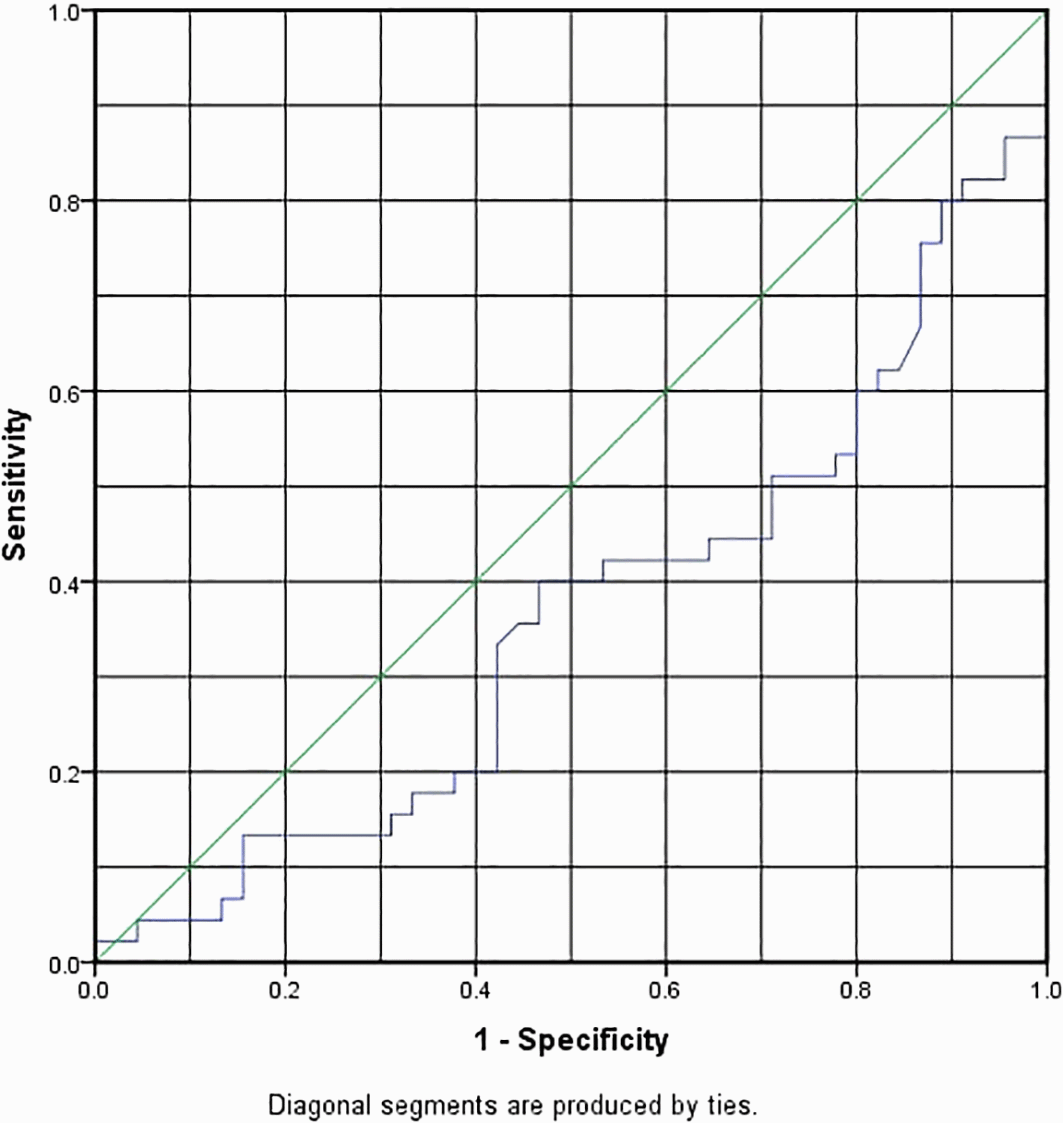
ROC, receiver operating characteristic; AUC, area under the curve.
Cardiovascular injury is a common cause of heart disease in COVID-19 patients. COVID-19 individuals with elevated cTn levels have one of three primary pathologies: myocarditis, microangiopathy, or myocardial infarction (MI).16 Myocarditis may be caused by either a direct viral invasion or injury to cardiomyocytes, which causes T-cell excitability and subsequent cardiac inflammation, or by indirect inflammation caused by the cytokine storm response as a result of the infection.17,18 The myocardium may have a high viral load or extensive inflammatory multifocal inflammatory cells on biopsy. Microangiopathy may also be caused by direct viral invasion of the endothelium through the angiotensin-converting enzyme 2 (ACE2) receptor, resulting in endothelial dysfunction. It may also occur indirectly as a result of these patients’ hypercoagulable and prothrombotic states, which produce endothelial dysfunction and, finally, cardiac damage as a result of perfusion deficits, vascular hyperpermeability, and vasospasms.19 cTnT levels rise 3 to 6 h after ischemic injury and peak between 12 and 24 h, with thresholds remaining elevated for four to five days.20,21 Admission levels are therefore useful for determining the presence of myocardial injury but not for determining the severity of the myocardial injury, which is more invariably related to the greatest rise in cTnT level.22 Thus, in this study we observed higher cTnT levels in the patient group than in the controls. The COVID-19 group showed a P-value of 0.025 (alpha 0.05) and the median (IQR) for cTnT levels was 0.0121 (0.0159) (ng/ml) compared to 0.0187 (0.0227) (ng/ml) for the control group, which was in agreement with a retrospective study done by Guo et al., in which patients with COVID-19 who had increased TnT levels had a 13% mortality rate during hospitalization.23 The rising mechanism of cTn in COVID-19 disease seems to represent non-coronary artery disease rather than acute coronary artery disease including MI. Furthermore, the underlying pathophysiology may be an inflammatory reaction, since in severe circumstances many people with COVID-19 have an increase in inflammation markers like C-reactive protein (CRP), which may manifest clinically as fulminant myocarditis.24 According to a prior study that examined the relationship among both AMI and COVID-19, there is also an influential independent association between increased cTn concentrations and COVID-19 disease severity, including death, and the relationship of varying degrees of severity with rising cTn concentrations may assist in determining how to treat a patient in the emergency department, and if cTn is raised. Even if the patient is not hospitalized, outpatient treatment may be considered.25 The intensity level of both hypolipidemia and COVID-19 displayed a parallel correlation.26 Furthermore, low HDL-C levels in COVID-19 are likely to cause severe complications.27 In COVID-19 infection, significant inflammatory process may develop, leading to a shift in lipid metabolism and vascular permeability that results in the transfer of cholesterol particles into the lungs, generating exudate, and causing a decrease in plasma LDL-C and cholesterol levels.28 There is additional evidence that COVID-19 can be triggered by sterol regulatory element-binding protein-2 inhibiting cholesterol biosynthesis, which results in a cytokine storm; therefore, the ratio of LDL-C to HDL-C is a powerful predictor of coronary heart disease and has greater predictive power than HDL-C and LDL-C alone for cardiovascular diseases (CVD).29,30 The LDL-C:HDL-C ratio was considerably higher in the COVID-19 infected group than in the controls (P-value = 0.00 0.05), with the median (IQR) for patients being 1.5919 (1.08) and 0.7346 for controls (0.67). The optimal LDL-C:HDL-C ratio cut-off value had a sensitivity and specificity of 86.7% and 77.8%, respectively; thus, the severity of patients in this study was associated with LDL-C:HDL-C ratio levels greater than 1.0089; the AUC for LDL-C:HDL-C ratio was 0.888, making it an excellent predictive marker for severe COVID-19 infection.31 In an investigation about the optimal cut-off value for LDL-C:HDL-C ratio in cardiovascular and metabolic disease, Chen et al. (2016) described 2.5 as an optimal cut-off point for LDL-C:HDL-C ratio to predict CVD risk factors in Uygur subjects over 35 years old and provided evidence that a higher LDL-C:HDL-C ratio was related to an undesirable cardio-metabolic risk level.32 This variation in optimal cut-off values may be due to a variety of factors, including genetic factors, especially those genes involved in lipid metabolism and overweight, such as apolipoprotein A-V (APOA5) and peroxisome proliferator-activated receptor (PPAR) genetic genotyping, as well as environments, race, lifestyle, and living habits. As a result, the optimal cut-off point of the LDL-C:HDL-C ratio may differ depending on a variety of factors, as discussed above.32
Because cTn is a marker of severity of illness and a strong independent predictor of unfavorable outcomes, it may aid in deciding how to manage a patient in the emergency room. Ambulatory treatment may be used if cTn is high but the patient is not hospitalized. The fluctuation of the LDL-C:HDL-C ratio level in COVID-19 patients’ blood suggested a significant association with the condition. Because the lipid profile is inexpensive and simple to do in all facilities, it may aid the clinician in determining the degree of COVID-19 infection.
Zenodo: Demographic data along with a comparison of cardiac troponin-T and LDL-C: HDL-C Levels. https://doi.org/10.5281/zenodo.6786306.33
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The authors would like to thank the participated subjects and the team of COVID-19 care units for their support during sample collection.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)