Keywords
Myocarditis, side effect, vaccination, mRNA, COVID-19
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Sociology of Health gateway.
This article is included in the Coronavirus (COVID-19) collection.
This article is included in the Sociology of Vaccines collection.
Myocarditis, side effect, vaccination, mRNA, COVID-19
Since early 2021, the COVID-19 vaccination program has been initiated in many regions.1 The program involves administration of several types of COVID-19 vaccines such as inactivated, protein subunits, messenger ribonucleic acid (mRNA)-based, and vector vaccines.2 It is widely known that a vaccine development usually requires 10-15 years before it is ready to use in human subjects. Moreover, it undergoes various steps including antigen identification and production, non-clinical (animal) testing, clinical trials (phase I-III), filing and licensing, and surveillance However, the vaccine development process for COVID-19 was completed in approximately a year. The fact that the COVID-19 pandemic resulted in very high mortality rates globally, led to an urgent need for the vaccine, which explains the short duration of vaccine development.3,4 However, this rapid process probably led to unfolding of various side effects gradually.5 Of all the available vaccines, mRNA-based COVID-19 vaccines have been associated with maximum side effects.6
mRNA vaccines have been investigated for several infectious diseases such as Cytomegalovirus, Zika virus, Human Metapneumovirus, Respiratory Syncytial Virus, Influenza, Chikungunya, and Rabies.7 Of these viruses, some serious side effects like toxic epidermal necrolysis were reported in the case of mRNA Rabies vaccine.8 Similarly, the mRNA-based COVID-19 vaccine also showed some minor side effects such as pain or redness at the site of injection, fatigue, fever, headache, nausea or vomiting, chest pain, and shortness of breath.9 Additionally, some fatal side effects such as acute kidney injury, anemia, and myocarditis have been reported.10 Of these, myocarditis is the most life-threatening condition due to associated high mortality rates (25%-56%).11 Various observational studies and case reports and series have shown the occurrence of myocarditis after mRNA-based COVID-19 vaccine administration;10,12–29 however, the findings vary across the studies. In the current scenario, global prevalence rate of myocarditis post mRNA-based COVID-19 vaccination should be established precisely, and the types of vaccines associated with this condition should be identified. Therefore, the present study aimed to assess the global prevalence and association between the risk of myocarditis and mRNA-based COVID-19 vaccination, using a networking meta-analysis approach.
A meta-analysis following the protocols of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)30 was conducted. The PRISMA checklist in our present study was provided in the supplementary files.31 To analyze our study objectives, a systematic search was conducted in PubMed, Scopus, and Web of Science and the required data were retrieved to calculate the cumulative prevalence and effect estimates.
Eligibility criteria were defined prior to the systematic search. The inclusion criteria for study sources were as follows: (1) Assessed the prevalence of myocarditis after mRNA-based COVID-19 vaccination and (2) provided the required information to calculate the prevalence and effect estimates. Reviews, commentaries, letters to the editor, and double publications were excluded from the study sources.
A systematic search was conducted in PubMed, Scopus, and Web of Science between January and February 2022; as of 3 February 2022 3rd, data collection was finished. Before searching for the primary outcomes, we searched for the type of mRNA-based COVID-19 vaccines involved in our study. The keywords adapted from medical subject headings used were as follows: [“mRNA COVID-19 vaccine” or “BNT162b1” or “Pfizer” or “mRNA-1273” or “Moderna”], [“Myocarditis” or “side effect”], and [“COVID-19” or “Coronavirus disease-19”]. The systematic search was limited to English language and original articles. In case of double publications, only studies with larger sample sizes were included. Moreover, a systematic search of the reference list of relevant systematic reviews was conducted to collect additional articles. Subsequently, the data of interest from the selected articles were extracted and included: (1) first author name, (2) publication year, (3) study design, (4) type of mRNA-based COVID-19 vaccines, (5) trade name of the vaccines, and (6) the incidence of myocarditis after mRNA-based COVID-19 vaccination. Two independent investigators (JKF and MI) performed the systematic search and extracted the data. Prior to performing the systematic search and data extraction, the assessments and ratings given by independent investigators were analyzed using the kappa agreement. Agreement was established if the coefficient of kappa agreement was greater than the p-value.
Before their inclusion in our analysis, the articles were assessed for quality using the Newcastle-Ottawa scale (NOS)32 with score ranging from 0 to 9. A score of 7-9, 4-6, and 0-3 indicated that the papers were of high, moderate, and low quality, respectively. Low-quality articles were excluded from the analysis. Using a pilot form, quality assessment was performed by two independent authors (JKF and MI) and any discrepancies were resolved through discussion.
The primary outcomes were cumulative prevalence and risk of myocarditis after mRNA-based COVID-19 vaccination. To identify the potential mRNA-based COVID-19 vaccines, an initial evaluation of the available data in PubMed, Scopus, and Web of Science was performed. We found that BNT162b1 (Pfizer) and mRNA-1273 (Moderna) were suitable for our analysis. Myocarditis cases data were retrieved according to the International Classification of Diseases, Tenth Revision (ICD-10) as follows: I40.0 (infectious myocarditis), I40.1 (isolated myocarditis), I40.8 (other acute myocarditis), I40.9 (acute myocarditis), I41 (myocarditis in diseases classified elsewhere), I51.4 (myocarditis unspecified), or B33.22 (viral myocarditis).
Before analyzing the data, potential publication bias and heterogeneity across the studies were assessed. Publication bias was determined using an Egger test, with a p-value ˂ 0.05 indicating a trend towards publication bias. Heterogeneity among studies was evaluated using the Q test with a p-value ˂ 0.10 suggesting heterogeneity and that a random effect model should be applied for data analysis; otherwise, fixed-effects model were be used. The cumulative prevalence of myocarditis after mRNA-based COVID-19 vaccination was determined using a single-arm meta-analysis with the dichotomous covariate method by calculating the event per sample size from each study. The effect estimate was presented as (logit) event rate. The analysis was performed using R software (RStudio version 4.1.1, MA, US). Confidence in Network Meta-Analysis (CINEMA 1.9.1, Bern, Switzerland) software was used to outline the network diagram of the comparison of the risk of myocarditis among patients administered with different types of mRNA-based COVID-19 vaccines. The effect estimate was presented in a forest plot as the pooled odd ratio and 95% confidence interval (OR 95% CI).
We collected 997 potential papers from the database and 16 papers from the reference list of related systematic reviews. Of these, 22 papers were excluded owing to the duplication of data and 942 papers owing to irrelevant topics. Subsequently, we included 49 full-text review papers. From these, 12 reviews, 16 case reports, and three papers were excluded owing to insufficient data. Finally, a total of 18 papers were analyzed to determine the cumulative prevalence and risk of myocarditis after mRNA-based COVID-19 vaccination.10,16–29 The process of article selection is shown in Figure 1 and the characteristics of the included papers are summarized in Table 1.
The data analysis revealed that the prevalence of myocarditis after mRNA-based COVID-19 vaccination was 859 cases in a population of 51,826,799 (logit event rate: ˗10.70; 95% CI: ˗11.24, ˗ 10.16) (Figure 2A). In subgroup analyses, the prevalence of myocarditis was 643 cases in a population of 33,088,209 (logit event rate: ˗10.61; 95% CI: ˗11.29, ˗9.93) (Figure 2B) after BNT162b1 (Pfizer) vaccination, 126 cases in a population of 10,710,913 (logit event rate: ˗10.13; 95% CI: ˗12.62, ˗7.64) (Figure 2C) after mRNA-1273 (Moderna) vaccination, and 90 cases in a population of 8,027,677 (logit event rate: ˗11.23; 95% CI: ˗12.44, ˗10.02) (Figure 2D) after combined vaccination with BNT162b1 and mRNA-1273. A summary of the cumulative prevalence of myocarditis after the mRNA-based COVID-19 vaccination is outlined in Table 2.
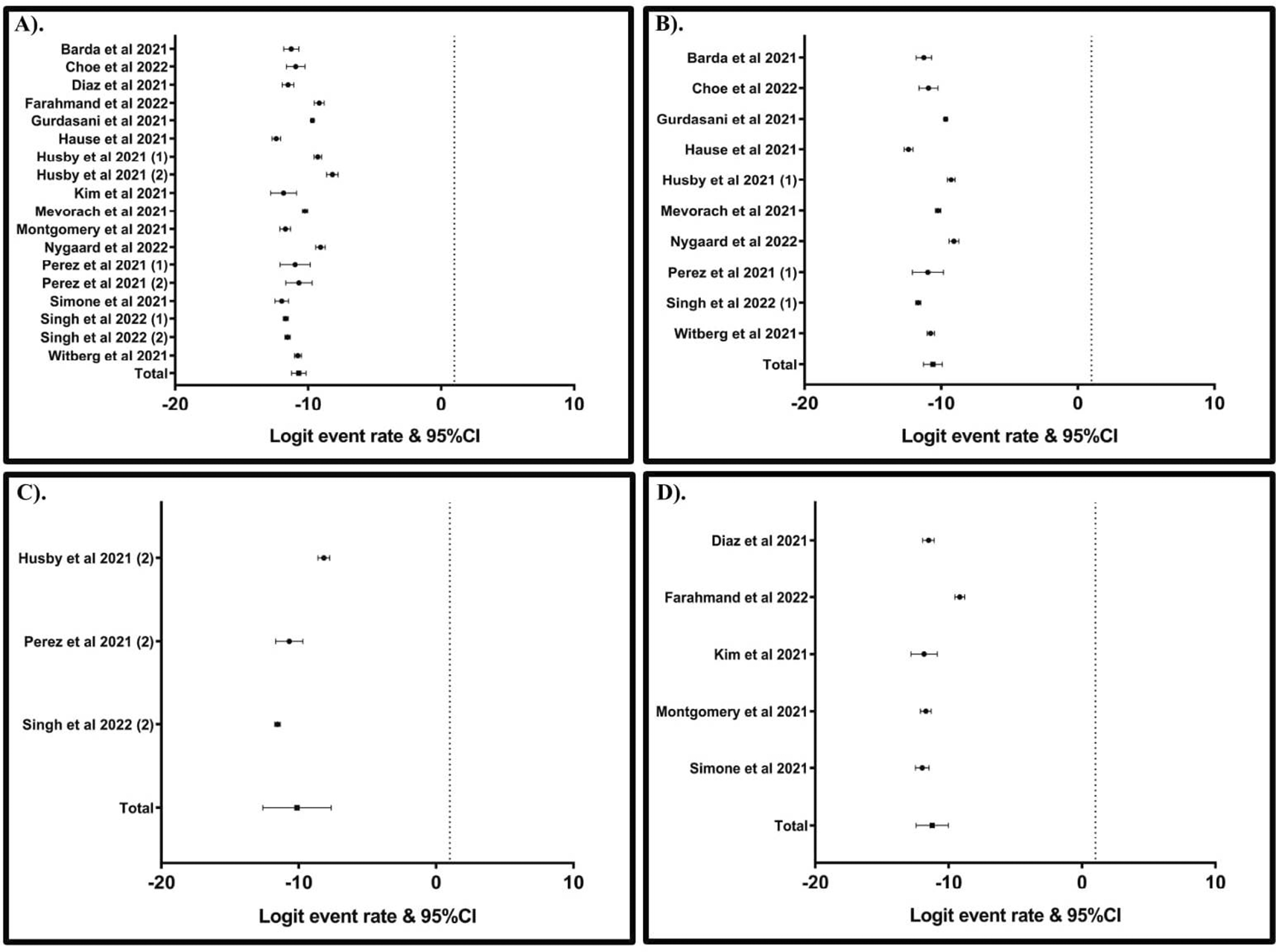
A). All type of mRNA COVID-19 vaccines. B). Pfizer mRNA COVID-19 vaccines. C). Moderna mRNA COVID-19 vaccines. D). The combination of Pfizer and Moderna.
The indirect comparison (Figure 3A) revealed that the use of BNT162b1 vaccination increased the risk of myocarditis by 1.63-folds compared to mRNA-1273 vaccination (OR: 1.63; 95% CI: 1.35, 1.97; p < 0.0001). Moreover, BNT162b1 was associated with an increased risk of myocarditis compared to the combination of BNT162b1 and mRNA-1273 vaccines (OR: 1.71; 95% CI: 1.37, 2.13; p < 0.0001). mRNA-1273 and the combination of BNT162b1 and mRNA-1273 showed a similar risk of developing myocarditis (OR: 1.05; 95% CI: 0.80, 1.38; p = 0.7270). A summary of the indirect comparison of the risk of myocarditis associated with different mRNA-based COVID-19 vaccines is presented in Table 3 and the network is illustrated in Figure 3B.
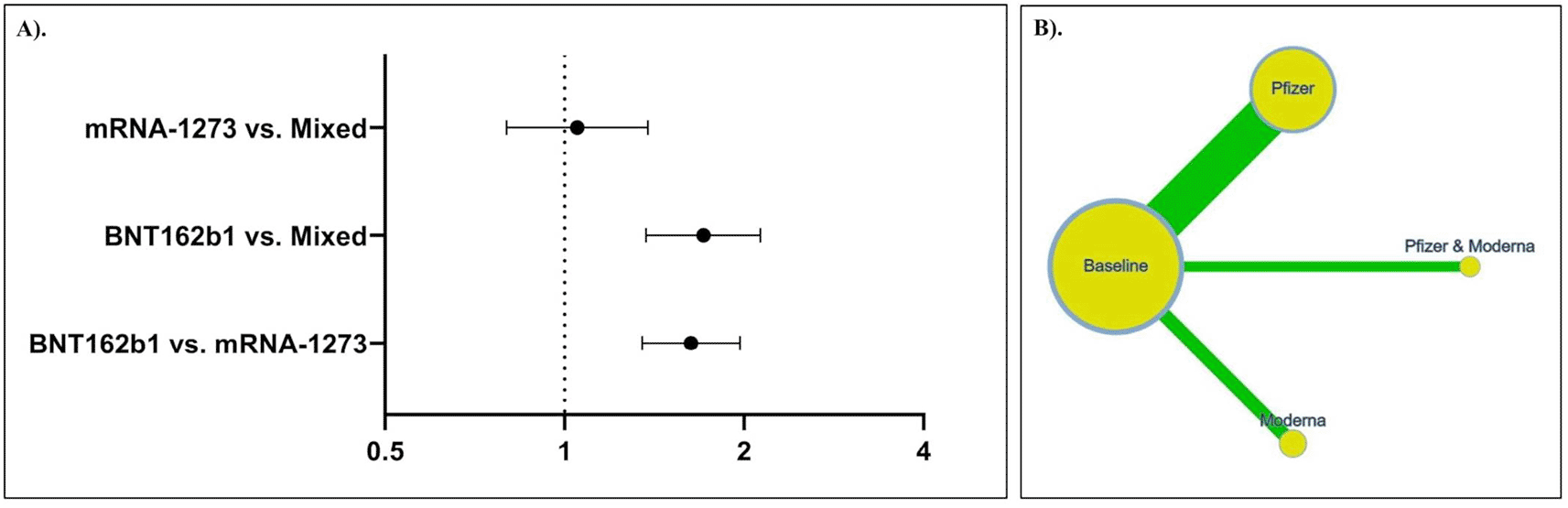
Heterogeneity was observed in all the models of analysis. Therefore, we used a random-effects model. Egger’s test was used to assess the potency of bias among the studies. Our pooled analyses revealed no publication bias (Table 2).
Our findings revealed that the pooled prevalence rate of myocarditis after mRNA-based vaccination was 1.7 cases per 100,000 population. Specifically, it was 1.9, 1.2, and 1.1 cases per 100,000 population after BNT162b1, mRNA-1273, and combination of BNT162b1 and mRNA-1273 vaccination, respectively. Our study is the first meta-analysis to report the prevalence of myocarditis after administration of different types of mRNA-based COVID-19 vaccines. Previously, a total of four meta-analyses in this context were conducted.33–36 However, these reports are limited to the crude prevalence rate and did not investigate different types of mRNA-based COVID-19 vaccinations. According to these reports the cumulative prevalence rate of myocarditis after mRNA-based COVID-19 vaccination was 1.2-11.9 per 100,000 population,35,36 which is consistent with our findings. In our study, we included a larger sample size; therefore, the present study might provide a better insight into the precise prevalence rate.
We also reported that BNT162b1 vaccination was associated with 1.6 and 1.7-folds higher risk of developing myocarditis compared to mRNA-1273 and the combination of BNT162b1 and mRNA-1273 vaccines, respectively. On the other hand, mRNA-1273 and the combination of BNT162b2 and mRNA-1273 shared similar risks of developing myocarditis. The underlying mechanism by which BNT162b1 vaccination leads to a higher risk of developing myocarditis remains debatable. Briefly, the principle of mRNA-based COVID-19 vaccination is to produce viral-mRNAs that can encode the perfusion stabilized full-length spike protein, a protein involved in the interaction with ACE2 receptors to infect the target cells. The production of antibodies with high affinity to spike protein may inhibit the interaction between spike protein and ACE2 receptors and therefore may provide protection against COVID-19 infection.37 Spike proteins of SARS-COV-2 have a molecular weight of 180-200 kDa.38 On the other hand, myocardial cells consist of actin and myosin proteins. These proteins have a molecular weight of 100-250 kDa.39 In the theory of cross-immunity, it is known that two different proteins with similar molecular weights, defined as molecular mimicry, may trigger cross-immunity.40–43 Therefore, due to the similar molecular weights of spike protein of SARS-COV-2 and actin-myosin, the antibodies may attack latter proteins in myocardial cells as well, resulting in development of myocarditis eventually. Moreover, studies also revealed that BNT162b1 consisted of highly purified ss-5-capped mRNA, and mRNA-1273 consisted of synthesis of ss-5-capped mRNA. The capability of spike protein production was reported higher in BNT162b1 than mRNA-1273.44,45 Furthermore, previous studies have also reported myocarditis in patients with COVID-19; however, the evidence of whether the myocarditis was caused by the direct infection or the systematic inflammation is not clear.46 On the other hand, a study found that COVID-19 patients with myocarditis showed no signs of injury in electrocardiography and echocardiography, indicating that myocardial damage was a result of systemic inflammation and not direct infection.47 This possible mechanism might explain the findings of our study, which indicate that the immunization with BNT162b1 had higher risk of developing myocarditis compared to other mRNA-based COVID-19 vaccines.
Our study is the first networking meta-analysis to report the prevalence rate and risk of myocarditis after mRNA-based COVID-19 vaccination. We revealed that immunization with BNT162b1 had the highest prevalence rate and risk of developing myocarditis compared to other mRNA-based COVID-19 vaccinations. Our findings may contribute to the development of a COVID-19 immunization policy. If an mRNA-based COVID-19 vaccine needs to be selected, we recommended the use of mRNA-1273 or the combination of mRNA-1273 and BNT162b1 over the use of BNT162b1 alone. Further investigations are required to assess the specific mechanism underlying association of BNT162b1 vaccine with high-risk of myocarditis compared to other mRNA-based COVID-19 vaccines. On the other hand, the knowledge of patients on the side effect of mRNA COVID-19 vaccination should be investigated to assess the willingness for COVID-19 vaccination.48–51
Our study had several important limitations. First, the potential confounding factors that might contribute to the development of myocarditis (e.g., infection due to common causative agents such as Coxsackie virus, group A streptococci, chlamydia, or Trypanosoma cruzi) were not included in the analysis.52 Second, the small prevalence of myocarditis after mRNA-based COVID-19 vaccination impeded the calculation of the precise prevalence and risk association. Third, the design of the included papers in our study was dominated by a retrospective study; therefore, further investigations with better study designs are required. Fourth, the proportion of myocarditis cases in each study was unequal. Fifth, the majority of papers in our analysis assessed BNT163b1 and the number of papers assessing mRNA-1273 was insufficient. Therefore, there is a possibility that calculation bias would have existed.
Our study revealed that the prevalence of myocarditis after mRNA-based COVID-19 vaccination was 1.7 cases per 100,000 population, and BNT162b1 was associated with the highest prevalence rate compared to other mRNA-based COVID-19 vaccines. BNT162B1 vaccination is also associated with a higher risk of myocarditis than other mRNA-based COVID-19 vaccines. Based on these results, we recommend the use of mRNA-1273 vaccine over the BNT162B1 vaccine.
Figshare: Supplementary files: The global prevalence and association between the risk of myocarditis and mRNA-based COVID-19 vaccination: A network meta-analysis, https://doi.org/10.6084/m9.figshare.19768498.v2.31
Figshare: PRISMA checklist for “The global prevalence and association between the risk of myocarditis and mRNA-based COVID-19 vaccination: A network meta-analysis”, https://doi.org/10.6084/m9.figshare.19768498.v2.31
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Idea/concept: MSR, JKF. Design: MSR, GS, JKF, LW. Control/supervision: MSR, GS, LW, MA, KD, HH. Data collection/processing: MI, DM, YP, APK, AA, DA, AP, EAP, HAM, YP, DA, PWMP, VAS, DS, ENP, ETF, OLP, RS, RY, KH, YSP, LN, LL, MDC, MI, II, ADS, FT, DAK, AIM. Extraction/Analysis/interpretation: JKF, MI. Literature review: MSR, JKF, MI. Writing the article: MSR, JKF, MI. Critical review: MSR, GS, LW, MA, KD, HH. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
We thank Lembaga Pengelola Dana Pendidikan (LPDP) Republic of Indonesia for supporting this project.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
Not applicable
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical medicine
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 29 Jul 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)