Keywords
COVID-19, World Health Organization, VigiBase, Hydroxychloroquine, pharmacovigilance, adverse events
This article is included in the Emerging Diseases and Outbreaks gateway.
COVID-19, World Health Organization, VigiBase, Hydroxychloroquine, pharmacovigilance, adverse events
The coronavirus disease 2019 (COVID-19) pandemic began in Wuhan, China, in December 2019. High body temperature, dry cough, headache, dyspnea, and diarrhea were all symptoms of this viral illness. The earliest occurrences of these symptoms were linked to a food market in Wuhan, China. Since then, some cases have developed into severe respiratory distress, with an approximate death rate of 2%.1–4 The global impact of the SARS-CoV-2 pandemic has been unprecedented, prompting the scientific community to investigate all potential remedies.5 Because hydroxychloroquine (HCQ) has previously been shown to be successful in the treatment and prevention of SARS-CoV,6 and because COVID-19 is similar to SARS-CoV, many investigators have advocated HCQ as a possible therapeutic drug.7 HCQ is a medication used in the treatment and prevention of malaria and autoimmune diseases such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE).8 In vitro investigations suggest that HCQ inhibits SARS-CoV-2 infection and multiplication.9–11 HCQ has antiviral action through entrance inhibition, replication inhibition, or immunological manipulation.12,13 Endosomal antigen processing is altered by HCQ, and innate and adaptive immune responses are modulated.14,15 This reduces the production of pro-inflammatory cytokines such as TNF-α, IL-1, and IL-6.
Furthermore, HCQ optimizes endothelial function and suppresses the prothrombotic condition. These characteristics may benefit people with severe COVID-19.16 In patients with malaria or autoimmune disorders, the most common adverse effects of HCQ encompass gastrointestinal distress (vomiting, diarrhea, stomach cramps), skin rash, headache, dizziness, and ophthalmic toxicity. In addition, these medications may potentially induce significant adverse effects such as arrhythmia, bronchospasm, angioedema, and seizures.17 Cardio-toxicity is the most devastating adverse effect of HCQ therapy in severely infected COVID-19 patients. A double-blind clinical study from Brazil revealed that medication dose may be a contributor in HCQ cardiotoxicity.18 The purpose of this research was to examine the adverse events related to HCQ administration (alone or in combination with other medicines) in COVID-19 patients in Iraq to determine any new/altered safety information connected to HCQ.
This was a descriptive analysis of the potential adverse events of HCQ during its use in the COVID-19 pandemic; data were collected from VigiBase™, the world’s most extensive database of more than 20 million Individual Case Safety Reports (ICSRs) submitted by members of the World Health Organization (WHO) Program for International Drug Monitoring since 1968, as well as the risk of multiple adverse events co-occurring. Sweden’s Uppsala Surveillance Centre (UMC) creates and maintains this database. VigiLyze™ was used to retrieve the reaction outcome, disproportionality measure (information component (IC)025 value), and other relevant variables. VigiLyze is a data mining and analytics tool developed for VigiBase. In addition, national centers like the Iraqi Pharmacovigilance Centre may gather adverse effects of medications and vaccines from users, healthcare personnel, and regulated pharmaceutical corporations using the e-Reporting module. E-Reporting enables the user or healthcare professional to generate, evaluate, and report adverse effects from anywhere with an internet connection using their smartphone or other devices. The reported side effects will be instantaneously accessible in VigiFlow™ as adverse event reports for further assessment.
The IC measures the disproportionality among observed and predicted reporting of adverse drug event pairing. A positive IC value implies that a specific drug adverse event combination is reported more often than expected based on all entries in the database. On the other hand, a negative IC value indicates that the drug adverse events pairing is recorded less often than predicted. The greater the IC value, the more the combination stands out from the background. The IC025 value is the lowest limit of the IC’s % credibility interval. The credibility interval indicates the stability of a particular IC value: the smaller the gap, the greater the stability.19
Descriptive statistics were used to analyze the data for this investigation. This research did not include any individual case evaluations. IBM SPSS Statistics (RRID: SCR 016479) version 27 software for Microsoft Windows was employed throughout the statistical analysis procedure.
The Iraqi Pharmacovigilance Centre reviewed and recorded 132 episodes of adverse events related to HCQ use in VigiBase over 2020 in the COVID-19 pandemic; the study involved 132 ICSRs, all cases were qualitatively screened, 49 reports were found for women, which accounted for 37.1% of cases, while 80 reports were found for men (60.6% of cases), and three unknown reports 2.3% were found. The age group of affected patients varied from 28 days to 75 years, as seen in Figure 1.
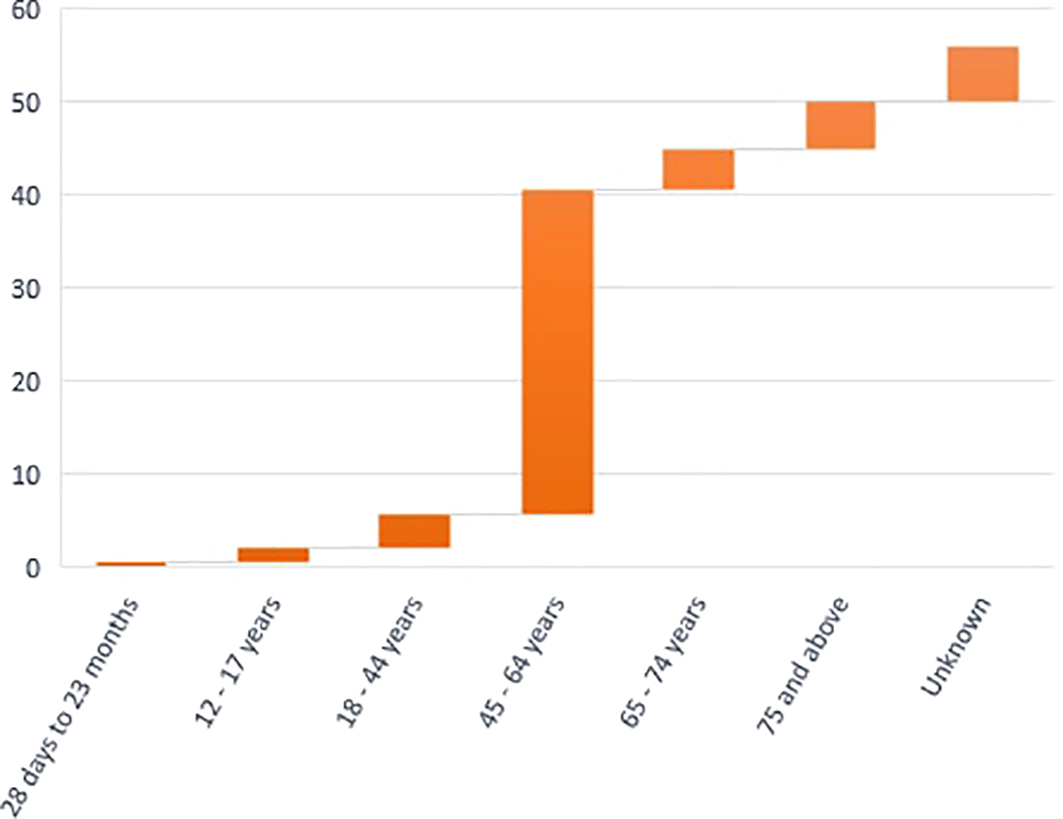
HCQ, hydroxychloroquine.
Azithromycin is the most often documented co-reported active component with HCQ, with 45 reports as suspected/interacting and five as a concomitant, totaling 50 reports (37.9% of cases). Oseltamivir has 29 suspected/interacting reports and seven concomitant reports, for a total of 36 reports (27.3%), paracetamol has one report as suspect/interacting and 21 reports as a concomitant, for a total of 22 reports (16.7%). Centrum (an American brand of multivitamins) has one report as suspected/interacting, and 20 reports were simultaneous, for a total of 21 reports (15.7%). Enoxaparin has three reports as questioned/interacting and nine as a concomitant, 12 reports in total (9.1%). Bromhexine received no reports as suspected/interacting and 10 reports as a concomitant, for a total of 10 reports (7.6%). Meropenem has four reports as suspected/interacting and four as a concomitant, eight reports in total. Clopidogrel has five reports as suspected/interacting and one as a concomitant, total of six reports (4.5%). In a quantitative assessment of the Iraqi database, the Iraqi Pharmacovigilance Centre found 44 combinations involving 177 adverse medication reactions for patients who took HCQ in various dosage strengths throughout 2020. There were 13 instances of QT prolongation on an electrocardiogram (ECG), 17 cases of upper abdominal pain, 21 cases of abdominal pain, nine cases of palpitation, five cases of dyspepsia, 13 cases of diarrhea, four cases of abdominal discomfort, 14 cases of nausea, and two cases of oral fungal infection. Many side effects, such as upper abdominal discomfort, palpitation, and dyspepsia, are more prevalent in Iraqi records than in foreign cases. The severity of these responses was assessed at 48%. Cardiac arrest, abdominal pain, fatigue, gastroesophageal reflux disease, diarrhea, headache, and dyspepsia were among the co-reported side effects as presented in Table 1.
The Iraqi number of observed cases was compared to the expected data in VigiBase for Iraq and the global registry, as well as the Iraq-IC025/Global-IC025 ratio, sex, severity, and patient age were enlisted in Table 2.
Findings from this study demonstrate how a medication’s safety profile depends on how it is administered. When administered outside of its marketing authorization, any medicine, even one that is well-known and is thought to be understood, may show higher frequent and serious adverse effects than anticipated. To guarantee patient safety, spontaneous reporting and pharmacovigilance are crucial in informing healthcare providers of possible issues.20 This research found that within the first wave of the pandemic, off-label use of HCQ in patients with COVID-19 has been related to greater reporting than in the preceding years. Adverse drug reactions (ADRs) are generally predicted and are stated in the French summary of product features for PLAQUENIL®, as previously documented in a study by P. Lory et al.21 Abdominal pain and QT prolongation were the most widely reported ADRs in individuals taking HCQ for the treatment of COVID-19, which agrees with the most consistently encountered ADRs recognized by Zekarias et al., throughout an evaluation of 2,573 reports on COVID-19-specific therapies from VigiBase.22 High dosages of the antimalarial23 and concomitant treatment with azithromycin have been associated with a greater risk of significant side effects. Furthermore, the HCQ pharmacokinetics are distinguished by their prolonged half-life and a large volume of dispersion.24 The most remarkable findings are the relatively high proportion of cardiac ADRs (ECG QT prolonged = 3.5/5 of overall ICSRs), which demonstrate that HCQ may directly damage the myocardium and cause cardiac rhythm disturbances. The relatively high incidence of toxicosis also explains the low therapeutic window of HCQ.25 The current findings further reveal that the cardiac signal (QT prolongation, arrhythmias, etc.) seen with HCQ in COVID-19 patients was previously prevalent in inflammatory arthritis or SLE patients.26,27 According to Funck-Brentano et al.,28 there are several potential causes for the elevated risk of cardiac toxicity. The first one could be connected to how SARS-CoV-2 affects the heart. In reality, individuals with coronavirus infections are more likely to have drug-induced rhythm abnormalities due to frequent hypokalemia and fever, which exacerbates the effects of drug-induced cardiac channel blocking.
Furthermore, elevated IL-6 levels following infection may contribute to the QT prolongation brought on by inflammation. According to Guo et al., myocardial damage linked to cardiac dysfunction and arrhythmias was seen in almost 30% of COVID hospitalized patients in a Chinese hospital. There is a strong correlation between these cardiac lesions and fatal results.29 Additionally, COVID-19 patients often received large dosages of HCQ in addition to other medications that cause QT prolongation (including azithromycin). HCQ and azithromycin were combined in 80% of the interaction situations, and the QT interval was lengthened in every single one. Other medications that interact with HCQ in a potentiating or additive way, such as spiramycin, fluoroquinolones, antivirals, or antidepressants, have also been linked to heart harm. Cardiac toxicity (QT prolongation, heart failure, and cardiac arrest) was the cause of the seven recorded fatalities during the COVID era. Our research points up several instances when a “drug interaction” with HCQ might negatively impact the heart.
Our investigation has some limitations and strengths, despite the inevitable biases of such research (under-reporting, absence of extensive information on dosages and exposure period in VigiBase, use of HCQ in simultaneous rheumatic or autoimmune illnesses, and not just COVID-19). Nevertheless, the findings have significant strengths: we utilized the world’s biggest pharmacovigilance database, which includes over 90% of people worldwide, to identify and analyze possible safety signals during the COVID-19 pandemic outbreak. These worldwide database results strengthen the external validity of our findings. Furthermore, unlike clinical trials, dealing with this data from VigiBase enables us to be in the setting of real-world practice. Data from the real world that were not analyzed in clinical trials allow for outcomes’ applicability.
The events reported to the Iraqi pharmacovigilance center include the concurrent use of other drugs, making it difficult to verify whether HCQ is responsible for these adverse effects. According to WHO global data, most of these impacts have minor differences. However, several have negative correlations, indicating that the predicted number of cases is greater than the actual number. In addition, some of the reported side effects, such as upper abdominal discomfort, abdominal pain, palpitations, and dyspepsia, showed a significant difference in IC025 between cases reported in Iraq and those reported internationally. This adverse effect may influence patient adherence; however, it seldom affects their use.
The raw data created and analyzed throughout the present research are still not publicly accessible due to commitments between data providers to the database utilized (VigiBase™) and the database administrator. National Iraqi pharmacovigilance center provides data to VigiBase, while the Uppsala Monitoring Centre serves as the holder in its position as a WHO cooperating center for worldwide drug monitoring. The dataset generated during the current study is not publicly available as it contains proprietary information that the authors acquired through a license. Information on how to obtain it and reproduce the analysis is available from the corresponding author on request for personal non-reproducible use to the following email address: yasiralkashab99@yahoo.com.
The authors express gratitude to the Iraqi Pharmacovigilance Centre for facilitating their access to VigiBase™.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Infectious diseases (COVID19), histopathology, molecular genetics, Oncopathology and Haematology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 11 Aug 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)