Keywords
Conditioned Medium - Mesenchymal Stem Cells, Mass Spectrometry, Chondrogenesis, Arkadia, Molecular Docking
This article is included in the Cell & Molecular Biology gateway.
Certain conditions can stimulate stem cells to produce secretory factors that differ from each condition, such as hypoxia. Umbilical Cord-derived Mesenchymal Stem Cells have been known to have higher proliferation rates, plasticity, and better self-improvement ability than MSCs from other sources.
The objective was to analyze the secreting proteins in the Conditioned Medium - Umbilical cord-derived Mesenchymal Stem Cells (CM-UCMSCs) which potentially has a role in the chondrogenesis of cartilage defect.
We used SDS PAGE combined with electrospray ionization mass spectrometry using mass spectrometer to perform a proteomic analysis of CM-UCMSCs which conditioned in a state of fasting (without serum albumin). Then, we continue to analyze to identify proteins of interest using Mascot sequence matching software. Then Analyzed using in silico / molecular docking to know the interaction of each protein with the cartilage repair factor.
SDS PAGE performed on all secreted products in CM-UCMSCs. We found one dominant band, then we used Mass Spectrometry continued Mascot sequencing protein that band, conclude that the protein was a D4A9T1/ RNF-111/ Arkadia Protein. And then using in silico/ molecular docking method analyzed that Arkadia Protein worked synergistically with TGF-β1 Protein through activation of TGF-β1 receptors to induce the chondrogenesis.
Our results show that the fasting state on CM-UCMSCs promoted secreted Arkadia protein. From in silico model prediction, this protein suspects to have an important role in the process of repairing cartilage defects. However, further research is needed before feasible and safe for the clinical application of CM-UCMSCs in cartilage repair.
Conditioned Medium - Mesenchymal Stem Cells, Mass Spectrometry, Chondrogenesis, Arkadia, Molecular Docking
We uploaded a new figure.
See the authors' detailed response to the review by William Mark Erwin
See the authors' detailed response to the review by Girish Pattappa
Currently, stem cells had provided promising potential as a substitute for cells, tissues, and organs, as well as for studying the biomolecular pathogenesis of the disease. Stem cell therapy in cartilage disease has been widely reported to have a good result in animal studies to clinical trials.1 Our previous research, in vivo application of Conditioned Medium - Umbilical cord-derived Mesenchymal Stem Cells (CM-UCMSCs) on white mouse cartilage defects, was found to increase significantly in the immunohistochemical examination of several proteins known to be markers in the process of chondrogenesis: Transforming growth factor-beta 1 (TGF-β1), SRY (sex-determining region y) box 9 (SOX9), Aggrecan, and Collagen Type II2 and also seen as significant in histological assessment using O’driscoll scoring.3
Mesenchymal Stem Cells or Mesenchymal stromal cells (MSCs) was understood could differentiate into regenerating tissue producing cells, but in recent research, MSCs has changed the name to Medicinal Signaling Cells (MSC) to more accurately reflect the fact that these cells secrete bioactive factors that are immunomodulatory and trophic (regenerative) meaning which home in on sites of injury or disease.4 MSCs also have an immunomodulatory effect, they can secrete anti-inflammatory cytokines which can be a pathway for the therapeutic mechanism in several immune and chronic diseases.5 MSCs isolated from the umbilical cord have been known to have higher proliferation rates, plasticity, and better self-improvement ability than MSCs from other sources.6 Wharton's jelly-derived stem cell-conditioned medium (WJ-SCs-CM) can increase the expression of cartilage-specific genes (aggrecan, Sox9, and collagen type II) by Chondrocytes and can be introduced as a promoting factor for cartilage regeneration.7 The derivatives of the stem cell are soluble factors that are secreted into the conditioned medium (CM).6 Certain conditions can stimulate stem cells to produce secretory factors that differ from each condition, such as hypoxia, hyperoxia, hypoglycemia, hyperglycemia, etc.8
A meta-analysis compared between secretome based therapy and stem cell therapy in articular cartilage regeneration, and the result is the secretome has a slightly higher potential than cell-based therapy.9 We assume that the free cell therapy approach will be safer and easier in the production and distribution process in the future. Therefore, this study aims to determine the components of the CM-UCMSCs in the state of fasting, which has suspected to play a role in the process of cartilage defect repair.
This research was conducted at the physiology laboratory of the veterinary medicine faculty. This study was experimental laboratory design with post-test only control group. There were thirty-six male Wistar rats aged 3-months, which were obtained from Gajah Mada University Yogyakarta, Indonesia (Letter number: 263/KEC-LPPT/V/2015). The sample size was calculated using Federer Formula:
n = sample size
t = number of experimental groups
There were 6 groups (t = 6), so we calculated the sample size were 4 for each groups. The homogeneity was confirmed through the balanced body weight (150-250 grams). All experimental procedures involving animals were carried out in keeping with guidelines from the National Institutes of Health Guide for the Care and Use of Laboratory Animals to ameliorate any suffering of animals.10 The animal models were acclimatized for a week at a temperature of 21-23 °C with controlled humidity (50 ± 5%) in a 12-hour artificial light cycle (8 am to 8 pm) to help them to adapt to the same conditions as they various origins. All rates were located individually in polycarbonate cages (0.90 × 0.60 × 0.60 m). Every animal model was fed with standard pellet and water was provided ad libitum with the husk replaced every three days. All animal models were routinely inspected and observed regarding their food consumption and fecal characteristics. Expected and unexpected adverse event were recorded to identify the deficiencies in procedures or study design,
The sample in each group was randomly chosen by giving each trial animal a tag number. Following that, the researcher randomly chose the tag numbers. They were divided into 6 groups, including control group receiving treatment for 2 months (C2), control group receiving treatment for 3 months (C3), and control group receiving treatment for 4 months (C4). Treatment group, receiving treatment for 2 months (T2), receiving treatment for 3 months (T3), and receiving treatment for 4 months (T4). Rats in treatment groups were injected with 1 mL/Kg body weight (BW) conditioned medium of UCMSCs 5 times with interval a week after creating cartilage defect. However, cartilage defect was created by surgery on the medial condyle area, followed by the destruction of cartilage through manual mechanical technique by drilling (750 rpm) using Kirschner Wire (D = 1.0 mm; h = 1.0 mm). There was no exclusion of animal during the experiment. The researchers also collaborate with veterinarian for group allocation in each stage of experiment, outcome assessment, and analysis.
Suffering and pain is minimized using appropriate anesthesia of 0.1 ml/10 grams body weight (BW) (Kepro, Netherlands), and xylazine 5 mg/kg BW (Xyla, Netherlands) were administered. General observations for signs of pain or suffering in the animal were conducted daily as needed. Moribund condition was used as a humane endpoint.10 However, because of the experiment using minimal invasive procedure and single treatment was used, there was no animal reached humane endpoint. Outcome measure was the histological changes of cartilage healing using O’driscoll scoring.11 Also the immunohistochemistry analysis for TGFβ-1, SOX-9, Aggrecan, and Collagen type II as a hallmark biomarker of cartilage regeneration. The results of this experiment are shown in previous study.3,12
This study has been approved by Ethical Clearance Commission of Gajah Mada University, Yogyakarta (Number: 263/KEC-LPPT/V/2015).
Stem cell culture was obtained from the umbilical cord of the Wistar rat (Rattus novergicus) aged 19 days washed in povidone-iodine 10% and normal saline in media transport (Dulbelco’s Eagle Modified Medium (DMEM) contained 200ug/ml penicillin, 200ug/ml streptomycin, dan 200u/ml fungizone). Then small pieces of the umbilical cord (around 20mm3) were dissolved with 0.25% EDTA collagenase and trypsin enzyme, then incubated for 30 minutes at 37 °C; added 2cc complete medium DMEM one time, 10% fetal bovine serum (FBS), 50ug / ml penicillin-streptomycin, and 2.5ug/ml fungizone), and then centrifuged at a speed of 3000 rpm for 10 minutes at 4 °C; then incubated again at 37 °C and 5% CO2, after 24 hours changed the medium. The culture was continued until approximately 80% of the confluence was achieved utilizing of the last suspension of each passage spread on the flask and cultured in an incubator, the medium was replaced every three days. This process was carried out consecutively until it reaches four passages. Morphologically, MSCs showed their spindle shape and fibroblast-like appearance using H&E and Giemsa staining. Cells exhibited a high nucleus to the cytoplasmic ratio on Giemsa staining. The adhered MSCs showed spindle-shaped morphology on H&E staining. The heterogeneity of the different MSC samples was demonstrated with Giemsa staining.
Mesenchymal stem cells that have reached 80% confluence were harvested using the warm trypsinization method. After the deneutralization of trypsin, the cell suspension was centrifuged at 3000 rpm for ten minutes; The supernatant was removed, the cell deposit is washed with PBS three times. Subsequent cell deposition was resuspended with a new medium with a concentration of 10,000 cells/ml. Subsequently, validated mesenchymal stem cells placed on culture plates were added with 10 ml of complete medium without serum or under fasting state with 5% CO2 levels during 48 hours and thereafter resupplied with serum FBS again. After fasting state, UCMSCs observed were in the normal-looking cell morphology, CM-UCMSCs produced metabolites or secretome in the secreted conditioned media and were harvested then processed to analyze.
The conditioned medium was analyzed by a mass spectrometer (MS). Protein samples were trypsin digested and peptides extracted according to standard techniques. Peptides were analyzed using Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS PAGE) then continue electrospray ionization MS. In SDS-PAGE, the use of sodium dodecyl sulfate (SDS, also known as sodium lauryl sulfate) and polyacrylamide gel largely separated solely based on polypeptide chain length and eliminates the influence of the structure and charge, and proteins. The conditioned medium was then collected, and cell residues were removed using centrifugation, then added sample buffer to samples, and mixed by flicking the tube. Heated the samples at 100 °C for three minutes in a heat block, then centrifuged at 15,000 rpm for one minute at 4 °C, and used the supernatant for SDS-PAGE.
Mass spectrometry using the Shimadzu Prominence nano HPLC system [Shimadzu] coupled to a 5600 TripleTOF mass spectrometer [Sciex]. Tryptic peptides were loaded onto an Agilent Zorbax 300SB-C18, 3.5 μm [Agilent Technologies] and separated with a linear gradient of water/acetonitrile/0.1% formic acid (v/v). Spectra were analyzed to identify proteins of interest using Mascot sequence matching software [Matrix Science] with MSPnr100 database by Proteomics International, Western Australia. And then protein found was analyzed using molecular docking or in silico model to visualized and tracked protein interactions with chondrogenesis markers used PyMol V.1 and webserver FireDock (http://bioinfo3d.cs.tau.ac.il/FireDock/), and the interaction between amino acid used LigPlot V.1. by Indonesian Bioinformatics and Biomolecular INBIO, Indonesia.
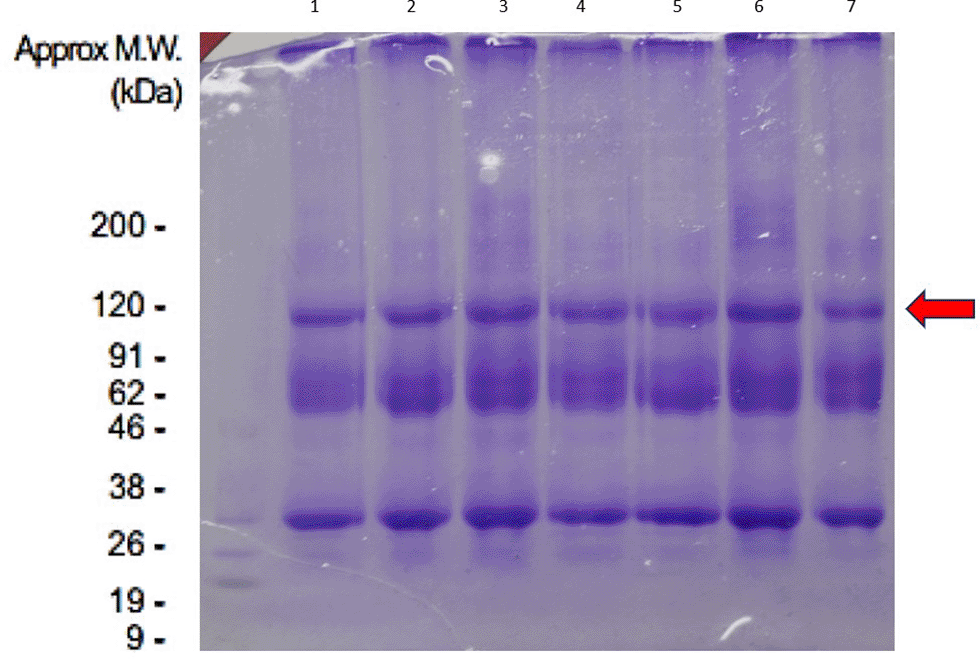
Peptides were analysed using SDS PAGE in eight tubes. The following results were obtained in Figure 1. Furthermore, the SDS PAGE results found dominant bands performed MS using the Shimadzu Prominence nano HPLC system [Shimadzu] coupled to a 5600 TripleTOF mass spectrometer, and identified proteins of interest using Mascot sequence matching software [Matrix Science] with MSPnr100 database found protein suitability as in Figure 2.
Based on our previous studies, we found to increased significantly in TGF- β1, SOX9, Aggrecan, and Collagen Type II.2 Therefore, after found D4A9T1/Rnf111/Arkadia Protein we analyzed interaction among these proteins using molecular docking or in silico. The docking process is carried out to predict the interaction of two molecules. This prediction is useful for knowing the potential interaction among molecules. We found there were interaction among Rnf-111, TGF-β1R, and TGF-β1 (Figure 3). The bond between TGF-β1R and RNF-111 has an affinity value (-27.52) while the interaction between TGF-β1R and TGF-β1 has an affinity value (-26.33).
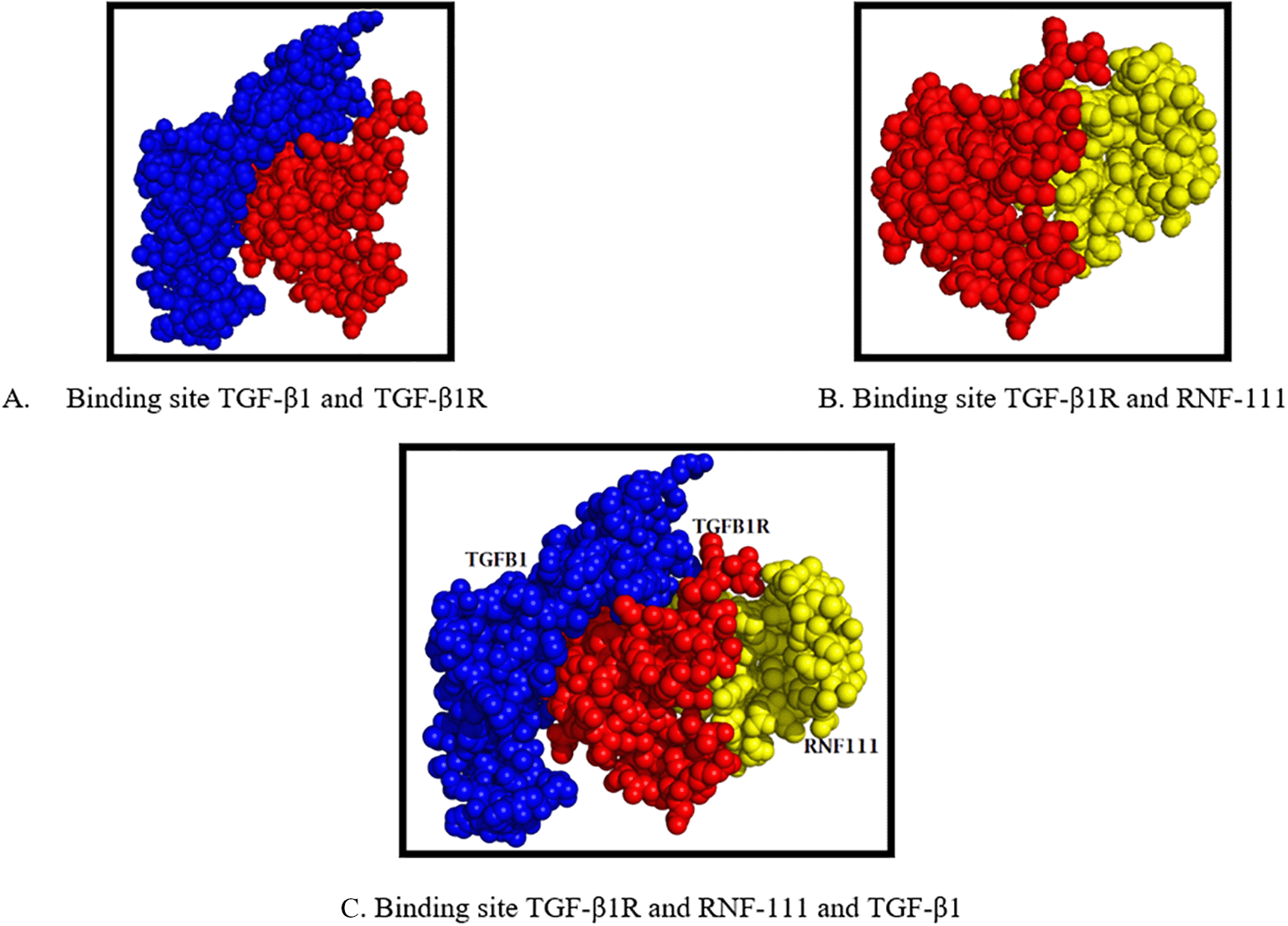
The bond between TGF-β1R and RNF-111 ligand is at a different site (Figure 3). The interaction of TGF-β1R and RNF-111 could trigger the process of chondrogenesis through the TGF-β1R pathways. This can be seen from the activation patterns that tend to be the same when viewed from binding amino acids were THR18 and ILE42 (Table 1).
In the present study, we found that one of the most dominant bands of CM-UCMSCs in the fasting state is Rnf-111. Rnf111 (ring finger 111) also known as Arkadia, is an E3 ubiquitin ligase degrade the negative TGF-b signal regulators, i.e. Smad7, c-Ski, and SnoN to amplify transforming growth factor (TGF)-b family signaling.13 TGF-β1 is one of the ligand proteins known to play a role in inducing the process of chondrogenesis.14 This is the basis for our hypothesis that the Arkadia/Rnf-111 protein suggests has a role in the process of chondrogenesis.
CM applied intraarticular knee of OA rats, it means this protein is in outside of chondrocytes, therefore, we assume this protein has an involvement in the process of chondrogenesis through the receptor-ligand pathway. From the result of in silico (Figure 3), the increasingly negative affinity value means that the higher the affinity which means the bond between the ligand and receptor is getting stronger. Based on these results, RNF-111 has a slightly higher activity than TGF-β1 in activating TGF-β1 receptors. The difference in binding position between TGF-β1 and RNF-111 on the TGF-β1R side shows that there is no competition in the activation process or there is no competitive binding, but rather the synergy process mechanism for activation affinity. Therefore, we suspect that RNF-111 as a part of CM-MSCs plays a synergistic role in the process of chondrogenesis through the TGF-β1R pathway.
Chen et al, shown that CM-MSCs had a satisfying effect on reducing the progression of OA in mice, by protecting microarchitecture from subchondral bones, balancing the MMP-13 and TIMP-1 ratios in cartilage, and promoting autophagy.15 CM-MSCs decreased matrix metalloproteinase (MMP) activity in cell supernatants as well as the levels of MMP-3 and MMP-13 proteins and mRNA while enhanced type II collagen expression in OA chondrocytes stimulated with IL-1𝛽.16 Another study, found that CM produced by MSC possess a well therapeutic effect on inflammatory chondrocyte, and the 10-fold concentrated MSC-conditioned medium could down-regulated chondrocyte synthesis inflammation-associated, and free-radical-related genes, such as TNF-α, IL-1β, IL-6, and iNOS even treated for 72 h OA.17 More specific research, found the exosomes from Embryonic MSCs has an effect on OA by balancing the synthesis and degradation of chondrocyte extracellular matrix (ECM), which in turn provides a new target for OA drug and potential drug-delivery system development.
Although TGFβ1 has been widely known to play a role in the process of chondrogenesis, in animal studies of mice and rabbits, there are reported side effects of growth factor-based therapy, such as supplementation of TGF-β1 which can trigger synovial proliferation and fibrosis, an attraction in pro-inflammatory lymphocytes, and induce the formation of osteophytes.18 This is the answer to why single growth factor supplementation is not recommended to treat articular abnormalities.19 We suspect those components and the environmental factors in CM-MSCs as whole support, especially in our study, to induce the process of chondrogenesis and repairing process of the cartilage defect completely.
A limitation of the study is that we did not analyze the concentration of every protein in CM-UCMSCs using immunoassay such as ELISA, and we cannot provide data of interaction with every single protein with the process of chondrogenesis. A further important weakness of this study is that we did not do the empirical methods (in knock out models), to verify the result of in silico analysis.
The conditioning medium has been demonstrated could promote MSCs secreting soluble protein in culture media. Using SDS-PAGE combined Mass Spectrometry we found the dominant protein in our CM-UCMSCs is Arkadia/RNF-111 protein. From in silico methods, we conclude that this protein as a dominant protein of CM-UCMSCs. However, we need further research to find out the important role of Arkadia/RNF-111 protein in the process of chondrogenesis through the TGF-β1R pathway and all of the secreted products in CM-UCMSCs, using knock out models to be feasible and safe for clinical application of CM-UCMSCs in cartilage repair. Then specific dose, duration, and technique of administration should be an attention in the future.
Bintang Soetjahjo: Conception and design, financial support, administrative support, provision of study material, collection and assembly of data, manuscript writing
Denny A: Data analysis and interpretation, manuscript writing, final approval of manuscript
Wibi Riawan: Data analysis and interpretation, manuscript writing, final approval of manuscript
Dryad. ARRIVE checklist and flowchart. DOI: https://doi.org/10.5061/dryad.zw3r228b6
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Stem cell research, particularly Mesenchymal Stem cells
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
No
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Mesenchymal stem cells and Immunomodulation
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Meniscus, Cartilage, Regenerative Medicine, Osteoarthritis, Animal models, Hypoxia, Mechanobiology.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: molecular biology, mass spectroscopy, intervertebral disc biology, stem cell biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 2 (revision) 06 Jun 24 |
read | read | ||
|
Version 1 23 Aug 22 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)