Keywords
DBLS3233, inflammatory marker, GLUT1, GLUT2, diabetes
This article is included in the Cell & Molecular Biology gateway.
DBLS3233, inflammatory marker, GLUT1, GLUT2, diabetes
Along with the establishment of more advanced molecular sciences, brand new approaches to managing diabetes mellitus (DM) are anticipated worldwide, especially in overcoming long-term complications. Kidney involvement in DM leads to one of the most common complications. Induced by many factors, inflammation is now known as one of the prime pathogeneses. Previous studies have shown that levels of inflammatory markers can predict the occurrence of DM and its microvascular complications, such as diabetic kidney disease (DKD).1 The inflammation pathways vary from oxidative stress, NF-κβ, janus kinase/signal transducers and activators of transcription (JAK/STAT) pathway, and inflammatory cytokines.1 Overexpression of these markers can lead to vascular dysfunction. NF-κβ, an apoptotic gene, will induce endothelial cell calcification and damage.2 In addition, one of the pro-inflammatory cytokines, TNF-α, is produced by mesangial, endothelial, glomerular, dendritic, and tubular cells in the kidney and majorly induces cytotoxicity, apoptosis, and necrosis. It was explained in a previous study that patients with diabetes mellitus had three to four times higher TNF-α levels compared to healthy adults.3
In addition to the inflammation control, improving insulin resistance is another main focus in preventing complications in the kidneys due to a prolonged hyperglycemic state. This is supported by the statement that insulin resistance is associated with microalbuminuria. One of the causes of albuminuria is the disruption of podocytes. Podocytes are insulin-sensitive and express glucose transporters, namely GLUT1, GLUT2, GLUT3, GLUT4, and GLUT8.4 GLUTs that are widely discussed are GLUT1 and GLUT4.4,5 GLUT4 is the main insulin-induced glucose transporter, whereas GLUT1 acts under basal conditions. Meanwhile, a less-discussed transporter, GLUT2, was also previously known to aid glucose uptake in cultured mouse podocytes.6 Understanding the insulin-signalling process and glucose uptake in podocytes through these GLUTs, especially the far less studied ones, can address new perspectives for novel drug development.6
DLBS3233, an Indonesian herbal product, which is a combined bioactive fraction of Cinnamomum burmanii and Lagerstroaemia speciosa, has demonstrated benefits in glucose control and upregulation of insulin signal transduction.7–12 Studies on Swiss Albino 3T3 pre-adipocytes mice showed that DLBS3233 works by increasing the expression of PI3-kinase, Akt, GLUT-4, peroxisome proliferators-activator receptor (PPAR)-γ, and PPAR-δ, and decreased the expression of the resistin gene at the mRNA level.12 Previous in vitro studies also reported that DLBS3233 increased GLUT-4 expression leading to increased glucose uptake in insulin-resistant 3T3-Swiss Albino adipocytes.13 Administration of DLBS3233 showed a decrease in HbA1c, fasting blood sugar and blood sugar one hour after fasting, as well as improved insulin resistance, adiponectin levels, low-density lipid (LDL), total cholesterol, and triglycerides.12 Following this discovery, further research is required to determine other beneficial effects of this agent, especially in preventing long-term complications in DM. In the present study, we observed in vitro effect of DLBS3233 in diminishing inflammatory markers, specifically TNF-α and NF-κβ, as well as in increasing glucose transporters, namely GLUT1 and GLUT2.
A total of 30 adult male Wistar rats (8-10 weeks old, 200-250 g) as experimental animals were obtained from D’Wistar (Bandung, Indonesia). All experimental procedures involving animals were carried out in accordance with the guidelines from the National Health Institute on the care and use of laboratory animals to ameliorate any suffering for the animals. All animal procedures were approved by the Animal Ethics Committee of Universitas Brawijaya, Malang, Indonesia (381/EC/KEPK-S3/2021). Animals were individually housed according to the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and maintained at a constant temperature of 25°C with a 12-hour light/dark cycle.
The animals were given free access to water. They had been acclimatized for two weeks before treatment. The rats were randomly divided into six groups (n=5 per group): non-diabetic rat group (negative control) with regular chow; five groups of high-fat diets (HFD) with a total calorific value 40 kJ/kg for four weeks, continued to receive low-dose (20 mg/kk BW) of streptozotocin (STZ) (Sigma – Aldrich cat#S0130) twice a week for three weeks by intraperitoneal injection and maintained on HFD (diabetic model groups). HFD can induce insulin resistance in rats and the multiple low-dose injection of STZ can induce the development of DM. Group 1 was the negative control, group 2 was the positive control (diabetic rats without treatment), and group 3 treated with DLBS3233 4.5 mg/kg body weight (BW)/day, group 4 with 9 mg/kg BW/day, group 5 treated with 18 mg/kg BW/day, and group 6 treated with pioglitazone 10 mg/kg BW/day for another two weeks. The rats were fasted for six to eight hours before they were given STZ. On the first day the blood sugar target is reached, the rats were given therapeutic agents according to the treatment group, while the rats in control groups received saline solution.
Two weeks after treatment, the rats were anesthetized before open-diaphragm and abdominal surgery, cardiac puncture for blood withdrawal, as well as pancreas and kidney removal for subsequent biochemical and histological analysis.
The kidneys were fixed in 10% buffered formalin, embedded in formalin, cut into 3-mm sections and stained with hematoxylin and eosin. The sections were viewed with a light microscope under 400x magnification. Glomerular area (GA) was assessed with image J 1.48 software. Sections containing forty glomeruli were photographed.
Three-mm tissue sections fixed in paraffin underwent xylene deparaffinization and ethanol dehydration. To inhibit endogenous peroxidase activity, the slices were treated with 4% hydrogen peroxide for 15 minutes at room temperature (RT). By soaking slides in 3% FBS + 0.25 percent Triton X-100, non-specific antibody binding was prevented (1 hr at RT). The sections were stained for 90 minutes at room temperature with rabbit NF-kBp65 antibody (Santacruz Biotech. cat: sc-8008), Glut-1 antibody (Santacruz Biotech. cat: sc-377228), Glut2 antibody (Santacruz Biotech. cat: sc-518022), and TNF-α antibody (Santacruz Biotech. cat: sc-130349). Slides were pre-incubated with biotinylated secondary antibody (one hour at room temperature) before being detected with diaminobenzidine (DAB) and counterstained with hematoxylin (40 min at RT). Slides were mounted and looked at with an Olympus light microscope, magnified 400 times. Sections containing ten glomeruli were observed randomly. The total number of DAB staining area was calculated and divided by 10 to get the average of each glomerulus per sample observed.
Immunohistochemistry (IHC) calculations were carried out referring to Soini et al., and Pizime and Cor, 2003, modified regarding the podocytes with TNF-α and NF-κβ expression, and pancreatic cells with GLUT1 and GLUT2 expression on each slide, which were counted at 20× magnification. Field of view was observed with a 1000× microscope magnification. Then, the average per field of view is taken. The Statistical Package for Social Science (SPSS) 24.0 version (IBM corporation, Illinois, Chicago, United State) software was used in this study to analyze the data. To compare the significant differences between the groups in the expressions GLUT1, GLUT2, TNF-α and NF-κβ ANOVA test was employed (p<0.05).
Quantitative IHC comparison between intervention groups is presented in Table 1.
| Groups | Mean±SD† | |||
|---|---|---|---|---|
| NfkBp65 | TNFα | Glut-1 | Glut-2 | |
| Group-1 | 3.00±1.58 | 5.80±1.92 | 5.20±2.78 | 8.60±1.14 |
| Group-2 | 10.60±1.67 | 11.40±2.07 | 2.60±1.52 | 2.60±1.52 |
| Group-3 | 7.60±1.34 | 9.40±1.14 | 10.00±1.58 | 11.40±2.51 |
| Group-4 | 3.20±0.84 | 5.00±1.58 | 10.40±2.30 | 12.60±2.41 |
| Group-5 | 3.80±1.48 | 4.60±1.67 | 10.60±2.61 | 12.40±1.82 |
| Group-6 | 4.00±1.58 | 2.80±1.48 | 11.40±2.07 | 15.00±2.35 |
| p-value ANOVA test | 0.000 | 0.000 | 0.000 | 0.000 |
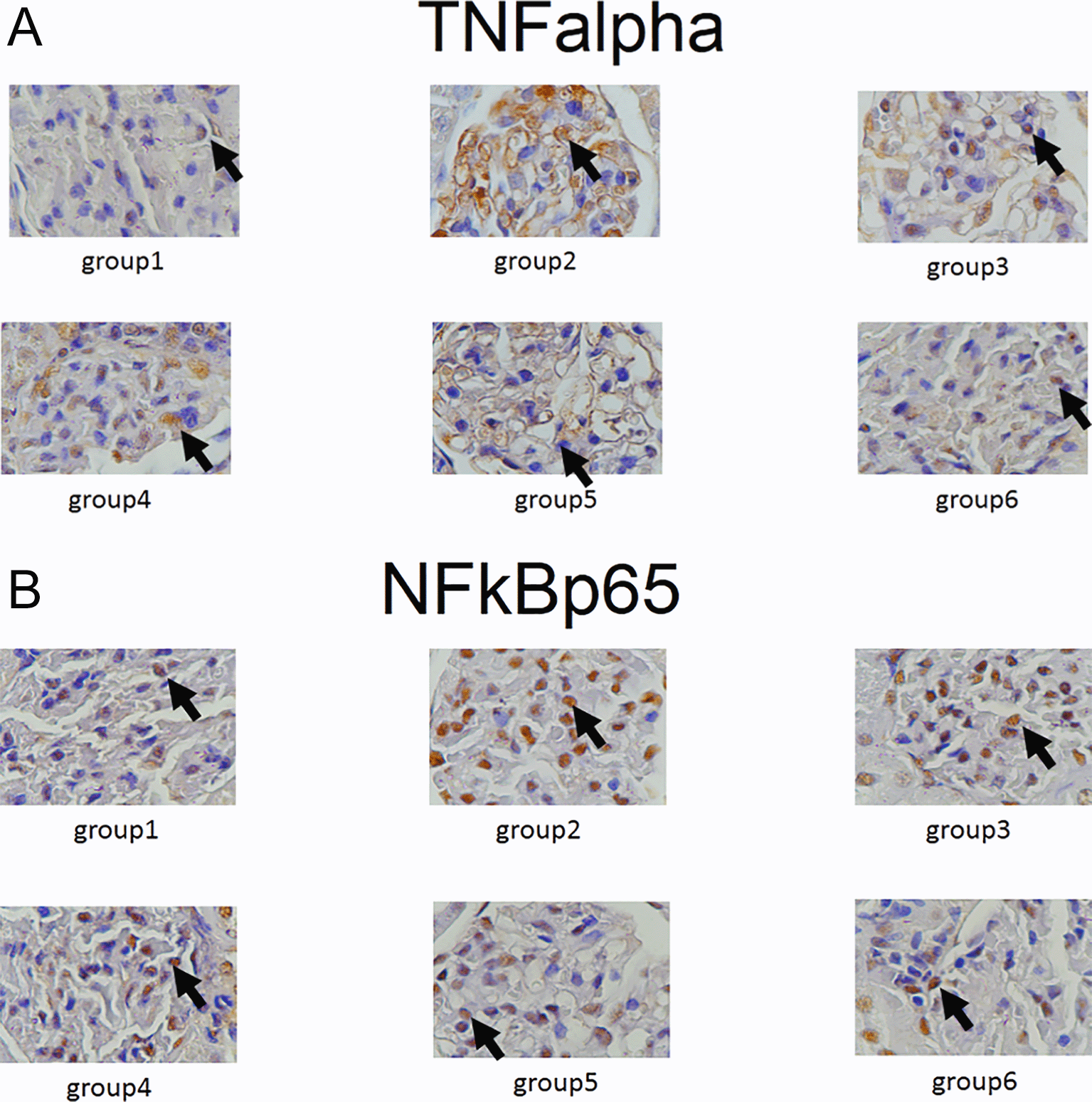
Expression of GLUT1, GLUT2, TNF-α and NF-κβ in IHC can been seen in Figures 1–4. Figures 1 and 2 show enhanced expression of pancreas GLUT1 and GLUT 2 in experiment groups 3-6, while Figures 3 and 4 display diminished expression of renal TNF-α and NF-κβ.
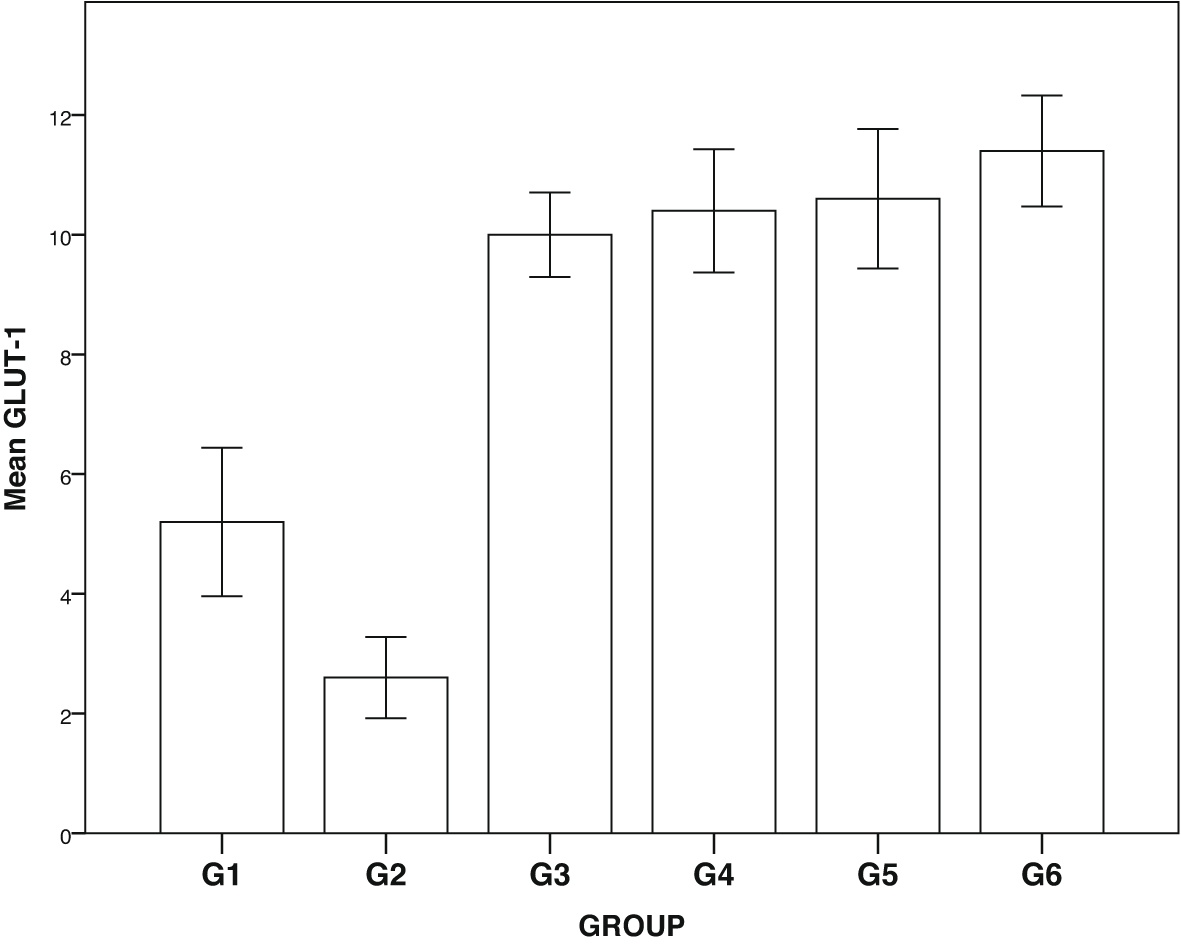
Group 1, negative control; group 2, positive control (diabetic rats without treatment); group 3, treated with DLBS3233 4.5 mg/kg BW/day; group 4, treated with DLBS3233 9 mg/kg BW/day; group 5, treated with DLBS3233 18 mg/kg BW/day; group 6, treated with pioglitazone 10 mg/kg BW/day.
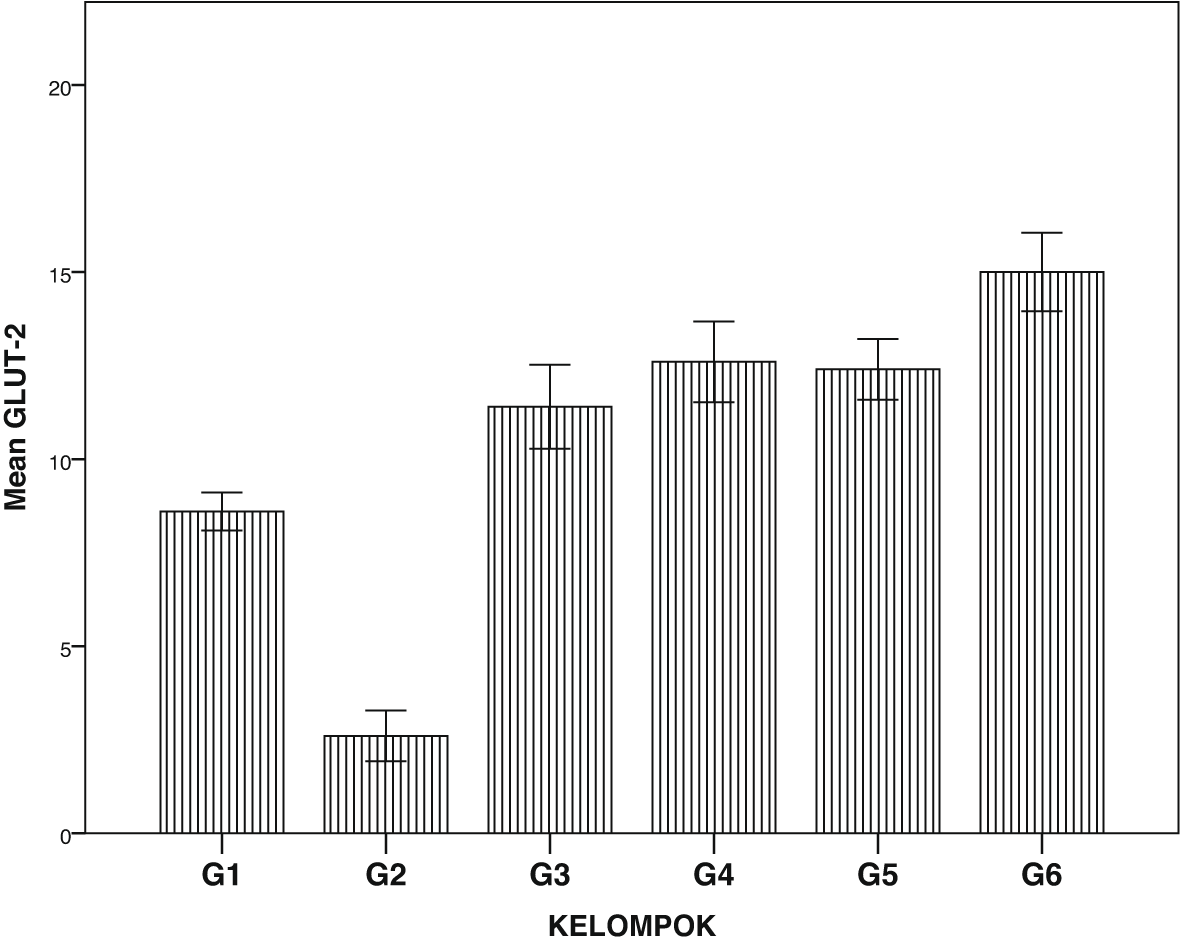
Group 1, negative control; group 2, positive control (diabetic rats without treatment); group 3, treated with DLBS3233 4.5 mg/kg BW/day; group 4, treated with DLBS3233 9 mg/kg BW/day; group 5, treated with DLBS3233 18 mg/kg BW/day; group 6, treated with pioglitazone 10 mg/kg BW/day.
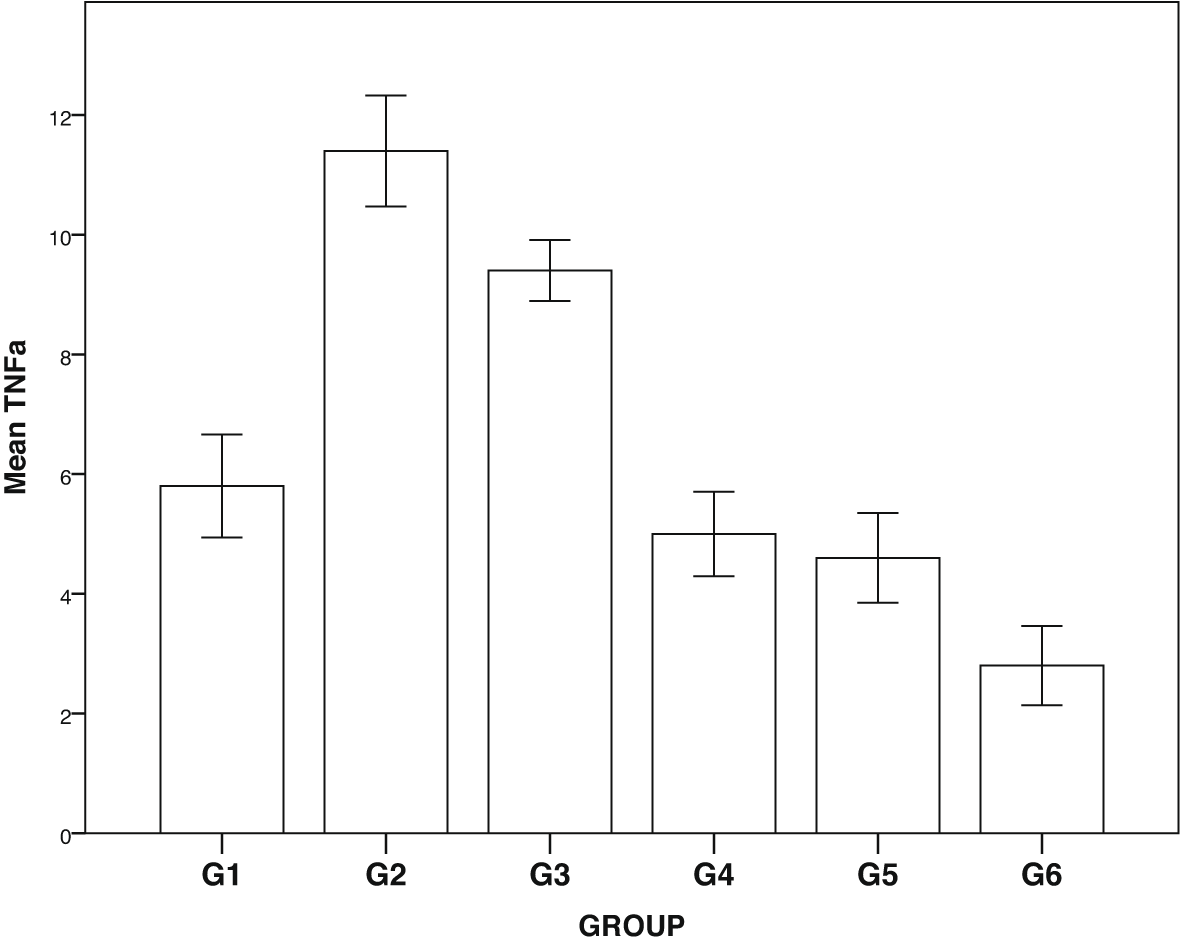
Group 1, negative control; group 2, positive control (diabetic rats without treatment); group 3, treated with DLBS3233 4.5 mg/kg BW/day; group 4, treated with DLBS3233 9 mg/kg BW/day; group 5, treated with DLBS3233 18 mg/kg BW/day; group 6, treated with pioglitazone 10 mg/kg BW/day.
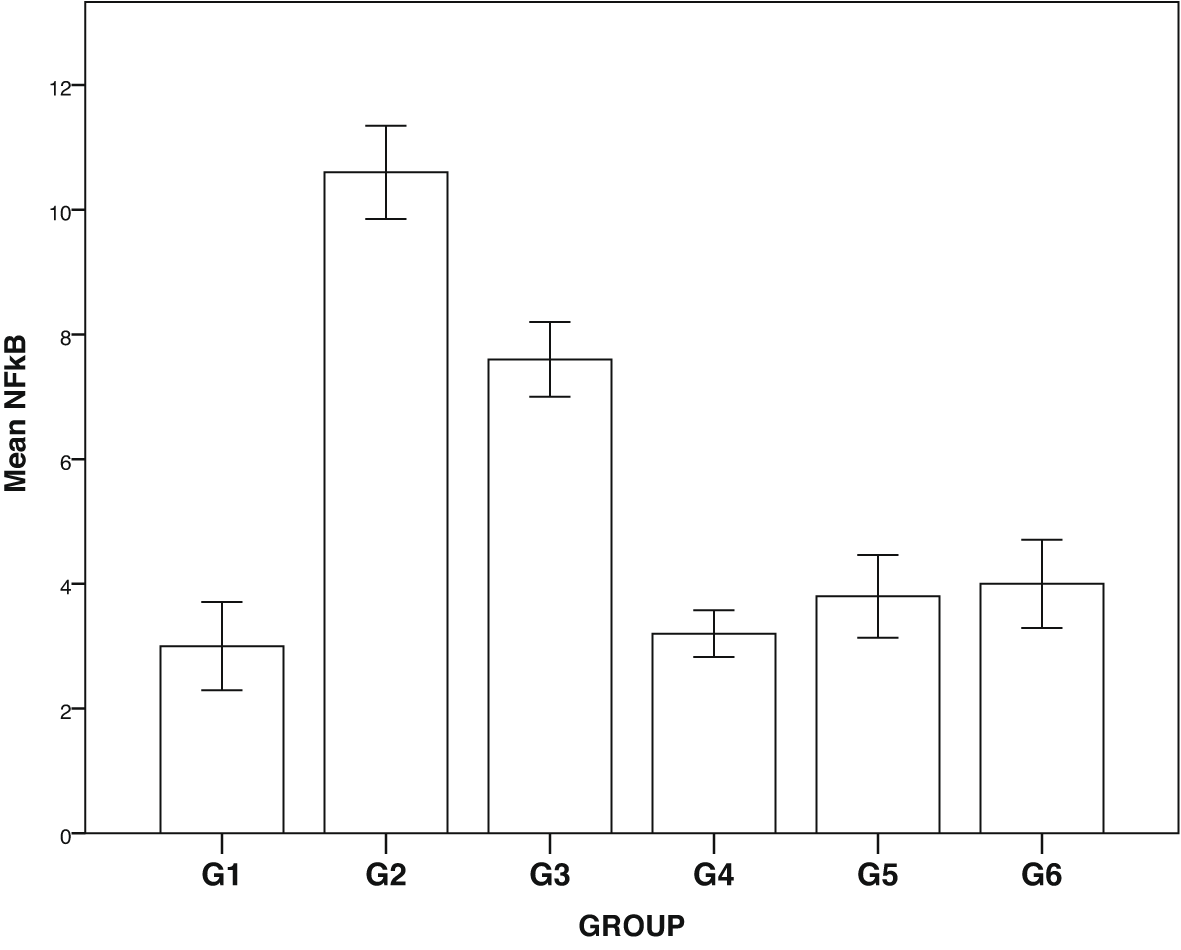
Group 1, negative control; group 2, positive control (diabetic rats without treatment); group 3, treated with DLBS3233 4.5 mg/kg BW/day; group 4, treated with DLBS3233 9 mg/kg BW/day; group 5, treated with DLBS3233 18 mg/kg BW/day; group 6, treated with pioglitazone 10 mg/kg BW/day.
The IHC analysis of GLUT1 and GLUT2 showed that group 2 experienced a decrease in expression compared to group 1, while GLUT 1 and GLUT2 increased in the treatment group (groups 3, 4, 5 and 6) compared to group 2 (p<0.05) (see Figure 5). Among six groups of intervention, group 2 showed a higher expression of TNF-α and NF-κβ compared to control (p<0.05). Moreover, TNF-αand NF-κβ significantly decreased in the treatment groups (groups 3, 4, 5, and 6) compared to group 2 (p<0.05) (see Figure 6).
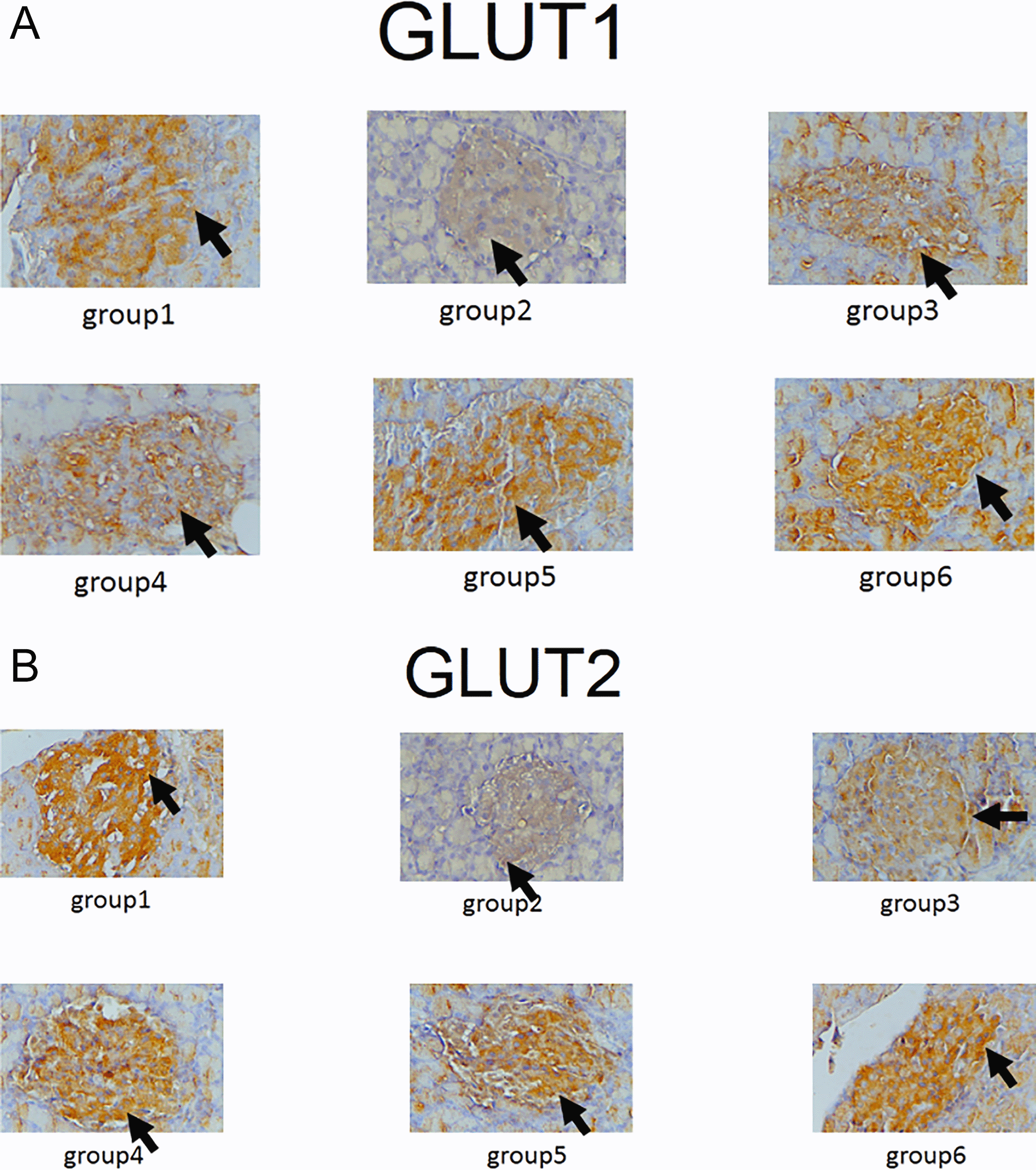
Group 1, negative control, Group 2, positive control (diabetic rats without treatment), Group 3, treated with DLBS3233 4.5 mg/kg BW/day, Group 4, treated with DLBS3233 9 mg/kg BW/day, Group 5, treated with DLBS3233 18 mg/kg BW/day, Group 6, treated with pioglitazone 10 mg/kg BW/day.
Group 1, negative control, Group 2, positive control (diabetic rats without treatment), Group 3, treated with DLBS3233 4.5 mg/kg BW/day, Group 4, treated with DLBS3233 9 mg/kg BW/day, Group 5, treated with DLBS3233 18 mg/kg BW/day, Group 6, treated with pioglitazone 10 mg/kg BW/day.
DLBS3233 is an Indonesian herbal product whose antidiabetic effect has been explored over the last 10 years. Preclinical studies in Switzerland showed that, at the mRNA level, DLBS3233 increased the expression of the PI3-kinase gene, Akt, GLUT-4, PPAR-γ and PPARδ, and decreased the expression of the resistin gene. Additionally, this substance was discovered to boost GLUT4 synthesis and activate adiponectin transcription at the protein level, consequently enhancing glucose uptake.14,15 However, little research has been done on how DLBS3233 affects the kidneys.
DM is a metabolic disease with a systemic effect on the kidneys, through an inflammatory process that damages the microvasculature, which in turn induces podocyte damage, characterized by proteinuria.3 TNF-alpha increases in diabetic patients, triggering inflammation which later damages podocytes. The inflammation also results in an endothelial dysfunction through NF-kb, causing endothelial cell calcification.2,3 This endothelial cell calcification might lead to the surge of oxidative stress and fibrosis in the process of diabetic nephropathy.16 The decrease in TNF-alpha and NF-kb in diabetes certainly prevents microvascular damage and fibrosis in the kidneys.
GLUT are glucose transporters that help insulin in the uptake of glucose to cells. GLUT1 acts on basal membrane, whereas GLUT2 acts on glucose uptake in podocytes. In diabetic patients, the decrease in each transporter indicates the process of insulin resistance.4–6 DLBS3233 has been reported to increase GLUT4 synthesis which improves the glycemic profile of diabetic patients.12 Our study showed that in the diabetic group, GLUT1 and GLUT2 were significantly lowered compared to the normal group. Administration of DLBS3233, showed an increase in the expression of GLUT 1 and GLUT2 through IHC examination of cells in the pancreas.
Besides, we also examined IHC for the expression of TNF-α and NF-κβ in the kidneys. In the group of diabetic rats, TNF-α and NF-κβ increased; on the other hand, there was a significant decline of both inflammatory markers in the treatment group. DLBS3233, apart from improving sugar uptake, has also been proven to improve inflammation in the kidneys.
Our experiment was a preliminary study and was still limited to the expression of GLUT1 and GLUT2 in the pancreas, with TNF- and NF-κβ expression in the kidney. Further research of other effects of DLBS3233 in the kidneys is underway.
DLBS3233 administration stimulated the decline of inflammatory markers (TNF-alfa and NF-kb) in the kidneys as well as enhancing the glucose uptake through increasing pancreatic GLUT1 and GLUT2 expression.
Figshare: DLBS3233, https://doi.org/10.6084/m9.figshare.20080094.v417
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank Faculty of Medicine, Brawijaya University, Malang, East Java, Indonesia for giving us an opportunity to do this research.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Mohani CI, Rudijanto A, Aulanni'am A, Soeharto S: DLBS3233 enhances nephrin and podocin expression also reduces oxidative stress marker and insulin receptor serine diabetic rats' podocytes.Ann Med Surg (Lond). 2023; 85 (8): 3894-3900 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: internal medicine, type 2 diabetes, metabolic syndrome, hypertension, obesity, diabetic kidney disease, inflammation, frailty, nutrition
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 23 Aug 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)