Keywords
Saccharomyces cerevisiae INVSc1 protein expression, EGFP protein, protein transductions.
This article is included in the Cell & Molecular Biology gateway.
Green Fluorescent Protein (EGFP) is widely used as a reporter gene, aiding in protein recovery and transduction studies. In this study, EGFP was tagged with eleven arginine residues (PolyR) and six histidine residues (His-tag) for purification. The aim was to enhance the synthesis of EGFP-PolyR in Saccharomyces cerevisiae and evaluate the effects of polyarginine modification on protein stability and expression levels. The expression of EGFP and EGFP-PolyR in S. cerevisiae was assessed through fluorescence measurements and protein levels. Structural analyses were conducted using in silico tools to investigate changes in beta strands and helices, which were validated through Western blots. Results showed that EGFP-PolyR maintained similar fluorescence levels to EGFP, but with notable structural changes. EGFP-PolyR's final beta strand terminates at Ala228, compared to Gly229 in EGFP, affecting the beta sheet's stability. Structural modifications also included altered helix lengths, with a longer helix 10 and shorter helix 9 in EGFP-PolyR. These alterations, along with shifts in helix-helix interactions, contribute to destabilization. Additionally, EGFP-PolyR exhibited unique gamma coils absent in EGFP, further differentiating its structure. The structural changes led to decreased protein expression and solubility, as indicated by Western blot analysis, with EGFP-PolyR showing significantly lower expression levels. The findings suggest that EGFP-PolyR is prone to aggregation and misfolding, characteristics often associated with aggregation-prone proteins.In conclusion, the polyarginine modification significantly impacts the structural integrity, stability, and solubility of EGFP. While fluorescence is retained, these changes hinder protein detectability and purification, highlighting the importance of considering structural alterations when modifying reporter proteins for experimental use.
Saccharomyces cerevisiae INVSc1 protein expression, EGFP protein, protein transductions.
In this version, There are several changes and additions to the paper :
1. We have added Dr. Syahputra Wibowo as an author because he conducted the bioinformatics analysis for this revision.
2. There are several parts that we decided to change this paper, namely:
- We have changed the title of this manuscript to reflect the additional data we have included.
- Introduction part, we changed it according to the bioinformatics analysis results.
- In the methodology section, we have incorporated protein structure analysis and structural analysis, thus completing the bioinformatic analysis.
- In the results section, we have incorporated additional bioinformatic analysis images and comprehensive structural analysis tables for EGFP and EGFP-PolyR.
- In the discussion section, the text has been modified and augmented in accordance with the incorporation of bioinformatic analysis data.
- In conclusion, the conclusion was also modified in accordance with the alterations made to other sections of the manuscript.
- In the extended data section, the figure previously published on figshare has been updated, and a new link has been included in this paper.
- In the reference section, some references were deleted and new ones were added in accordance with the revisions made to this paper.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Enhanced green fluorescent protein (EGFP) has often been used as a reporter for protein transport, especially in eukaryotic cells such as mammalian and yeast cells. EGFP was derived from green fluorescent protein (GFP), a commonly used reporter protein that does not require a substrate for it to fluoresce. The EGFP gene is a mutated version of the GFP gene, with P64L and S65T mutations. These mutations cause a higher emission intensity in EGFP relative to that of the wild-type GFP, and better thermal stability of the EGFP protein, especially in eukaryotic cells.1 EGFP can be used as a marker in optimizing protein isolation procedures and their functions. The fluorescence makes it easier for us to observe the localization of the expression of the protein. The intensity of EGFP fluorescence can also indicate the level of expression of the protein in a system. EGFP can also be used to study protein transfer, such as analysis of the work of a protein transduction system for cell reprogramming purposes.
One of the uses of cell reprogramming is manufacturing induced pluripotent stem cells. Induced pluripotent stem cells are expected to be used as therapeutic cells for patients. Cell reprogramming can be done using protein transduction, viral transduction, or chemicals. Cell reprogramming by viral transduction can activate oncogenic genes, whereas chemical methods are highly unspecific and cannot be interpreted clearly.2,3 On the other hand, protein transduction is reputed as a harmless method to deliver protein precisely. However, protein transduction has its disadvantages, such as low transduction efficiency in host cells. Therefore, research is needed to increase transduction proteins by modifying the target protein.
Polyarginines are short polypeptides classified as non-amphipathic cell-penetrating peptides (NaCPP) and have cationic characteristics, including binding anionic lipid membranes; therefore, polyarginine can enter through the plasma membrane of eukaryotic cells. As a result, the NaCPP class is less toxic than the primary amphipathic and secondary cell-penetrating peptide (CPP). The NaCPP class uses endocytosis mechanisms with low concentrations for cellular uptake.4 The amount of added arginine amino acid residues affect the cellular uptake’s efficiency level, whereas more arginine residues have a better efficiency level. Adding more than 15 amino acid residues decreases intracellular delivery efficiency.5 Proteins fused to polyarginine are more efficiently transduced into human iPS cells compared to those without the addition of CPP.6
In this research, we designed and constructed two proteins, EGFP and EGFP-PolyR. EGFP-PolyR is an EGFP protein with the addition of eleven arginine residues (polyarginine – polyR) at the C terminal of EGFP to be used as a transduction protein model for cell reprogramming purposes. The gene encoding EGFP and EGFP-PolyR was synthetically constructed and expressed in Saccharomyces cerevisiae INVSc1. The study aimed to investigate the pattern of EGFP-PolyR protein expression in S. cerevisiae INVSc1 compared to EGFP. This paper aims to contribute to the current understanding of structural modifications when optimizing reporter proteins for experimental applications.
The gene encoding EGFP (GenBank Accession #U55762) and EGFP- PolyR (was synthetically made by IDT Custom Gene Synthesis cloned in a plasmid called pEGFP- PolyR, in such a way that it allowed sub-cloning of the gene with and without the polyR sequence, by using a different combination of restriction enzymes. In addition to the 11× arginine residues (polyR), codons encoding 6× histidine residues (6×His-tag) were also added downstream to the polyR codons. The synthetic gene was sub-cloned into pYES2CT, a Saccharomyces cerevisiae expression plasmid, during which the polyarginine codon either remained fused to the EGFP gene or was removed, creating recombinant pYES2CT pYES2CT-EGFP-PolyR or pYES2CT- EGFP, respectively. Since the pYES2CT plasmid has its own 6×His-tag downstream to its Multiple Cloning Sites (MCS), positioned in-frame with the EGFP and EGFP-PolyR codons, the expressed EGFP was expected to have one 6×His-tag, whereas the EGFP-PolyR were expected to have two.
The intended digested fragments sized 821 bp (EGFP-PolyR) and 733 bp (EGFP), were gel-purified using GenepHlow Gel/PCR kit (DFH004) (GeneAid, Taiwan) with 20 μL elution buffer. The DNA concentration was measured using a BioDrop-Duo UV/Vis spectrophotometer (Thomas Scientific, United States). Then, the DNA was stored at -20°C. Ligation was carried out following the T4 DNA ligase procedure as described by the manufacturer (Promega, United States). The ligation was performed at 4°C overnight, with a plasmid-insert volume ratio of 1:10.
The transformation into Escherichia coli TOP10 was carried out following the method of Abyadeh et al. (2017).7 Recombinant E. coli cells were cultured in LB with 100 μg/mL ampicillin. Transformation by heat shock was carried out using the Scilogex SCI-100HCM-Pro Digital Thermal Mixer (United States) at 42°C for 90 seconds. The transformants were selected on LB agar with100 mg/mL ampicillin (LB Agar+Amp). Colony PCR was performed on selected transformants with pYES2CT-specific primers (Forward: 5′-AATATACCTCTATACTTTAACGTC-3′; Reverse 5′-GAGGGCGTGAATGTAAGCGTGAC-3′) to verify the presence of intended inserts. PCR was done using Biometra Tone Thermal Cyclers (Analytik Jena AG, Germany) and carried out following the procedure from MyTaq Red Mix (Bioline, UK) manufacturer, for 35 cycles, with predenaturation (95°C) for 1 minute, denaturation for 15 seconds, annealing (55°C) for 15 seconds, elongation (72°C) for 10 seconds, and post elongation for 5 minutes.
pYES2CT-EGFP- PolyR and pYES2CT-EGFP plasmids were isolated using ‘WizardPlus SV minipreps DNA purification kit (A1330) (Promega, United States)’ after being propagated in E. coli TOP10. Isolated plasmids were used to transform Saccharomyces cerevisiae INVSc1 with the lithium acetate (LiAc) method.8 Transformants were selected on minimal medium (MM) agar without uracil. Plasmids were isolated from transformant colonies using the MP Biomedicals yeast plasmid isolation kit (MP Biomedicals, United States, Cat. 112069400). PCR was carried out to confirm the success of the transformation using Biometra Tone Thermal Cyclers (Analytik Jena AG, Germany) and carried out following the procedure from MyTaq Red Mix (Bioline, UK) manufacturer, for 35 cycles, with an annealing temperature of 55°C. Colony PCR was performed on selected transformants with pYES2CT-specific primers (Forward: 5′-AATATACCTCTATACTTTAACGTC-3′; Reverse 5′-GAGGGCGTGAATGTAAGCGTGAC-3′), to verify the presence of intended inserts. PCR was done using Biometra Tone Thermal Cyclers (Analytik Jena AG, Germany) and carried out following the procedure from MyTaq Red Mix (Bioline, UK) manufacturer, for 35 cycles, with predenaturation (95°C) for 1 minute, denaturation for 15 seconds, annealing (55°C) for 15 seconds, elongation (72°C) for 10 seconds, and post elongation for 5 minutes.
S. cerevisiae’s fast-growing diploid strain INVSc1 (Invitrogen Corporation) was used as a host cell for protein expression. The yeasts were cultured in yeast-extract peptone dextrose (YEPD) medium. Recombinant cells of S. cerevisiae INVSc1 were cultured in a minimal medium (MM) without uracil. Protein expression followed the user guide of the pYES2CT plasmid (Invitrogen, 2003), with several modifications. A single colony of recombinant S. cerevisiae INVSc1 carrying pYES2CT-EGFP or pYES2CT-EGFP- PolyR was inoculated into 3 mL of MM medium; the culture was then incubated at 30°C 230 rpm for 48 hours. After that, a 1-mL culture was taken to measure its OD600 value. Culture with OD600 = 0.4 were subcultured into 100 mL of induction medium (MM medium containing 2% galactose and 1% raffinose). The cultures were further incubated after adding the induction medium for 48 to 72 hours. The culture was centrifuged at 5000 rpm for 10 minutes to harvest and stored in a freezer at -80 °C until further use.
A 1 mL 10× diluted culture was centrifuged at 5000 rpm for five minutes. The pellet was then fixed with 500 μL 3.7% formaldehyde for 30 minutes. The cell suspension was centrifuged at 5000 rpm for 5 minutes, and the supernatant was discarded. as the remaining pellet was resuspended in 1 mL PBS 1×. A 50-μL fixed-cell suspension was placed in a well of 96-well plates (Thermo Scientific) to visualize the EGFP fluorescence under an inverted fluorescent microscope (Zeiss Axiovert 40 CFL, UK), (40× magnification, λex 488 nm and λem 507 nm). Fluorescent intensity was measured using ImageJ software, and delta intensity was calculated as follows:
Intensityt is the fluorescent intensity at time t post-inoculation to induction medium
Intensity0 is the fluorescent intensity at time 0 post-inoculation to induction medium
All data were expressed as mean ± standard deviation. All statistical analyses were performed using SPSS software (IBM SPSS Statistics 24). The differences between groups were analyzed using one-way analysis of variance (ANOVA) after passed the homogeneity test and normality test, then proceeded to the Bonferroni post-hoc test. A p-value < 0.05 was considered as statistically significant.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out following the modified Iqbal and Ahmad (2016) method. The protein sample was mixed with 6× SDS gel loading buffer (600 mM Tris-Cl pH 6.8, 12% SDS, 30% glycerol (v/v), 0.03% bromophenol blue, and 20 mM DTT) and ran on 10 % SDS-PAGE gel at 100 V for 110 minutes until the bromophenol blue dye reached approximately 0.5 cm from the lower limit of the gel.9
Western blotting was also carried out following the method of Iqbal and Ahmad (2016), with several modifications. Protein was transferred from gel to membrane (transfer buffer: 300 mM glycine, 300 mM Tris, 0.05% SDS) using Pierce Power Blotter (Thermo Scientific, United States) set at 25 kV, 1.3 A for 25 minutes. Then, the membrane was washed six times with PBST (PBS, 1% Tween-20) before applying the primary antibody (Anti-6×His-Tag monoclonal antibody (Invitrogen, catalog number MA1-21315) in blocking buffer (5% skim milk (Merck, Germany) in PBST), with 1:3000 ratio). Then the membrane was incubated at 4°C overnight before applying the secondary antibody (Goat anti-mouse IgG HRP, Invitrogen, catalog number 31430 in blocking buffer, with a ratio of 1:20,000 ratio) for 60 minutes with shaking (70 rpm). Then, the substrate (SuperSignal West Femto Luminol, Thermo Scientific, United States) was used. The duration of the exposure was 20 minutes.
Protein structures were predicted using AlphaFold3,10 a deep learning-based tool for accurate protein structure prediction. The input sequence for each protein was provided to AlphaFold3, which was run using the default settings. The predictions were performed on a high-performance computing cluster to ensure computational efficiency and accuracy. The output from AlphaFold2 included a predicted structure with confidence scores, which were used to assess the reliability of the model.
Ramachandran Plot Analysis: The stereochemical quality of the AlphaFol3-predicted structures was assessed using the Ramachandran plot, which was generated using PROCHECK. PROCHECK is a widely used tool for evaluating the geometry of protein structures, specifically analyzing the distribution of phi (φ) and psi (ψ) dihedral angles. The structural models generated by AlphaFold2 were analyzed using PROMOTIF, a tool available through the PDBSum web server.11 PROMOTIF was employed to examine key structural features and motifs within the predicted protein structures. This included the identification of secondary structure elements, analysis of protein-protein and protein-ligand interfaces, and evaluation of the overall geometry of the model.
After digests and ligation processes were successfully performed (Figure 1 and Figure 2A), the ligation products, hereby called pYES2CT-EGFP and pYES2CT-EGFP-PolyR, for plasmid carrying egfp and egfp-poly R respectively, were propagated in E. coli TOP10. Plasmids isolated from this E.coli TOP10 that had been confirmed to have the correct size ( Figure 2B) and carried the genes ( Figure 2C) were then used to transform S. cerevisiae INVSc1. Screening of S. cerevisiae INVSc1 transformants was performed by PCR of purified plasmids. PCR results of positive colonies are presented in Figures 2D and 3.

Insertion of EGFP-PolyR gene was performed using the enzymes BamH1 (position 519) and Not1 (position 577) (A), whereas EGFP gene was inserted into pYES2CT plasmid using BamHI and EcoRI, thus leaving the PolyR fragment out (B).
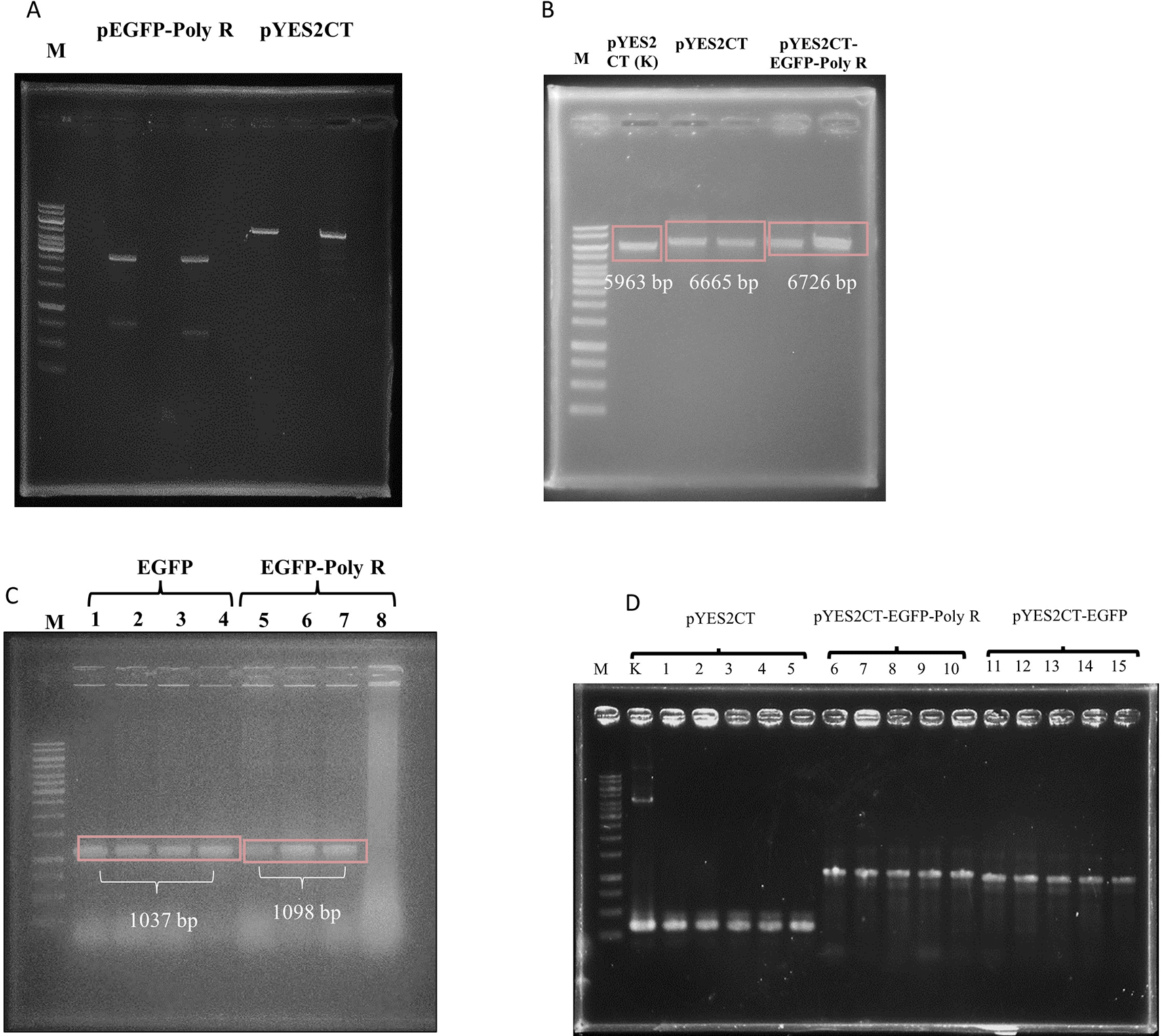
pEGFP-Poly R (lane 5) and pYES2CT (lane 7) were cut with restriction enzymes EcoRI and BamHI, where pEGFP-Poly R produced Egfp gene (733 bp band), and pYES2CT produced vector (5905 bp band) (A). The 822 and 733 bp DNA were isolated from gel, and ligated to the 5932 and 5905 bp bands, respectively. Single digest (BamHI) of plasmids isolated from E.coli TOP10 transformed with pYES2CT-EGFP (lane 3 and 4) and pYES2CT-EGFP-Poly R (lane 5 and 6), in comparison to wild type pYES2CT (lane 2), showed that the plasmid sizes were correct (B). Also, colony PCR of E.coli confirmed the presence of the intended genes as 1037bp and 1098bp bands, representing the Egfp and Egfp-polyR , respectively (C). Transformed yeast colony screening was performed by PCR of plasmids purified from the yeast, using pYES2CT-specific primers. As a control, pYES2CT plasmid was used (lane K). Lanes 1-15 showed PCR amplification results of plasmids isolated from S. cerevisiae culture, transformed with pYES2CT (lanes 1-5), pYES2CT-EGFP-Poly R (lanes 6-10), and pYES2CT-EGFP (lanes 11-15). Amplification of pYES2CT without insert (lane 1-5) shows the same size amplicons as a positive control (K), which are 334 bp, amplification of pYES2CT-EGFP-Poly R produced amplicons of 1097 bp (lane 6-10), while the pYES2CT-EGFP produced amplicons of 1036 bp (lane 11-15) (D). Lane M is GeneRuler 1kb DNA Ladder (Thermo Scientific SM0312).
The growth curve showed that post-induction S. cerevisiae INVSc1 stayed longer at the exponential phase when not carrying any plasmid in comparison to the same strain with pYES2CT plasmids ( Figure 4). The plasmid-carrying yeasts seemed to reach stationary phase 24 hours after inoculation to induction medium ( Figure 4B and C), whereas the wild-type culture stayed at exponential phase for up to 64 hours ( Figure 4A) before slowing down its growth. There is a significant decrease of the growth curve in S. cerevisiae INVSc1 carrying the plasmids EGFP and EGFP-PolyR, compared to the wildtype. The significant decrease was seen from 48 – 72 hours ( Figure 4D).

Optical densities were measured at λ=660nm, 48 hours, and 72 hours post-inoculation to induction medium of the wild-type and recombinant S. cerevisiae (D). The result reported are mean of four replicates while the error bar indicates its standard deviation. Mean data accompanied by different letter in each time point are significantly different (Bonferroni test, p < 0.05).
The fluorescence produced post-inoculation to induction medium by each recombinant yeast and wild-type yeast is presented in Figure 5A. The Δintensity was calculated and presented as a graph in Figure 5B. There was a significant difference between wildtype fluresens expression with recombinant yeast carrying pYES2CT EGFP and pYES2CT EGFP PolyR-plasmid on 48 hours and 72 hours. The addition of pYESC2 EGFP and pYES2CT EGFP PolyR plasmids significantly inhibited the proliferation of S. cerevisiae starting at 48 hours.
Western blotting using anti-his-tag mouse monoclonal antibody (primary antibody) and Goat anti-mouse IgG HRP (secondary antibody) proved that the EGFP-PolyR and EGFP proteins were expressed in S. cerevisiae INVSc1 at similar ( Figure 6).
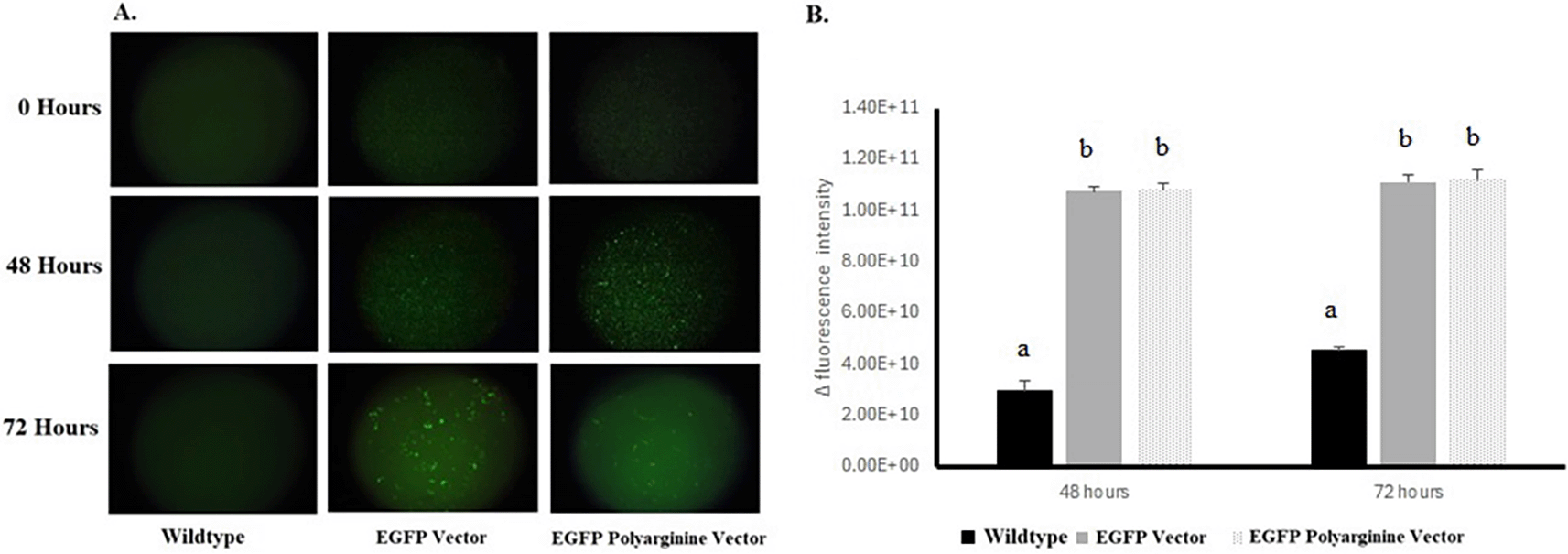
The result reported are mean of six replicates while the error bar indicates its standard deviation. Mean data accompanied by different letter in each time point are significantly different (Bonferroni test, p < 0.05).
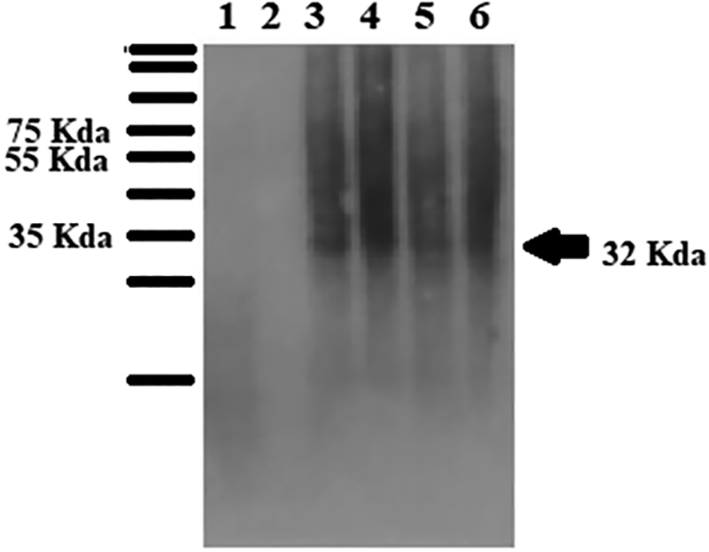
The Western blot was performed using an anti-his-tag antibody as the primary antibody.
The beta strand structures of EGFP-Poly R and EGFP exhibit a critical difference in the length of the final beta strand. In EGFP-Poly R, the last beta strand ends at Ala228, whereas in EGFP, it extends one residue further to Gly229. This difference could impact the structural stability of the beta sheet, as the absence of the glycine residue in EGFP-Poly R might reduce the flexibility and stability of this structural element. In addition to differences in beta strands, there are notable variations in the helical structures of EGFP and EGFP-Poly R. EGFP features a total of 11 helices, including both G and H types, while EGFP-Poly R has only 10 helices, missing one compared to EGFP (Table 1 and Table 2). This reduction in helices could significantly affect the overall stability and integrity of the protein structure, as helices play a crucial role in maintaining protein stability. Notably, helix 10 in EGFP spans from Leu251 to Ser253, comprising 3 residues, whereas in EGFP-Poly R, it extends to 5 residues, from Pro265 to Leu269 (Figure 7 and Figure 8). This extension may reflect a structural adjustment due to the additional arginine residues in the Poly R sequence. Conversely, helix 9 in EGFP-Poly R, spanning from Pro245 to Trp248, shows decreased length and stability with a lower pitch and rise per residue compared to EGFP, suggesting potential instability. Both EGFP and EGFP-Poly R deviate from ideal helical parameters, but EGFP-Poly R exhibits slightly greater deviations in helix 9, indicating possible structural strain from the Poly R modification. The extension of helix 10 by two additional residues in EGFP-Poly R might be a direct consequence of this modification, altering the local structural environment. These differences in the beta strands and helices between EGFP and EGFP-Poly R likely contribute to the overall destabilization of the protein. The shortening of the final beta strand, combined with changes in helix lengths and stability, particularly the extension of helix 10 could lead to misfolding or reduced structural integrity of EGFP-Poly R (Figure 8). Additionally, the interactions between helices show some important differences. The helix-helix interactions between EGFP and EGFP-Poly R reveal subtle yet significant differences that could impact the stability of the protein. Both proteins share similarities in certain interactions, such as those between helices A1 and A5, A1 and A6, A2 and A3, and A5 and A6, with nearly identical distances and angles. However, differences arise in the interactions between helices A5 and A8, and A8 and A9. In EGFP, the A5-A8 interaction has a distance of 10.6 Å with an angle of -102.9°, while in EGFP-Poly R, this distance shortens to 10.0 Å with an angle of -100.5°. More notably, the A8-A9 interaction shows a distance of 2.6 Å with an angle of -79.3° in EGFP, but in EGFP-Poly R, the distance increases to 3.2 Å with a significant angle shift to -94.9°, and an increase in the number of interacting residues from 4 to 6. These differences suggest altered packing and potential destabilization of the protein structure in EGFP-Poly R. The changes in helix-helix interaction, particularly between A8 and A9, could contribute to a less stable conformation in EGFP-Poly R.
The comparison of beta turns between EGFP and EGFP-Poly R reveals several unique structural differences that highlight how modifications in the sequence can influence the protein's conformation. One notable difference is observed in the Leu65-Gly68 turn. EGFP exhibits a phi angle of -89.7° and a psi angle of -39.1°, while EGFP-Poly R shows a significantly more negative phi angle of -102.1° and a psi angle of -35.1°. This change in the phi angle suggests a more pronounced deviation in the turn's geometry in EGFP-Poly R compared to EGFP. Additionally, the chi1 angles are 51.1° for EGFP and 47.1° for EGFP-Poly R, reflecting an adjustment in the side-chain orientation due to the extended polyarginine sequence. Another distinct difference is found in the Phe115-Asp118 turn. EGFP has a phi angle of -125.8° and a psi angle of 100.9°, whereas EGFP-Poly R shows a less negative phi angle of -121.3° and a psi angle of 98.3°. The chi1 angles for EGFP are -65.5°, while for EGFP-Poly R, they are -66.0°. The shift in the phi and psi angles in EGFP-Poly R indicates a possible alteration in the turn’s local conformation, potentially affecting interactions with neighboring regions. The Asp211-Glu214 turn presents another unique aspect. EGFP has a phi angle of -63.7° and a psi angle of -16.3°, whereas EGFP-Poly R shows slightly different angles with a phi of -64.6° and a psi of -16.1°. This minimal difference in phi and psi angles, along with chi1 angles of 26.5° for EGFP and 26.8° for EGFP-Poly R, highlights subtle adjustments in the beta turn’s configuration. Another differences in the Leu65-Gly68 turn, in EGFP, this turn is of the I type, suggesting a more regular and stable loop structure at this position. In contrast, in EGFP-Poly R, the same region forms an IV type turn, indicating increased flexibility or a less well-defined structure, which could lead to differences in the local stability of the protein. The I type turn is a common secondary structure element in proteins where four consecutive residues create a turn in the polypeptide chain. In this type of turn, the first and fourth residues are hydrogen-bonded, creating a tight loop. The phi (Φ) and psi (Ψ) angles typically found in I type turns are around -60° for Φ and -30° to -60° for Ψ. These angles contribute to the formation of a stable turn, often found in beta-hairpins or at the end of beta strands. The IV type turn is a more flexible and less common turn compared to type I. It doesn’t conform to a specific set of phi and psi angles and is often seen as a more irregular or less tightly constrained turn. This flexibility means that IV type turns can vary more in their structure, leading to different folding patterns or conformations in the protein. Because of its variability, an IV type turn can introduce local flexibility or disorder in a protein structure, potentially affecting the overall stability of the region. Flexible or irregular turn like the IV type could make the protein more prone to misfolding or less resistant to environmental stressors.12
The last one is the difference of gamma turns between EGFP and EGFP-Poly R reveals significant structural differences that likely influence their stability and functionality. EGFP features seven gamma turns (Table 1), whereas EGFP-Poly R contains nine, indicating that the poly-arginine modification introduces additional structural elements (Table 2). Among the common gamma turns, the His82 to Phe84 turn shows minor differences. In EGFP, the phi angle is -85.6°, psi is 82.4°, chi1 is -168.5°, with a CA distance of 6.0 Å. For EGFP-Poly R, the phi angle is -86.3°, psi is 83.0°, chi1 is -169.3°, and the CA distance remains 6.0 Å. Similarly, the Glu143 to Asn145 turn in EGFP has a phi angle of -84.5°, psi of 65.2°, chi1 of -171.6°, with a CA distance of 5.6 Å. In EGFP-Poly R, the corresponding values are phi -83.9°, psi 65.6°, chi1-170.0°, and CA distance 5.6 Å. EGFP also has unique gamma turns not present in EGFP-Poly R. The Gly268 to Asp270 turn, with a phi angle of -84.6°, psi of 69.5°, chi1 of -70.8°, and a CA distance of 5.8 Å, is missing in EGFP-Poly R. Its absence may lead to reduced flexibility and altered local folding. Similarly, the Thr272 to Thr274 turn, with phi of -72.7°, psi of 62.5°, chi1 of -78.9°, and CA distance of 5.4 Å, is also not found in EGFP-Poly R. The Gly275 to His277 turn, with phi of -65.9°, psi of 95.2°, chi1 of -86.4°, and a CA distance of 5.8 Å, is absent in EGFP-Poly R. On the other hand, EGFP-Poly R includes several unique gamma turns not observed in EGFP. The Phe257 to Gly259 turn, with phi of -61.9°, psi of 76.0°, chi1 of -74.2°, and a CA distance of 5.0 Å, introduces new conformational possibilities. The Leu269 to Ser271 turn, with phi of -75.5°, psi of 58.9°, chi1 of -152.5°, and a CA distance of 5.5 Å. The Thr272 to Thr274 turn in EGFP-Poly R, with phi of -56.1°, psi of 83.6°, chi1 of -80.3°, and a CA distance of 5.7 Å, differs from its counterpart in EGFP. Then Gly275 to Arg277 turn, with phi of -82.1°, psi of 91.4°, chi1 of -94.9°, and a CA distance of 6.0 Å, reflects a structural modification that might influence the protein's overall folding.
In this study, PolyR was added to EGFP protein to function as a cell-penetrating peptide (CPP), which is commonly used as a carrier of protein into cells. Arginine-rich CPP has been used as a carrier for the intracellular delivery of various bioactive molecules such as proteins, peptides, and nucleic acids. The number of arginine residues used as CPP can affect the efficiency of cellular uptake and cytosolic release. It is well understood that high cellular uptake efficiency is achieved with 8-12 residues.13 Polyarginine as CPP also plays an essential role in forming hydrogen bonds with cell membrane protein groups.14
The expression of EGFP protein, as inferred from the fluorescence and Western blot data, indicated that the induction of the recombinant cultures was successful. Analysis performed at 48 and 72 hours post-inoculation to induction medium showed no increase in fluorescent intensity with time (Figure 5B). Thus, in the future, this could be used as a reference supporting that it would only take 48 hours of culturing for expression, hence being more time-efficient.
It is known that the addition of a CPP sequence can have a negative influence on recombinant protein yield. 15 It was reported that ten arginines (R10) residues were added to the superfolder GFP (sfGFP) N-terminal in pBAD plasmid containing arabinose-inducible sfGFP gene with a C-terminal His tag. No recombinant R10-sfGFP was expressed by E. coli TOP10. However, the unmodified sfGFP gene expressed over 30 mg/L protein under the same condition.16 It was also reported that although the fluorescent protein obtained after purification showed a single band on SDS-PAGE, mass spectrometry (MS) analysis revealed truncation of the R10, leaving only two arginine residues present at the C-terminus upon expression when the position of R10 and His-tag was swapped relative to the sfGFP gene (i.e. His tag at the N-terminus and R10 at the C-terminus). 17
In contrast to these previous findings, our result indicated that the addition of a PolyR group to the C-terminal of EGFP carrying C-terminal 6×His-tag did not seem to interfere with protein expression in S. cerevisiae INVSc1, as shown by the similar fluorescence (Figure 5B) and protein (Figure 6) levels expressed by yeasts carrying pYES2CT-EGFP band pYES2CT-EGFP-PolyR. In combination with the data from the proteins tertiary structural predictions and alignment, this expression result indicated that the conformational change caused by the addition of PolyR did not affect the protein expression.
It would be interesting to observe whether relocating the 6×His-tag or the PolyR to different terminals would improve the protein expression as well as provide ease of purification since it is feared that the PolyR can interfere with the binding of 6×His-tag to Ni-NTA resin to be used for purification or the anti-His-tag antibody used for detection of expression.
The lower growth curve of the transformant S. cerevisiae compared to the wild-type can be due to the growth inhibition factors of S. cerevisiae activated due to the addition of MM induction medium (2% galactose and 1% raffinose). Adding raffinose to the growth medium of S. cerevisiae can activate the programmed cell death formation of acetic acid. Guaragnella's study (2013) suggests that S. cerevisiae is resistant to programmed cell death via acetic acid because S. cerevisiae can compensate by activating retrograde mitochondria. Although programmed cell death is activated, they are not necessarily inhibited by growth.16 In addition, previous studies have shown that high-level expression of a EGFP protein localized to an intracellular compartment is expected to cause cellular defects because it overloads localization processes and growth defects.17,18 This causes a significant decrease in the growth curve of yeast containing plasmids EGFP and EGFP PolyR. Even so, the low growth curve of the transformant S. cerevisiae seems does not affect the expression of EGFP fluorescence proteins (Figures 5A and 5B and 2). Previous research has shown that PolyR expression in cells can cause protein translation stalls.19 Results in experiments showed that the addition of PolyR at the C terminal did not cause the EGFP protein translation stall, only a decrease of the growth curve significantly. This is possible because our plasmid vectors have been designed to express them in sufficient quantities within the cell.
The reduced detection of EGFP-Poly R in Western blot assays, compared to native EGFP, stems from significant structural alterations caused by the poly-arginine modifications. EGFP-Poly R exhibits a truncated final beta strand and a reduced number of helices, leading to decreased stability and increased susceptibility to misfolding and aggregation. Additionally, changes in the lengths and angles of helices, as well as the introduction of new gamma turns, contribute to structural strain and potential instability.20 These modifications result in reduced solubility and altered epitope accessibility, making EGFP-Poly R more prone to proteolytic cleavage during sample preparation. Consequently, while EGFP-Poly R is expressed within cells, its aggregated and unstable forms are less detectable in Western blots, unlike the more stable and detectable native EGFP.21 Histag addition can cause protein instability. It would seem prudent to suggest that some cases of histag may result in increased aggregation, disruption to protein folding, proteolytic changes of cleavage, and a combination of these factors, which could be 8X more significant than 6X.22–24
It is imperative to stress that poly-arginine causes significant changes with regard to the structure of EGFP and affects the stability and solubility of the protein. However, it is rather reassuring that the fluorescence of EGFP-PolyR is preserved at similar levels. However, the changes in the secondary structure elements, such as beta-strands, helices, and turns, as well as the increase in protein instability, should not be disregarded as they do influence the detectability and overall usefulness of the antibody in protein immobilization and isolation/analysis. Which proves the fact that structural alteration should be taken into account when optimizing the reporter proteins for the experimental purposes.
Figshare: Growth curve of Saccharomyces cerevisiae with and without plasmid insertion. A. Growth curve of Saccharomyces cerevisiae without plasmid, https://doi.org/10.6084/m9.figshare.19759018.v1.25
Figshare: The fluorescence of Saccharomyces cerevisiae without transformation and with transformation (pYES2CT- EGFP/ EGFP plasmid, pYES2CT-EGFP- PolyR/EGFP Polyarginine plasmid) at OD 600 on 48 hours and 72 hours, https://doi.org/10.6084/m9.figshare.19762699.v2.26
Figshare: Figure 1-7, https://doi.org/10.6084/m9.figshare.25388371.27
Figshare: Attachment, https://doi.org/10.6084/m9.figshare.19767589.v8.28
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank Program SAME Direktorat Sumber Daya, DRPM Ditjen Diktiristek, Kemendikbud Tahun 2022, and LPPM UPNVJ for their support in this study.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Fuchs SM, Raines RT: Polyarginine as a multifunctional fusion tag.Protein Sci. 2005; 14 (6): 1538-44 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast, molecular biology, cell biology, plant science, plant-microbe interactions
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast engineering, GFP expression in yeast, fluorescence microscopy, microbial growth, biochemistry, microbial food processes
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pichia pastoris, Recombinant expression, Protein engineering, Applied microbiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Dos Santos NV, Saponi CF, Ryan TM, Primo FL, et al.: Reversible and irreversible fluorescence activity of the Enhanced Green Fluorescent Protein in pH: Insights for the development of pH-biosensors.Int J Biol Macromol. 2020; 164: 3474-3484 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast engineering, GFP expression in yeast, fluorescence microscopy, microbial growth, biochemistry, microbial food processes
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Kintaka R, Makanae K, Moriya H: Cellular growth defects triggered by an overload of protein localization processes.Sci Rep. 2016; 6: 31774 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Yeast, overexpression, plasmid construction, systems biology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Protein chemistry, protein engineering, enzymology, protein therapeutics, biocatalysis and biotransformation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 25 Nov 24 |
read | read | |||
|
Version 2 (revision) 27 Mar 24 |
read | read | |||
|
Version 1 03 Jan 23 |
read | read | |||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)