Keywords
Acute submissive pulmonary embolism, Balloon angioplasty, Simultaneous thrombolysis, Maceration
Background: Acute sub-massive pulmonary embolism is a common clinical condition, and it is associated with high mortality and morbidity. This condition is commonly associated with various co-morbidities and clinical circumstances.
Methods: This is a case report series of 4 patients, wherein the thrombolysis and balloon angioplasty was performed simultaneously using a Cordis 6F diagnostic catheter and later exchanged with a 6F guide catheter in the respective pulmonary artery when a larger balloon was required.
Results: All these four patients achieved successful revascularization of the respective pulmonary artery. One patient expired 30 hours after the procedure with a significant reduction in the symptoms suddenly, which was likely a second episode of pulmonary embolism. No bleeding manifestations were observed in any of the patients. The other three patients are on follow-up.
Conclusion: Simultaneous coronary balloon dilatation and thrombolysis is a useful method in the treatment of high risk submassive acute pulmonary embolism. If needed, higher caliber balloons can be used for the same technique using guide catheters.
Acute submissive pulmonary embolism, Balloon angioplasty, Simultaneous thrombolysis, Maceration
The new version is modified based on the reviewers comments. All sections of the manuscript have been updated with more details about the patients, and emphasis on the clinical significance, novelty and application of the study have been added.
See the author's detailed response to the review by Carlos Jerjes-Sanchez
See the author's detailed response to the review by Raja Sekhar Varma
Acute pulmonary embolism is a common medical condition, and the treatment is often challenging. The incidence of acute pulmonary embolism is in the range of 0.6/1000/year.1 The estimated incidence of deep vein thrombosis is 1-2/1000 persons every year.2 It is the 3rd leading cause of cardiovascular mortality. The burden of deep vein thrombosis and related pulmonary embolism are high.3 These also pose a large economic burden for the health care providers irrespective of the system worldwide.3 The mortality in patients with acute pulmonary embolism is significant, and the exact burden is high when quantified. At present robust data about this condition is not available and the magnitude of the problem is an underestimate. Common challenges are encountered in treating this condition is associated severe breathlessness, co-morbidities and the patient is often moribund. Associated comorbidities or predisposing conditions preclude the treatment methods due to the associated risk of bleeding and mortality. Hence, controversies exist in choosing the method of treatment of this condition, and the best treatment methods at present are catheter directed thrombolysis and low dose thrombolysis. Among the costs involved, the catheter directed thrombolysis is associated with the lowest cost.4 This is by considering the risk of bleeding vs. the benefits achieved, which is primarily the mortality. With the advent of the COVID-19 pandemic, the incidence of venous thrombosis has increased significantly, especially in severe cases of COVID-19.5 At present catheter directed thrombolysis has the best results, and even this is associated with 6.5% cardiac arrest and about 5% hemoptysis.6
Catheter directed thrombolysis is associated with least mortality6 though there are some studies which show definite conclusions regarding the treatment methods is not possible.7 Some studies show similar results with all methods of treatment modalities,8 though many studies claim the benefits9–11 and safety12 of catheter directed thrombolysis. Catheter based thrombolysis is performed by placing a catheter in the pulmonary artery and thrombolytics are infused over 24 to 30 hours. Catheter interventions without thrombolysis include aspiration thrombectomy, mechanical thrombectomy, rheolytic thrombectomy etc. The presence of many approaches prompted the author to use a method based on expertise and experience with maximization of local resources.
Prompt treatment of this condition is always advised, and treatment delays are associated with increased mortality.13 This case series comprises four cases wherein the treatment of acute high-risk sub-massive pulmonary embolism was performed in a controlled manner effectively, safely, and swiftly without any complications during the procedures.14 Also, coronary balloons and 6F coronary diagnostic catheters were used in the procedures predominantly to simplify the treatment process of this life-threatening vascular event, and to prevent complications like sudden cardiac death, cardiogenic shock, and hemoptysis during the treatment procedures. The current report is descriptive about four male patients encountered with acute high risk sub-massive/severe pulmonary embolism, who were treated with balloon angioplasty/maceration and thrombolysis. To our knowledge, no similar case report or series were published in the past.
Informed written consent was taken from patients for all procedures. Institutional ethical committee approval has been obtained from Pondicherry Institute of Medical Sciences (PIMS) Institutional ethical review committee, Puducherry. The consent to publish the individual clinical details described in this report was obtained from each patient.
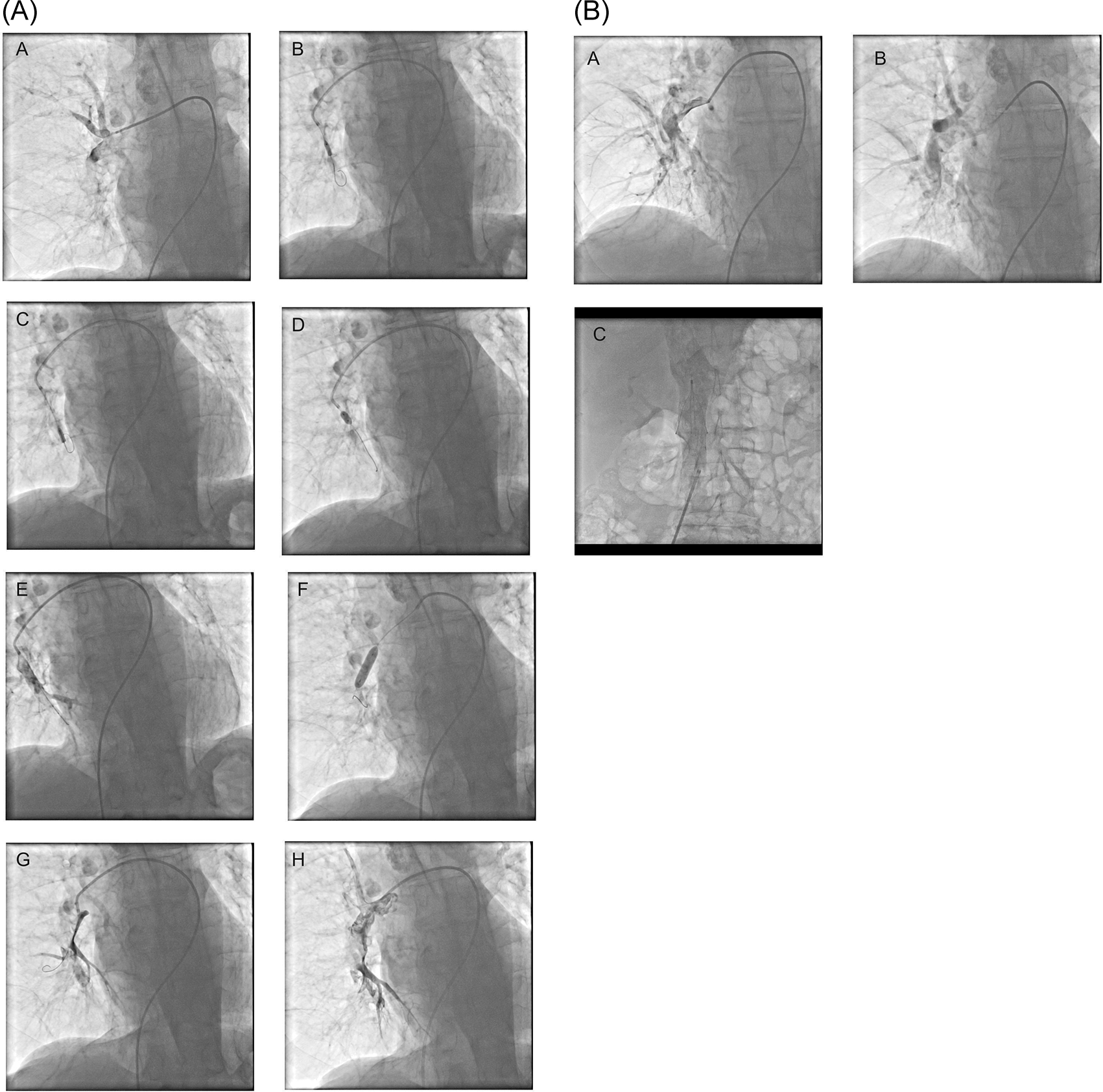
The patient was a 45-year male who developed acute onset of breathlessness and presented to the emergency ward with acute breathlessness and chest pain. At presentation the patient had sinus tachycardia with heart rate of about 110/min, respiratory rate 34/min, blood pressure was 90/60 mmHg, oxygen saturation of about 87 to 89%, and qualitative troponin I estimation was positive. Electrocardiogram (ECG) showed non-specific T inversions in inferolateral leads and sinus tachycardia. The patient was initially treated as unstable angina with congestive cardiac failure and angiogram was performed, and the diagnostic coronary angiogram was normal. Angiogram was performed, and the coronary angiogram was normal. As the patient had persistent tachycardia, the echocardiogram performed showed dilated right atrium, ventricle, and pulmonary artery. Before the pulmonary angiogram the patient was started on dopamine, which was tapered and stopped 6 hours after the procedure. Since the patient was started on loading dose of antiplatelets - aspirin and clopidogrel, low molecular weight heparin and a low oxygen saturation and acute corpulmonale, a high risk of pulmonary bleeding was contemplated by the author and hence pulmonary angiogram was performed with an intention of catheter-based thrombolysis. Pulmonary angiogram, which showed dilated right atrium and right ventricle and near total occlusion of the left pulmonary artery (Figure 1, panels A). The patient underwent balloon dilatation, and thrombolysis was performed simultaneously (Figure 1, panels B to F). Accuforce balloon, 4.5 mm, was used for balloon dilatation through Cordis 6F diagnostic catheter, and thrombolysis was performed with Tenecteplase. 35 mg of Tenecteplase was used with boluses given by hand injections simultaneously with balloon dilatations. There was an immediate improvement in breathlessness, reduction in tachycardia, and the oxygen saturation also improved to 99 percent when the procedure was completed in the cardiac catheterization lab. Flow in the left pulmonary artery improved (Figure 1, panels G and H). The patient had improvement in the general condition, and he was discharged on day three. The right atrium and right ventricular dilatation were also reduced. Deep veins were normal by Doppler evaluation, and hence inferior vena-cava filter was not placed in this patient. This patient is under follow-up for one year, and at present on rivaroxaban and currently asymptomatic.
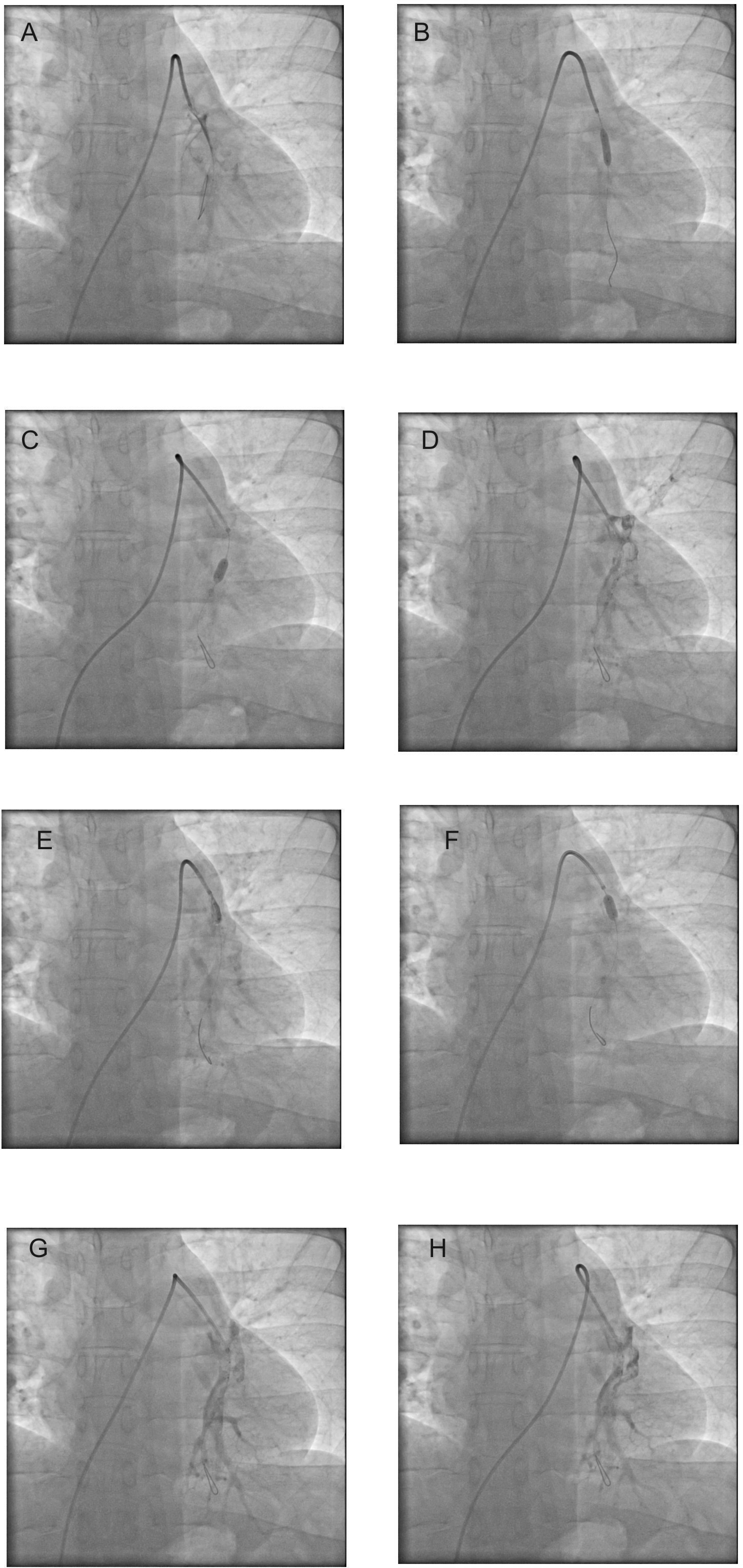
The 85-year male patient developed breathlessness and chest pain, which was sudden in onset. The patient recently underwent a hip operation for an intertrochanteric fracture on the right side, and recently he was ambulant. The patient was diaphoretic with pulse rate of 140 to 150/min, blood pressure of 100/80 mmHg and the patient had severe breathlessness with oxygen saturation 85% to 87%. By echocardiography, the right atrium and right ventricle were dilated with ratio 1.2 when compared to the left ventricle. ECG showed sinus tachycardia with right axis deviation, and T inversions in inferior and anterolateral leads. The troponin I test was negative. A clinical diagnosis of pulmonary embolism was made, and systemic thrombolysis with a 60% dose of streptokinase was advised. The advised dose was less due to recent major surgery within one week and his advanced age. However, the physician on duty refused to perform thrombolysis due to the high risk of bleeding in the perioperative period, and hence the patient was taken up for pulmonary angiogram and interventions at midnight. Patient was started on dopamine and noradrenaline throughout the procedure and the infusion was gradually tapered and stopped 12 hours after the procedure. A pulmonary angiogram showed near total occlusion of the right pulmonary artery (Figure 2A, panel A). The patient underwent thrombolysis with balloon dilatation in the right pulmonary artery, where the clots were visualized (Figure 2A, panels B to E). A 5 mm quantum apex balloon was used initially, and it was improvised with a 6 mm Sterling balloon (Figure 2, panel F). Tenecteplase 35 mg was used in the procedure and was given as boluses after dilution with saline. At the end of the procedure, the clot volume was reduced, and pulmonary artery blood flow improved (Figure 2A, panels G and H). Throughout the procedure the patient had a pulse rate of about 140/min and patient had breathlessness and cough. The patient’s symptoms improved, and tachycardia mildly reduced to about 130/min immediately after the procedure. Subcutaneous low molecular weight heparin was given with good results. On day two, the tachycardia and breathlessness were reduced by about 60%. A repeat pulmonary angiogram performed on the third day showed significant clearance of the clots in the right pulmonary artery (Figure 2B). Venous doppler showed clots in left iliofemoral veins. Inferior venacava filter was inserted, and this patient is on regular follow-up for 2 years, and he is on rivaroxaban.
A 59-year male presented with breathlessness and orthopnea for ten days, and this patient had mild pericardial effusion and corpulmonale and severe pulmonary artery hypertension. At presentation the pulse rate was about 120/min, blood pressure 110/80 mmHg respiratory rate was 36/min, and the oxygen saturation was about 85%. The patient also had crepitations and collapse of the entire right lung by chest roentgenogram. Since the patient was having symptoms for 10 days, and collapse-consolidation of right entire lung fields, a failure of systemic thrombolysis was contemplated by the author and hence pulmonary angiogram was performed with an intention of thrombolysis. Echocardiography showed a dilated right atrium and right ventricle with severe pulmonary artery hypertension. The electrocardiogram showed sinus tachycardia with T inversions in inferior and anterior leads with right axis deviation. The patient was started on a small dose of noradrenaline at the beginning of the pulmonary angiogram which was stopped 4 hours after the procedure. Pulmonary angiogram showed a large clot in the right pulmonary artery (Figure 3, panels A and B). Initially, 035 Terumo wire was inserted, and using a peripheral - 5×40 mm balloon, angioplasty was performed in the right pulmonary artery. Thereafter, 014 wire was inserted (Figure 3, panel E), and balloon dilatations using coronary balloons, 2×10 mm, and 4.5×8 mm, were performed (Figure 3, panel F). The patient underwent serial balloon dilatations and simultaneous thrombolysis with streptokinase by hand injection. Partial recanalization of the right pulmonary artery was achieved (Figure 3, panels G to I). Investigations for deep vein thrombosis and any associated malignancies did not reveal any findings. A pulmonary angiogram was repeated after two days, and balloon dilatation with heparin was given during the second intervention for residual thrombi in the right pulmonary artery. Also, the oxygen saturation improved to about 90%. The patients’ general condition and breathlessness improved, and he was discharged. One month follow-up showed mild lung expansion compared to the previous results on the right side, and he had mild breathlessness on exertion with pulmonary artery systolic pressure of 45 mmHg. After that, the patient went for a follow-up at his neighboring district hospital.
This was a 47-year male who presented with breathlessness and cough for one day and had a history of occupation-related long travels. At presentation the patient was obese, the pulse rate was 110/min, respiratory rate of 34/min, blood pressure 100 to110/80 mmHg, and oxygen saturation was about 88%. Troponin I qualitative test was not reactive. ECG showed non-specific ST-T changes with sinus tachycardia, creatinine was 1.2mg/dl. Echocardiography revealed a corpulmonale and right ventricular dysfunction. The patient was taken up for catheter-based thrombolysis at midnight with an idea for better results and reduced bleeding manifestations. The patient was started on low dose of dopamine before the catheter interventions, and it was tapered 8 hours after the procedure. An inferior vena-cava angiogram showed clots in the left iliac artery (Figure 4, panel A). The patient had large clots in the left pulmonary artery with near-total occlusion (Figure 4, panels B and C). Using Cordis 6F diagnostic catheter, serial balloon dilatations were performed to clots in the left pulmonary artery (Figure 4, panels D and E), and its mid and lower branches using a 5 mm Quantum apex balloon and thrombolysis was performed simultaneously using streptokinase in the left pulmonary artery. A significant improvement in the blood flow of the left pulmonary artery was observed (Figure 4, panels G and H). The patient had relief in breathlessness and chest tightness immediately after the procedure and a significant reduction in oxygen requirement. He was started on low molecular weight heparin and clopidogrel 75mg once a day. For 24 hours post-procedure patient had substantial clinical improvement. 1.3M units of streptokinase were used by dilution in saline and were administered as hand injections. On day two, the patient, being asymptomatic with minimal oxygen support, developed sudden onset of breathlessness and rapid deterioration and death subsequently. The patient possibly could have had an episode of another pulmonary embolism from the deep veins or from the primary source, which was only clinical speculation. No bleeding manifestations were observed before deterioration.

All the interventional procedures were performed in the right femoral approach, and after initial iliac vein and inferior vena cava visualization to look for clots. Unfractionated heparin 5000 units were administered before the start of the procedure. The dose of streptokinase was 1.5 million units, which is a single vial diluted and given as small boluses during the procedure, which usually was about 30 to 40 min in both these patients. In both the patients 90% of the above dose was used. An inferior vena cava filter (Cook Celect) was placed in one of the cases. Through the right femoral vein approach, the filter was taken and placed in the infrarenal segment of the inferior vena cava after an initial angiogram. NTpro-BNP was not performed in any of the cases. D Dimer was elevated in all the cases, and only a one-time measurement was performed. All the patients had a tricuspid annular plane excursion (TAPSE) <15 mm, and all patients at presentation had a dilatation of the right ventricle with a right ventricle/left ventricle ratio ≥ of 1.0. Troponin I was performed in two patients, and on only patient 1 it was positive.
In all the above cases, balloon angioplasty/maceration and thrombolysis were performed simultaneously to achieve recanalization of the respective pulmonary artery. In all these cases, either Cordis 6F diagnostic catheter or, in the late stages, exchanged with 6F guide catheters were used. The advantages of these procedures are a reduction in the contrast volume load and easy availability of catheters for the same. The 5 mm coronary balloons are commonly available, and if higher balloons are required, exchange with larger balloons was used during the procedures. When a balloon size >5 mm is required, the 6F guide catheter is used. Other advantages of this method include the early withdrawal of the catheter after the procedure. When a thrombolysis catheter is placed for a longer duration in the pulmonary artery, arrhythmias, infections, and thrombosis tend to occur, and thereby, the mortality would increase.
5 mm balloons are average-sized, and the pulmonary arteries are in the range of 8 to 10 mm. The advantage of a 5 mm balloon is inside the clot, the balloon can be maneuvered safely inside the smaller pulmonary artery branches without difficulty. In the late stages of the interventional procedure, when the clarity of the distal pulmonary artery or the target artery is good, large balloons like 6 mm or 7 mm Sterling balloons can be used for this purpose. Larger balloons may result in dissection, spasms and proximal dislodgement of clots which may be detrimental.
The novelty in these case series is the use of coronary balloon maceration and simultaneous thrombolysis using easily available thin calibre coronary diagnostic catheters (6F) to effectively, quickly, and safely treat this life-threatening and high-risk clinical condition. The smaller or average-sized coronary balloons have the advantage of playing inside the pulmonary artery branches without damaging the vessel. Larger balloons tend to damage the vessels and endothelium, which release signal peptides/proteases, and cause vasovagal and neurocardiogenic responses during the procedures, which could be life-threatening. Hence, being ‘average’ in approach may yield better results in uncertainty as definite treatment approaches are not well defined for this clinical condition.
Systemic thrombolysis was the treatment of choice for pulmonary embolism in our center, which is also the current recommendation by guidelines. Numerous cases of systemic thrombolysis were performed as a routine in the past 16 years by the author whenever a large pulmonary embolism was diagnosed with good results and some failures and complications. Usually, the associated comorbidities with this condition are high, and many times, the patient cannot be shifted to a catheterization lab due to associated sick clinical conditions like surgeries, fractures, multiorgan disorders, etc.
Streptokinase was used in two patients, and slowly, the use of streptokinase is becoming obsolete, and it is being replaced by newer generation tissue-specific thrombolytic agents like Tenecteplase.15 Streptokinase, though considered weaker compared to tissue-specific fibrinolytic agents, it has the advantages of lesser bleeding manifestations, 1/10th of the cost of newer generation thrombolytic agents, and still works well in myocardial infarctions, though not preferred.16 There are theoretical concerns about streptokinase antibodies, especially in the Asian population, which can reduce their efficacy.17 However, the differences are only marginal, and in real-time clinical observations, streptokinase is still used routinely and widely in acute myocardial infarctions with success in Asian countries. There are minor allergy concerns during streptokinase use. Commonly observed allergies include febrile reactions, shivering and minor occasional fall in blood pressure. In both the patients in which streptokinase was used there was no allergy reactions. In the first two patients, the Tenecteplase dose was 35 mg, though the weight-adjusted dose was slightly higher - 40 mg and 45 mg. In the past experiences of the author, the two major complications where the patients’ condition goes out of control are intracranial bleeding and hemoptysis.18 As the balloon maceration was performed the injections were simultaneously injected. When the clot volume was adequately reduced, the injection was stopped (the ‘enough’ decision), and during the procedures, the patients had moderate to severe breathlessness, cough, and lower oxygen saturation, which was a setting for hemoptysis. This dosage decision was based on the intuition of the operator.19
Antiplatelets like clopidogrel or aspirin are not currently recommended in the treatment of acute pulmonary embolism, though some studies have demonstrated benefits in acute deep vein thrombosis.20,21 Occasional use of clopidogrel in small doses in this series is prompted by the observations in the treatment of clots in conditions like acute myocardial infarctions. In this series, in two of the cases, small tirofiban boluses were given of 10 ml in each patient as desperate measures, which is learned by the good resolution of clot volumes during primary angioplasties with tirofiban boluses.
Inferior vena cava filters were used in one of the patients, though guidelines do not recommend them for routine use. The studies on which guidelines are based do not come to definitive conclusions, and there is also a lacuna in knowledge regarding the use of inferior vena cava filters.22–24 The presence of varied associated comorbidities, complex clinical situations, a lesser number of pulmonary embolism cases compared to acute coronary syndromes, and varied long-term compliance with drugs in the patients are some significant differences across the population section, which preclude generalization in treatment. In the author’s previous experiences, the inferior vena cava filters are very safe, and we have yet to see a filter occlusion/thrombosis, etc., even when placed above the renal arteries in a few cases of massive deep vein thrombosis.
The raw data is available in the data availability section below. The number of fluoroscopy and cine acquisitions can be significantly reduced in future similar interventions, and this will reduce contrast usage indirectly.
Many treatment methods are available for physicians to choose for patients with acute pulmonary embolism.25–28 Commonly used methods are systemic thrombolysis, low dose thrombolysis, catheter-directed thrombolysis, ablation of thrombus, Inari flow retriever method, ultrasonic treatment and other embolectomy devices available for this purpose. Though consensus statements are available about the treatment strategy, the choice varies based on the scenario. The scenario of the patient, the clinical manifestations, severity, and co-morbidities like recent surgeries determine the clinicians’ decision.29,30 Also, a classification of the sub-massive/massive differentiation at the bedside is not always possible easily, even though various criteria exist for such a classification.25,29–31 Catheter-directed interventions are especially suited for individuals where the systemic thrombolysis is contraindicated or at high risk for bleeding manifestations. Surgical treatment options have been explored with good results. However, surgery is associated with long bypass time, and the need for a ventilator precludes this method as a routine and easy treatment option.32,33 Contrast reduction will give advantages in these patients as renal failure is commonly associated in an overt or subtle form in these patients. Reducing contrast load and fluoroscopy times are very useful in coronary and peripheral angioplasties.34,35 Renal failure, coronary artery diseases, cancer, and diabetes are commonly associated in patients with deep vein thrombosis.30 Simultaneous balloon dilatation and thrombolysis would be better for greater penetration of the thrombolytic agents and concurrent mechanical clearance of the clots, and the hardware is readily available in all cardiac catheterization labs.
Using an IVC filter in patients with deep vein thrombosis or venous thromboembolism poses many dilemmas. Metanalysis and NICE guidelines indicate the benefits of the IVC filter in these patients. In the current case discussion, in the opinion of the author, an early deployment of an IVC filter would have benefitted patient-4 by preventing the recurrence of pulmonary thromboembolism.36 It was the practice of the author to insert an IVC filter after a few days or before the discharge of the patients, as some patients would require reintervention of the pulmonary artery. In this case series, all four patients were males. The incidence of pulmonary thromboembolism is higher in the female gender,37 and the treatment availability and outcomes tend to be worse among females.37–39 Hence, this method needs to be evaluated in a large patient population, including the female gender, for a better assessment of the results. These observations were made in a few numbers of patients only. Further studies, including a large number of patients with acute sub-massive pulmonary embolism, needs to be performed for validation of the current observations.
Balloon angioplasty/maceration and thrombolysis simultaneously can be used as a treatment option in patients with acute high-risk sub-massive pulmonary embolism using coronary balloons and catheters. If larger balloon dilatation is required, 6F guide catheters can be used.
Mark Christopher Arokiaraj. (2023). Treatment of Acute Sub-massive Pulmonary Embolism with Balloon Angioplasty and Thrombolysis Simultaneously (Version 1). Zenodo. DOI: https://doi.org/10.5281/zenodo.8159042.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC BY 4.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulmonary embolism and thrombolysis.
Is the background of the cases’ history and progression described in sufficient detail?
No
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
No
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
No
Is the conclusion balanced and justified on the basis of the findings?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Pulmonary embolism and thrombolysis.
Is the background of the cases’ history and progression described in sufficient detail?
Yes
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Yes
Is the conclusion balanced and justified on the basis of the findings?
Yes
References
1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, et al.: Thrombosis: a major contributor to global disease burden.Arterioscler Thromb Vasc Biol. 2014; 34 (11): 2363-71 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Complex cardiovascular interventions
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 09 Jul 24 |
read | |
|
Version 1 05 Oct 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)