Keywords
Nitric oxide, eNOS 894G>T, polymorphism, cancer, stroke, meta-analysis, safe work
This article is included in the Oncology gateway.
Although numerous case-control studies have examined the role of nitric oxide, particularly the 894G>T polymorphism in the eNOS gene, in increasing the risk of stroke and cancer, there remains a need for a comprehensive meta-analysis to clarify these associations. This study aims to address this gap by thoroughly evaluating the relationship between the eNOS 894G>T polymorphism and the risks of cancer and stroke.
We conducted an exhaustive search across digital databases including Science Direct, PubMed, and Google Scholar for studies published between 2012-2023. A rigorous selection process was employed to include relevant studies, which were then analyzed using robust meta-analytical techniques to determine the association between the eNOS 894G>T polymorphism and the risks of cancer and stroke.
In this meta-analysis, we combined data from 2,013 cases and 2,187 control subjects for cancer risk assessment and 1,006 cases with 1,146 control subjects for stroke risk evaluation. Our findings indicate that the eNOS 894G>T polymorphism is significantly associated with an increased risk of cancer when comparing GG vs. GT+TT genotypes. Additionally, there is a notable correlation between this polymorphism and stroke incidence under various genetic models (T vs. G, TT vs. GG + GT, GG + GT vs. TT).
The results of this meta-analysis suggest a significant association between the eNOS 894G>T polymorphism and increased risks of cancer and stroke. These findings underscore the importance of conducting future studies with larger sample sizes and more comprehensive analyses further to elucidate the role of nitric oxide in these diseases. This study addresses some concerns but further detailed and non-repetitive research is necessary for conclusive evidence.
Nitric oxide, eNOS 894G>T, polymorphism, cancer, stroke, meta-analysis, safe work
We have endeavored to reduce repetition, deepen analyses, and provide more meaningful insights in line with reviewers' comments. Titles have been corrected to include specific genes and polymorphisms for clarity. The study objectives have been appropriately placed in the Background section instead of the Methods section. The article has also provided clearer and more detailed information regarding the number of cases and controls, as per the reviewers' feedback.
To read any peer review reports and author responses for this article, follow the "read" links in the Open Peer Review table.
Nitric oxide (NO) is a critical signaling molecule involved in various physiological and pathological processes in mammals. In humans, NO is produced enzymatically by nitric oxide synthase (NOS) from L-arginine, resulting in the formation of L-citrulline and NO itself (Korde Choudhari et al., 2013). The NOS family comprises three isoforms: endothelial NOS (eNOS), neuronal NOS (nNOS), and inducible NOS (iNOS) (Sachdev, 1999). NO plays a significant role in coagulation, neuronal activity, and cerebral blood flow regulation (Korde Choudhari et al., 2013). Moreover, it can induce cellular inflammation, potentially delaying stroke onset, and acting as a carcinogen, thereby increasing cancer risk.
Recent studies have highlighted the role of NO in the progression and metastasis of cancer through various mechanisms, including polyamine synthesis and inhibition of NO-mediated tumor cytotoxicity (Gào and Schöttker, 2017; Gào et al., 2019). NO’s involvement in hypoxic responses further promotes angiogenesis and cancer cell defense, which are attributed to its mutagenic properties. Specifically, iNOS has been implicated in carcinogenesis due to its production during chronic inflammation (Utispan and Koontongkaew, 2020).
The endothelial NOS (eNOS) isoform is vital for maintaining normal vascular tone under physiological conditions and has been extensively studied in the context of carcinogenesis and neuroprotection during stroke (Lim et al., 2008). Among the single nucleotide polymorphisms (SNPs) identified in the eNOS gene, the 894G>T (rs1799983) variant has been widely associated with cardiovascular diseases and cancer (Yang et al., 2019). This SNP results in structural and functional changes in the eNOS protein, contributing to the pathogenesis of these diseases.
Despite the recognition of multiple SNPs in the eNOS gene, this study focuses on the 894G>T variant due to its significant association with cancer and stroke risks. This meta-analysis aims to provide a comprehensive understanding of the relationship between eNOS 894G>T polymorphism and the risks of developing cancer and stroke.
The research method used was a meta-analysis. Our meta-analysis adhered to the criteria recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, or PRISMA. To discover relevant primary articles, we performed a comprehensive search of digital databases, including PubMed, Science Direct, and Google Scholar, to identify all relevant studies on the correlation of NO especially eNOS 894>GT, and risk to cancer and stroke. Articles sought must be published between 2012-2023. We applied the following keywords: “NO” or “NOS” or “eNOS” or “eNOS 894G>T” AND “polymorphism” AND “cancer risk” AND “stroke risk”. To ensure a comprehensive review of the literature, we conducted a thorough examination of the reference lists included in the recognized literature.
Determination of inclusion and exclusion criteria followed PICOS (Problem, Intervention, Comparison, Outcome, and Study design). The included studies were carefully examined to ensure their relevance and quality for our study. No national restrictions were imposed, meaning studies from all countries were considered eligible for inclusion. The research that met the eligibility criteria was carefully selected for our analysis: 1) Articles published in the English language that investigated the correlation between NO, especially eNOS 894G>T, and the risk of cancer and stroke; 2) Designed as a case-control study; 3) Articles that provided detailed data on genotype and allele frequencies of eNOS gene polymorphisms, which has sufficient data for the calculation of the odds ratio (OR) and the confidence interval of 95%. Therefore, studies were excluded according to as the following criteria: 1) Qualitative research; 2) No available genotype frequency; 3) Studies without control; 4) Meta-analysis studies; and 5) Animal studies.
The data in all studies were extracted when sufficient criteria were met. We then used Microsoft Excel to record the year of publication, the last name of the authors, control, and case sample sizes, and the country of the study. The results were then compared after being extracted, and an assessment was carried out along with the resolution of matters that were not appropriate through consensus. We extracted data from the nine articles meeting eligibility criteria for cancer risk and the seven articles for stroke risk association with NO.
A statistical review was implemented using RevMan, Cochrane with version 5.4 to investigate the association between NO and the risk of stroke and cancer. Crude ORs and a CI of 95% were utilized. Pooled ORs were computed for various genetic models of the eNOS G894T gene polymorphism, including GT+TT versus GG, GT versus GG, TT versus GG, and T versus G. The eNOS gene encodes for endothelial nitric oxide synthase and has a polymorphism at position 894G>T that can result in GG, GT, or TT variants (Buldreghini et al., 2010). G represents the homozygous wild-type genotype, where the individual has two copies of the G allele. GT represents the heterozygous genotype, where the individual has one copy of the G allele and one copy of the T allele. TT represents the homozygous variant genotype, where the individual has two copies of the T allele (Hinz et al., 2013). The calculation of pooled ORs allowed to perform a Z test with a significance level of p≤0.05.
The presence of heterogeneity among the studies included was assessed with a Q test score. If there was no significant hterogeneity, i.e., p>0.10, the effect model was applied consistently. Otherwise, when (p<0.10), the random-effects model was utilized. The diversity of the included research was assessed using the I2 test, which quantifies the degree of heterogeneity. If the I2 value was less than 25%, it indicated no heterogeneity. If the I2 value ranged from 25% to 50%, it showed moderate heterogeneity. If the I2 was greater than 50%, it indicated extreme heterogeneity. The 50% p-value indicating the existence of heterogeneity between the studies and a random effects model (Mantel-Haenszel technique) was implemented; conversely, if no significant heterogeneity was found, the fixed effect model is applied.
The flow diagram in Figure 1 summarises the study workflow. A total of 145 articles were identified in the databases. After removing duplicates, a total of 109 studies remained. 36 studies were screened, and 21 studies were excluded for various reasons. Ten other studies (da Costa Escobar Piccoli et al., 2012; Jang et al., 2013; Rah et al., 2013; Akhter et al., 2014; Kang et al., 2014; Özçelik et al., 2014; Ben Chaaben et al., 2015; Hung et al., 2019; Lee, 2019; Tsay et al., 2019) were also excluded because of unavailable genotype frequency. Six studies (Hao, Montiel and Huang, 2010; Yao et al., 2013; Guo, 2014; Zhao et al., 2014; Abedinzadeh et al., 2020; Akbar et al., 2022) were not included in the analysis because they were meta-analysis studies.
Finally, fifteen studies met the eligibility criteria and were included in the meta-analysis. These consisted of nine case-control studies examining the association between nitric oxide (NO) and cancer risk, and six studies analyzing the association between NO and stroke risk. The characteristics of these studies are summarized in Table 1.
| First Author/Year | Country | Risk | Case/Control | Case | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Allele | Genotype | Allele | ||||||||||
| GG | TT | GT | T | G | GG | TT | GT | T | G | ||||
| (Adibmanesh et al., 2020) | Iran | Cancer | 100/100 | 28 | 28 | 44 | 100 | 100 | 57 | 6 | 37 | 49 | 151 |
| (Aouf et al., 2019) | Tunisia | Cancer | 259/169 | 149 | 20 | 90 | 130 | 388 | 73 | 18 | 78 | 114 | 224 |
| (Branković et al., 2013) | Serbia | Cancer | 150/100 | 76 | 9 | 65 | 83 | 217 | 54 | 6 | 40 | 52 | 148 |
| (Carkic et al., 2020) | Serbia | Cancer | 50/110 | 21 | 5 | 24 | 34 | 66 | 61 | 7 | 42 | 56 | 164 |
| (Koçer et al., 2020) | Turkey | Cancer | 107/100 | 74 | 1 | 32 | 34 | 180 | 65 | 1 | 34 | 36 | 164 |
| (Su et al., 2018) | Taiwan | Cancer | 1044/1200 | 825 | 10 | 209 | 229 | 1859 | 935 | 15 | 250 | 280 | 2120 |
| (Verim et al., 2013) | Turkey | Cancer | 66/88 | 7 | 10 | 49 | 69 | 63 | 31 | 13 | 44 | 70 | 106 |
| (Yadav et al., 2019) | India | Cancer | 179/173 | 88 | 20 | 64 | 104 | 240 | 96 | 8 | 59 | 75 | 251 |
| (Yanar et al., 2016) | Turkey | Cancer | 58/147 | 18 | 11 | 29 | 51 | 65 | 31 | 35 | 81 | 151 | 143 |
| (Anliaçik et al., 2019) | Turkey | Stroke | 112/160 | 21 | 14 | 77 | 44 | 40 | 38 | 19 | 103 | 57 | 61 |
| (Diakite et al., 2014) | Morocco | Stroke | 165/182 | 83 | 16 | 66 | 30 | 70 | 117 | 7 | 58 | 20 | 81 |
| (El Gohary, El Azab and Kamal El-Din, 2017) | Egypt | Stroke | 30/10 | 18 | 6 | 6 | 45 | 15 | 5 | 3 | 3 | 12 | 8 |
| (Kaur, Uppal and Kaur, 2015) | India | Stroke | 120/101 | 84 | 6 | 30 | 18 | 83 | 83 | 1 | 17 | 9 | 91 |
| (Kumar et al., 2016) | India | Stroke | 250/250 | 164 | 12 | 74 | 20 | 80 | 186 | 5 | 59 | 14 | 86 |
| (Shyu et al., 2017) | Taiwan | Stroke | 229/243 | 151 | 16 | 62 | 21 | 50 | 185 | 7 | 51 | 13 | 87 |
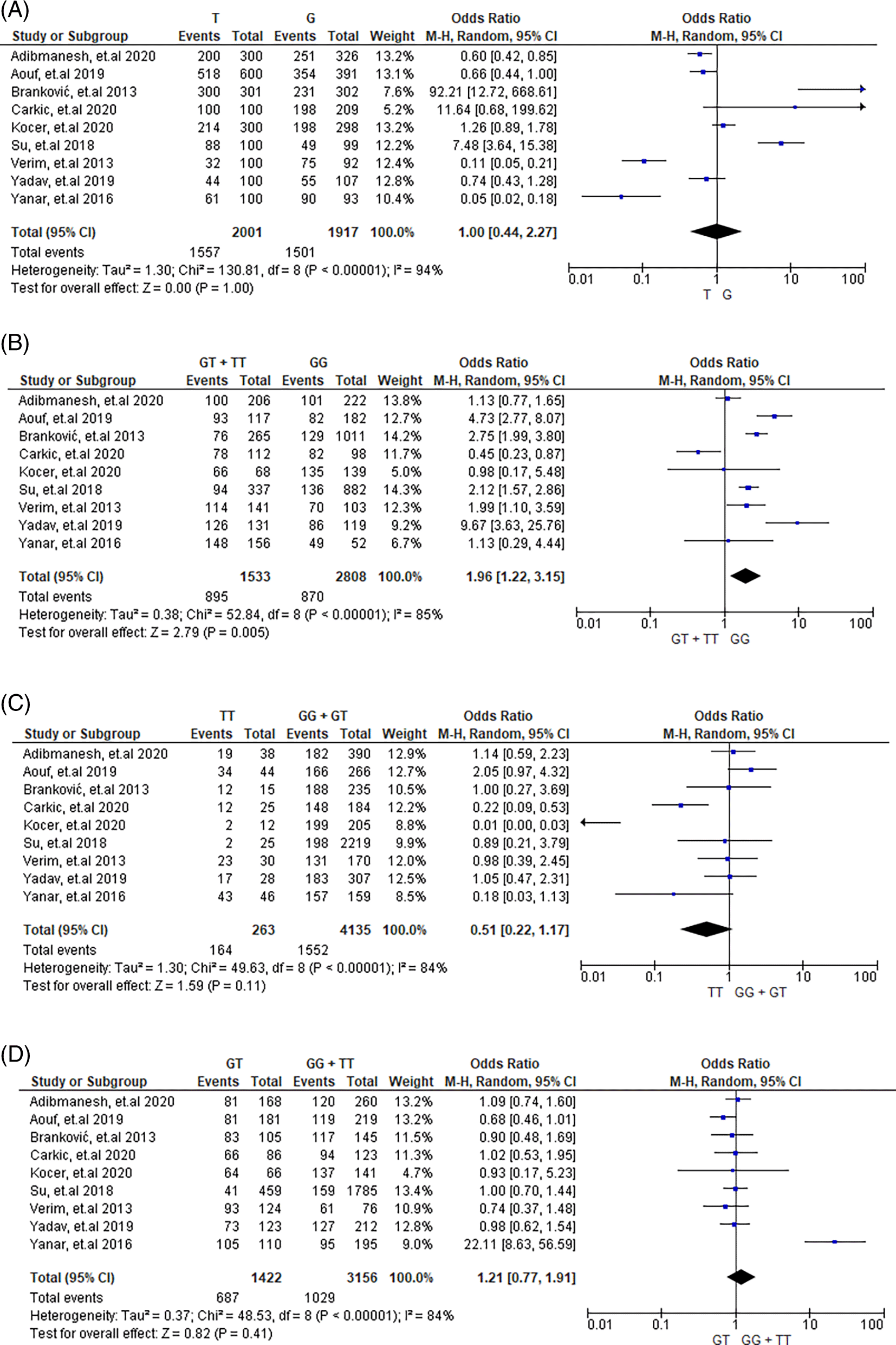
The meta-analysis incorporated data from a total of 2,013 cases and 2,187 control subjects for cancer risk assessment, and 1,006 cases with 1,146 control subjects for stroke risk evaluation. Table 2 presents the aggregated outcome polymorphism through meta-analysis and its association with cancer risk. The results showed that the genetic model “GT+TT vs GG” showed a significant association with cancer risk, as it had a significant p-value (<0.05) and a high OR (1.96). Other genetic models did not show a significant association with cancer risk, indicating that specific genotype combinations might play a more crucial role in cancer susceptibility.
| Genetic Models | NS | Pooled ORs (95% CI) | p-valuea (Z test) | I2 (%) | pH | pE | Method |
|---|---|---|---|---|---|---|---|
| T vs G | 9 | 1.00 (0.44,2.27) | 1.00 | 94 | <0.00001 | 0.17497 | Random model |
| G vs T | 9 | 1.00 (0.44,2.27) | 1.00 | 94 | <0.00001 | 0.34809 | Random model |
| GT+TT vs GG | 9 | 1.96 (1.22,3.15) | 0.005 | 85 | <0.00001 | 0.42245 | Random model |
| TT vs GG+GT | 9 | 0.51 (0.22-1.17) | 0.11 | 84 | <0.00001 | 0.03886 | Random model |
| GT vs GG+TT | 9 | 1.21 (0.77-1.91) | 0.41 | 84 | <0.00001 | 0.13326 | Random model |
Frequencies of T versus G, GT+TT versus GG, GT versus GG+TT, and TT versus GG+GT genotypes were 78% versus 78%, 58% versus 31%, 48% versus 33%, and 62% versus 38%, respectively in cases and controls.
Table 3 analysis revealed that individuals with the T allele (either in GT or TT genotypes) have a higher risk of stroke compared to those with the G allele (p < 0.05). This supports the hypothesis that the eNOS 894G>T polymorphism may be a significant factor in stroke risk.
| Genetic models | NS | Pooled ORs (95% CI) | p-valuea (Z test) | I2 (%) | pH | pE | Method |
|---|---|---|---|---|---|---|---|
| T versus G | 6 | 1.20 (1.01,1.43) | 0.04 | 45 | 0.11 | 0.11583 | Fixed model |
| G versus T | 6 | 0.88 (0.74,1.05) | 0.15 | 0 | 0.86 | 0.17541 | Fixed model |
| GT+TT versus GG | 6 | 0.68 (0.24-1.93) | 0.47 | 91 | <0.00001 | 0.17523 | Random model |
| TT versus GG+GT | 6 | 0.09 (0.03-0.30) | 0.0001 | 94 | <0.00001 | 0.44879 | Random model |
| GT versus GG+TT | 6 | 1.03 (0.40-2.64) | 0.95 | 92 | <0.00001 | 0.29785 | Random model |
Frequencies of T versus G, GT versus GG+TT, GT+TT versus GG, and TT versus GG+GT genotypes were 37% versus 27%, 76% versus 74%, 78% versus 81%, and 29% versus 84%, respectively in cases and controls.
Heterogeneity was observed among the studies in every allele and gene, as depicted in Figures 2 and 3 (T versus G, G versus T, GT+TT versus GG, TT versus GG + GT, and GT versus GG + TT). Tables 2 and 3 provide information on the selected model (random or fixed effect) utilized in order to review universal genetic model correlations.
Both funnel plot images (Figure 4) show the possibility of publication bias. The left side of the plot is more “full” than the right side (asymmetry of the funnel plot). This indicates that there are more studies with positive results (OR>1) compared to studies with negative results (OR<1). Egger’s test results show a p-value<0.05, which means there is statistical evidence that the funnel plot is significantly asymmetrical.
NO acts as a crucial part of numerous pathological and psychological processes, including cancer development and stroke (Zhao et al., 2014). This meta-analysis aimed to elucidate the association between the eNOS 894G>T polymorphism and the risk of cancer and stroke. Our findings highlight several important points that contribute to the current understanding of NO’s involvement in these diseases. Our findings suggest a potential link between the polymorphism and increased cancer susceptibility, particularly in individuals with the TT or GT genotypes compared to GG. This association appears consistent across various ethnicities (African, Asian, European). However, some studies reported no significant association, highlighting the potential influence of other factors. Similarly, the eNOS 894G>T polymorphism might be associated with an increased risk of stroke, especially in the “T versus G” and “TT versus GG+GT” genetic models. However, findings here were more varied, with some studies showing no significant association.
Figure 5 and Figure 6 depict the potential roles of NO in cancer and stroke development. Reduced eNOS activity and subsequent NO deficiency might contribute to tumor growth and impaired blood flow in stroke. External factors like chronic stress, known to affect eNOS activity, could further influence cancer and stroke risks. Based on Figure 5, overproduction of NO can facilitate tumor angiogenesis and metastasis.
Based on Figure 6, low levels of NO derived from eNOS may exert neuroprotection in stroke by promoting vasodilatation and increasing cerebral blood flow (Yang et al., 2019). However, at the same time, there is an enhancement in superoxide production due to eNOS uncoupling. When eNOS is uncoupled, nitric oxide (NO) is not produced, and peroxynitrite is formed instead. This occurs when the enzyme is unable to convert L-arginine into NO due to a lack of cofactors or substrates. Peroxynitrite damages lipids, proteins, and DNA and can trigger the activation of polyadenosine diphosphate ribose (ADP-ribose) polymerase (PARP), all of which contribute to neurotoxicity in stroke.
However, this study has several limitations that need to be considered. Firstly, significant heterogeneity between the studies included in the analysis may affect the validity and generalisability of the results. Furthermore, interpretation of the results should be done with caution as additional uncontrolled factors, such as differences in the sample population, and environment, may affect the estimation of genetic effects. Furthermore, in some cases, the limited number of studies may have limited the statistical power of the analyses and affected the ability to draw strong conclusions. Therefore, more extensive and well-controlled studies are needed to confirm these findings and understand more about the role of genetics in the pathogenesis of stroke and cancer diseases.
In conclusion, the recent meta-analysis found that nitric oxide-related polymorphisms with the eNOS 894G>T gene are associated with a substantial risk of cancer in the total population based on the GG vs. GT+TT genetic model and significantly correlated with the manifestation of stroke in the genetic models T vs. G, TT vs. GG + GT, and GG + GT vs. TT. Considering the conclusion, these results should be reassessed in the coming days through studies with a larger sample population.
All underlying data are available as part of the article and no additional source data are required.
Zenodo: PRISMA Checklist for “Association between nitric oxide and cancer and stroke risk: A meta-analysis”, https://doi.org/10.5281/zenodo.8031323 (Tualeka et al., 2023).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
No
Is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
If this is a Living Systematic Review, is the ‘living’ method appropriate and is the search schedule clearly defined and justified? (‘Living Systematic Review’ or a variation of this term should be included in the title.)
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Breast cancer, lung cancer, cancer immunology, cancer metabolism, microbiome
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
No
Competing Interests: No competing interests were disclosed.
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: NO and cancer
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: NO and cancer
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gene polymorphisms, meta-analysis, risk association, molecular epidemiology, medical biotechnology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 10 Jun 24 |
read | read | ||
|
Version 2 (revision) 28 Mar 24 |
read | |||
|
Version 1 13 Nov 23 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)