Keywords
Nitric oxide, eNOS 894G>T, polymorphism, cancer, stroke, meta-analysis, safe work
This article is included in the Oncology gateway.
Nitric oxide, eNOS 894G>T, polymorphism, cancer, stroke, meta-analysis, safe work
Nitric oxide, or NO, is a chemical compound found in organisms such as mammals. For example, in humans, NO acts as a signaling molecule in various physiological and pathological processes in the body and simultaneously, becomes a diatomic free radical, produced by the enzymatic activity of NOS itself on the L-Arginine compound, yielding the production of L-citrulline along with NO (Korde Choudhari et al., 2013). The NOS family consists of three members, namely eNOS, nNOS, and finally iNOS (Sachdev, 1999). NO influences the coagulation process, neuronal activity, and cerebral blood flow (Korde Choudhari et al., 2013). NO has the potential to induce cellular inflammation, which can delay the onset of stroke. Additionally, NO can act as a carcinogen, increasing cancer risk.
NO plays a substantial role in cancer’s progression and development. NO promotes cancer progression and metastasis via polyamine synthesis or inhibition of NO-mediated tumor cytotoxicity (Gào and Schöttker, 2017; Gào et al., 2019). The roles and functions of NO have been extensively investigated in numerous types of cancer. The response to hypoxia involves NO, which plays a crucial role in inducing angiogenesis and promoting cancer cell defense, and is attributed to the mutagenic behavior exhibited by NO. When cells are exposed to NO for a considerable duration, it is commonly a result of iNOS being produced during chronic inflammation, which gives it a role in carcinogenesis (Utispan and Koontongkaew, 2020). iNOS is considered to have an impact on the mechanism of carcinogenesis.
iNOS plays a multifaceted role in tumor building through its involvement in genetic changes, angiogenesis, proliferation, metastasis, and immunosuppression (Erlandsson et al., 2018). Several studies have proven NO’s influence on both illnesses. NO has been demonstrated to contribute to lung cancer’s progression (Chen et al., 2008). NO can potentially promote the progression of pulmonary carcinoma through a process called protein nitration. NO can also cause head-neck cancer in smokers and people with alcohol use disorders (Patel et al., 2009). The subtype NO, which is iNOS/NOS2, is considered to be correlated with a raised risk of development of prostate cancer (Aaltoma, Lipponen and Kosma, 2001). NO can also result in the onset of breast cancer (Loibl et al., 2002).
NO has a critical part in regulating cerebral circulation and modulating neuronal activity. Microvascular endothelial cells in the brain, also known as the endothelium, are capable of producing and releasing various vasoactive substances, among which NO. The continuous production of NO by the endothelium in basal situations and its reactions to vasoactive stimuli provide knowledge of the complex regulation of cerebral circulation and the maintenance of vascular health in the brain. This dysfunction in NO production and release could be a factor in the progression of stroke. Stroke refers to a clinical condition characterized by unexpected loss of cerebral responsibility as a result of vascular pathology in the brain (Demaerschalk et al., 2016). NO is essential to stroke as an important signaling molecule. The harmful effects of NO derived from iNOS and nNOS primarily stem from the generation of nitrates and free radicals (Zhao et al., 2000). nNOS and iNOS are involved in causing nerve injury during both the beginning and final stages of brain ischemia. Conversely, when eNOS is activated, it has a neuroprotective effect (Chen et al., 2017).
The NOS isoform responsible for producing NO in the vascular endothelium is known as endothelial NOS (eNOS), which in its isoform is expressed through cells and actively contributes to normal vascular tone in physiological conditions. eNOS has also been extensively investigated in the context of carcinogenesis, particularly its involvement in mediating tumor maintenance (Lim et al., 2008). Furthermore, limited levels of NO produced by eNOS can have a neuroprotective effect on stroke through increased vasodilation and cerebral circulation (Yang et al., 2019). Recently, several single nucleotide polymorphisms have been found in eNOS, among which 894G>T in exon 7.
Several case-control studies aimed to check whether the NO correlation with cancer and stroke risk exists, especially its polymorphism eNOS, as well as how these factors may impact the development of cancer and stroke risk. Hence, we conducted a meta-analysis to provide a clearer understanding of the association between NO levels and both cancer risks.
The research method used was a meta-analysis. Our meta-analysis adhered to the criteria recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, or PRISMA. To discover relevant primary articles, we performed a comprehensive search of digital databases, including PubMed, Science Direct, and Google Scholar, to identify all relevant studies on the correlation of NO especially eNOS 894>GT, and risk to cancer and stroke. The search period was limited from December 2022 to January 2023. We applied the following keywords: NO or NOS or eNOS or “eNOS 894G>T” AND “polymorphism” AND “cancer risk” AND “stroke risk”. To ensure a comprehensive review of the literature, we conducted a thorough examination of the reference lists included in the recognized literature.
Determination of inclusion and exclusion criteria followed PICOS (Problem, Intervention, Comparison, Outcome, and Study design). The included studies were carefully examined to ensure their relevance and quality for our study. No national restrictions were imposed, meaning studies from all countries were considered eligible for inclusion. The research that met the eligibility criteria was carefully selected for our analysis: 1) Articles published in the English language that investigated the correlation between NO, especially eNOS 894G>T, and the risk of cancer and stroke; 2) Designed as a case-control study; 3) Articles that provided detailed data on genotype and allele frequencies of eNOS gene polymorphisms, which has sufficient data for the calculation of the odds ratio (OR) and the confidence interval of 95%. Therefore, studies were excluded according to as the following criteria: 1) Qualitative research; 2) No available genotype frequency; 3) Studies without control; 4) Meta-analysis studies; and 5) Animal studies.
The data in all studies were extracted when sufficient criteria were met. We then used Microsoft Excel to record the year of publication, the last name of the authors, control, and case sample sizes, and the country of the study. The results were then compared after being extracted, and an assessment was carried out along with the resolution of matters that were not appropriate through consensus. We extracted data from the nine articles meeting eligibility criteria for cancer risk and the seven articles for stroke risk association with NO.
A statistical review was implemented using RevMan, Cochrane with version 5.4 to investigate the association between NO and the risk of stroke and cancer. Crude ORs and a CI of 95% were utilized. Pooled ORs were computed for various genetic models of the eNOS G894T gene polymorphism, including GT+TT versus GG, GT versus GG, TT versus GG, T versus G, and G versus T. The eNOS gene encodes for endothelial nitric oxide synthase and has a polymorphism at position 894G>T that can result in GG, GT, or TT variants (Buldreghini et al., 2010). G represents the homozygous wild-type genotype, where the individual has two copies of the G allele. GT represents the heterozygous genotype, where the individual has one copy of the G allele and one copy of the T allele. TT represents the homozygous variant genotype, where the individual has two copies of the T allele (Hinz et al., 2013). The calculation of pooled ORs allowed to perform a Z test with a significance level of p≤0.05.
The presence of heterogeneity among the studies included was assessed with a Q test score. If there was no significant hterogeneity, i.e., p>0.10, the effect model was applied consistently. Otherwise, when (p<0.10), the random-effects model was utilized. The diversity of the included research was assessed using the I2 test, which quantifies the degree of heterogeneity. If the I2 value was less than 25%, it indicated no heterogeneity. If the I2 value ranged from 25% to 50%, it showed moderate heterogeneity. If the I2 was greater than 50%, it indicated extreme heterogeneity. The 50% p-value indicating the existence of heterogeneity between the studies and a random effects model (Mantel-Haenszel technique) was implemented; conversely, if no significant heterogeneity was found, the fixed effect model is applied. Publication bias assessment was not conducted in this study based on the limited number of articles included in meta-analysis (less than 10).
The flow diagram in Figure 1 summarises the study workflow. A total of 145 articles were identified in the databases. After removing duplicates, a total of 105 studies remained. These studies were further screened by reviewing the titles and abstracts, leading to the exclusion of 56 articles that did not meet the predetermined exclusion and inclusion conditions. After carefully examining the full text of the remaining 43 records, an additional 21 articles were excluded based on both exclusion and inclusion conditions. Ten other studies (da Costa Escobar Piccoli et al., 2012; Jang et al., 2013; Rah et al., 2013; Akhter et al., 2014; Kang et al., 2014; Özçelik et al., 2014; Ben Chaaben et al., 2015; Hung et al., 2019; Lee, 2019; Tsay et al., 2019) were also excluded because of unavailable genotype frequency. Six studies (Hao, Montiel and Huang, 2010; Yao et al., 2013; Guo, 2014; Zhao et al., 2014; Abedinzadeh et al., 2020; Akbar et al., 2022) were not included in the analysis because they were meta-analysis studies.
Finally, 15 qualified articles met the eligibility criteria. Nine case-control studies examined the association between NO and cancer risk, and six case-control studies analyzed the association between NO and stroke risk. Table 1 shows the features of the 15 studies incorporated in our analysis.
| First Author/Year | Country | Risk | Case/Control | Case | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotype | Allele | Genotype | Allele | ||||||||||
| GG | TT | GT | T | G | GG | TT | GT | T | G | ||||
| (Adibmanesh et al., 2020) | Iran | Cancer | 100/100 | 28 | 28 | 44 | 100 | 100 | 57 | 6 | 37 | 49 | 151 |
| (Aouf et al., 2019) | Tunisia | Cancer | 259/169 | 149 | 20 | 90 | 130 | 388 | 73 | 18 | 78 | 114 | 224 |
| (Branković et al., 2013) | Serbia | Cancer | 150/100 | 76 | 9 | 65 | 83 | 217 | 54 | 6 | 40 | 52 | 148 |
| (Carkic et al., 2020) | Serbia | Cancer | 50/110 | 21 | 5 | 24 | 34 | 66 | 61 | 7 | 42 | 56 | 164 |
| (Koçer et al., 2020) | Turkey | Cancer | 107/100 | 74 | 1 | 32 | 34 | 180 | 65 | 1 | 34 | 36 | 164 |
| (Su et al., 2018) | Taiwan | Cancer | 1044/1200 | 825 | 10 | 209 | 229 | 1859 | 935 | 15 | 250 | 280 | 2120 |
| (Verim et al., 2013) | Turkey | Cancer | 66/88 | 7 | 10 | 49 | 69 | 63 | 31 | 13 | 44 | 70 | 106 |
| (Yadav et al., 2019) | India | Cancer | 179/173 | 88 | 20 | 64 | 104 | 240 | 96 | 8 | 59 | 75 | 251 |
| (Yanar et al., 2016) | Turkey | Cancer | 58/147 | 18 | 11 | 29 | 51 | 65 | 31 | 35 | 81 | 151 | 143 |
| (Anliaçik et al., 2019) | Turkey | Stroke | 112/160 | 21 | 14 | 77 | 44 | 40 | 38 | 19 | 103 | 57 | 61 |
| (Diakite et al., 2014) | Morocco | Stroke | 165/182 | 83 | 16 | 66 | 30 | 70 | 117 | 7 | 58 | 20 | 81 |
| (El Gohary, El Azab and Kamal El-Din, 2017) | Egypt | Stroke | 30/10 | 18 | 6 | 6 | 45 | 15 | 5 | 3 | 3 | 12 | 8 |
| (Kaur, Uppal and Kaur, 2015) | India | Stroke | 120/101 | 84 | 6 | 30 | 18 | 83 | 83 | 1 | 17 | 9 | 91 |
| (Kumar et al., 2016) | India | Stroke | 250/250 | 164 | 12 | 74 | 20 | 80 | 186 | 5 | 59 | 14 | 86 |
| (Shyu et al., 2017) | Taiwan | Stroke | 229/243 | 151 | 16 | 62 | 21 | 50 | 185 | 7 | 51 | 13 | 87 |
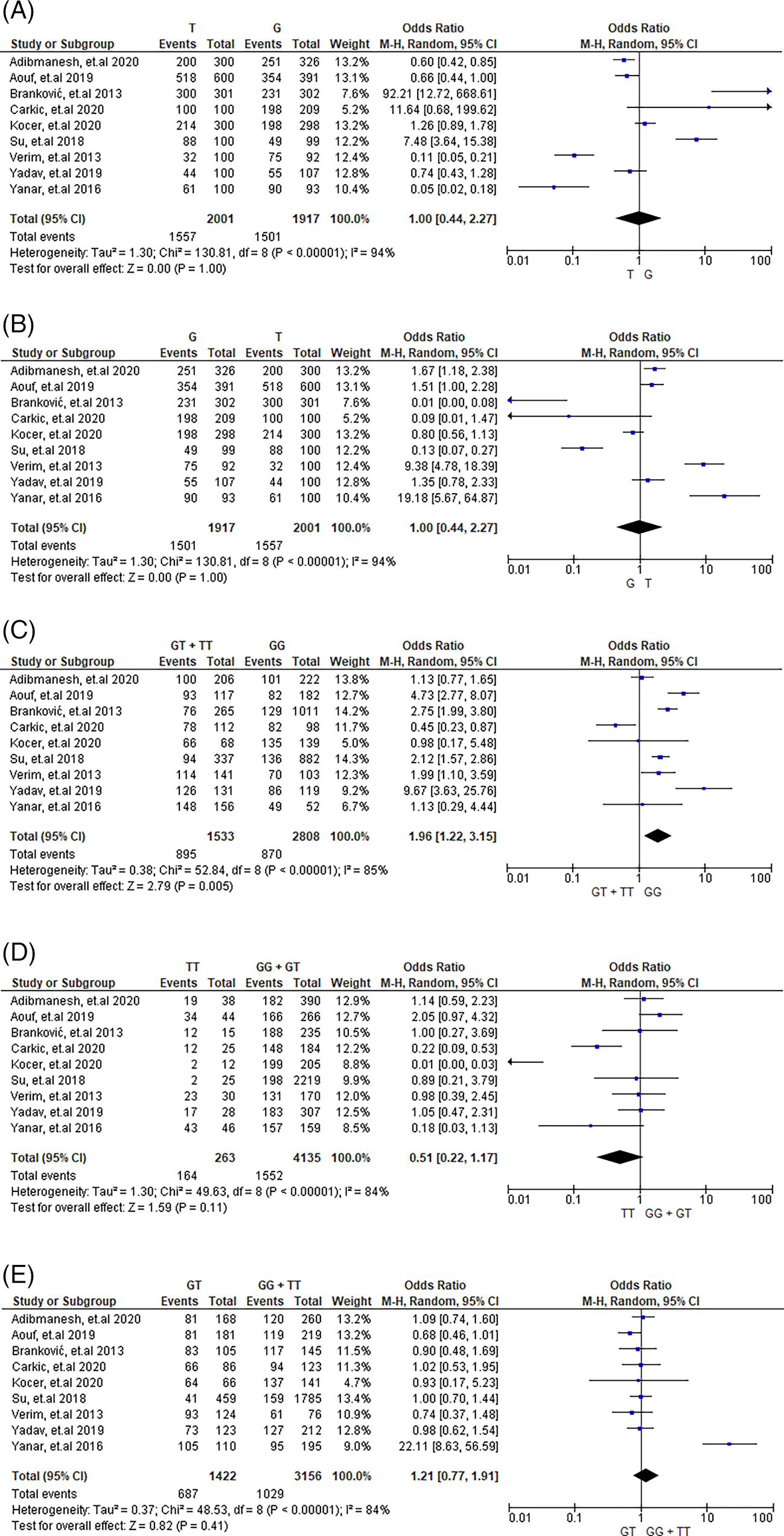
A total of 15 studies investigating an association between NO, cancer, and stroke risk, especially, the endothelial nitric oxide G894T polymorphism, were included. In our analysis, we identified a total of 2,013 cases and 2,187 control subjects for assessing cancer risk, as well as 1006 cases and 1146 control subjects for evaluating stroke risk. Table 2 presents the aggregated outcome polymorphism through meta-analysis and its association with cancer risk. The results showed a substantial correlation of cancer risk and the eNOS polymorphism G894T, with comparisons of T versus G yielding an OR of 1.00 (95% CI 0.44 to 2.27, where p=1.00), G versus T, where the OR was 1.00 (95% CI 0.44 to 2.27) with p=1.00, and TT versus GG+GT, where the OR was 0.51 (95% CI is 0.22 to 1.17 and p=0.11), comparison of GT versus GG+TT with an OR of 1.21; 95% CI is 0.77 to 1.91, and p=0.41).
| Genetic Models | NS | Pooled ORs (95% CI) | p valuea (Z test) | I2 (%) | pH | pE | Method |
|---|---|---|---|---|---|---|---|
| T vs G | 9 | 1.00 (0.44,2.27) | 1.00 | 94 | <0.00001 | 0.17497 | Ramdom model |
| G vs T | 9 | 1.00 (0.44,2.27) | 1.00 | 94 | <0.00001 | 0.34809 | Ramdom model |
| GT+TT vs GG | 9 | 1.96 (1.22,3.15) | 0.005 | 85 | <0.00001 | 0.42245 | Ramdom model |
| TT vs GG+GT | 9 | 0.51 (0.22-1.17) | 0.11 | 84 | <0.00001 | 0.03886 | Ramdom model |
| GT vs GG+TT | 9 | 1.21 (0.77-1.91) | 0.41 | 84 | <0.00001 | 0.13326 | Ramdom model |
Table 3 presents the consolidated findings of the meta-analysis, demonstrating the significant association between the eNOS G894T gene and the risk of stroke where the comparison is T versus G, namely the OR of 1.20 (95% CI=1.01 to 1.43) with a p of 0.04 and the TT versus GG+ GT comparison with an OR of 0.08 (95% CI, namely 0.03 to 0.30), where p is 0.0001. However, a significant correlation was not found in G versus T, where the OR is 0.88 (95% CI, namely 0.74 to 1.05) with p=0.15, and in TT versus GG+GT, where the OR was 0.68 (95% CI was 0.24 to 1.93 and p=0.47) compared with GT versus GG+TT, where the OR was 1.03 (95% CI was 0.40 to 2.64, and p=0.95).
| Genetic models | NS | Pooled ORs (95% CI) | p valuea (Z test) | I2 (%) | pH | pE | Method |
|---|---|---|---|---|---|---|---|
| T versus G | 6 | 1.20 (1.01,1.43) | 0.04 | 45 | 0.11 | 0.11583 | Fixed model |
| G versus T | 6 | 0.88 (0.74,1.05) | 0.15 | 0 | 0.86 | 0.17541 | Fixed model |
| GT+TT versus GG | 6 | 0.68 (0.24-1.93) | 0.47 | 91 | <0.00001 | 0.17523 | Ramdom model |
| TT versus GG+GT | 6 | 0.09 (0.03-0.30) | 0.0001 | 94 | <0.00001 | 0.44879 | Ramdom model |
| GT versus GG+TT | 6 | 1.03 (0.40-2.64) | 0.95 | 92 | <0.00001 | 0.29785 | Ramdom model |
Heterogeneity was observed among the studies in every allele and gene, as depicted in Figures 2 and 3 (T versus G, G versus T, GT+TT versus GG, TT versus GG + GT, and GT versus GG + TT). Tables 2 and 3 provide information on the selected model (random or fixed effect) utilized in order to review universal genetic model correlations.
NO acts as a crucial part of numerous pathological and psychological processes. NO is extensively implicated in various events related to cancer, including angiogenesis, metastasis, invasion, and apoptosis, which various studies have investigated; results have provided evidence that increased concentrations of NO within cancer cells can effectively suppress tumor angiogenesis and metastasis (Zhao et al., 2014). Conversely, low levels of NO in tumor cells may facilitate tumor growth by reducing NO-induced apoptosis (Heller, 2008). These observations suggest that the effects of NO on cancer development may be strongly dependent on the local NO concentration. NO is the result of three types of NOS isoforms, namely nNOS, eNOS, and iNOS. These enzymes facilitate the conversion of l-arginine to l-citrulline through oxidation (Vanini, Kashfi and Nath, 2015). eNOS is one of the three isoforms of NOS responsible for synthesizing NO in humans. Moreover, this particular isoform is closely linked to angiogenesis, which is associated with NO synthesis in both normal and cancerous cells (Song et al., 2013). A polymorphism was found in the gene encoding eNOS that could alter the production of NO. The G894T variation, also known as the guanine polymorphism to thymine with position 894 and exon 7 (rs1799983), is of particular interest (Akbar et al., 2022).
From several studies that were selected for our meta-analysis, the polymorphism was correlated with increased cancer risk in both African, European, and Asian countries. Adibmanesh et al. (2020) stated that the eNOS polymorphism 894G>T showed a significant correlation with colorectal cancer existing in the T allele genotype. Aouf et al. (2019) stated that NOS3 G894T substantially increases the risk of nasopharyngeal carcinoma (NPC) in a population from Tunisia. Furthermore, research conducted by Branković et al. (2013) suggested that NOS3 894G>T genetic polymorphisms were not associated with the risk of prostate tumor in a community in Serbia but may be relevant as prognostic factors for the progression of prostate cancer and patients’ outcome. In the study by Carkic et al. (2020), the results proved that eNOS had a significant impact on oral squamous cell cancer (OSCC) in the Serbian population. Koçer et al. (2020) stated that there was no significant association between the eNOS G894T gene and the risk of lung cancer, where p was greater than 0.05. Research by Su et al. (2018) found no relationship between OSCC and eNOS holotypes in Taiwan. Research by Verim et al. (2013) proved the existence of NOS3 is crucial in increasing the susceptibility to bladder cancer within the Turkish population. Based on the research by Yadav et al. (2019), it is suggested that eNOS 894G > T polymorphisms play a role in influencing the risk of epidermoid cell cancer of the head and neck in the population of North India. Additionally, a study conducted by Yanar et al. (2016) suggests a potential association between the G894T variation of NOS3 and the possibility of laryngeal cancer (LC), possibly due to the involvement of impaired redox homeostasis.
The role of nitric oxide in cancer can be seen in Figure 4. Based on Figure 4, overproduction of NO can facilitate tumor angiogenesis and metastasis. The NOS isoforms that produce NO in the vascular endothelium are defined as endothelial NOS (eNOS), which is found in the endothelium and carries out a crucial role in regulating vascular tone under normal conditions, which is involved in carcinogenesis and contributes to tumor protection (Lim et al., 2008). One possible explanation for the role of this enzyme in cancer progression is that reduced eNOS enzyme activity may lead to a functional decrease in NO levels within the tumor microenvironment, thereby promoting tumor growth. Recently, single nucleotide polymorphisms (SNPs) have been discovered in the eNOS gene. One of these SNPs, located at exon 7 (894G>T). Regarding to the functional role of NO in regulating angiogenesis in cancer, it is possible that this SNP might be positively correlated with the cancer progress by affecting NO synthesis.
Stroke ranks as the second-leading major contributor to mortality and disability in adults, after coronary heart disease (WHO, 2020). Stroke is a multifactorial disease; Epidemiological studies and animal experiments have provided indications of a genetic impact on the development of ischemic stroke (IS) (Hassan and Markus, 2000). Family history also serves as a crucial factor in assessing the potential for stroke. Endothelial NO, synthesized by eNOS, acts as a significant part of regulating blood flow and exhibits anti-proliferation and anti-inflammatory substances. eNOS polymorphism has a significant impact on endothelial dysfunction. The G894T variant of eNOS has been implicated in the development of diverse conditions, consisting of cardiovascular diseases and erectile dysfunction. A compromised NO-dependent vasomotor response is believed to be involved in the pathophysiology of stroke (Kaur, Uppal and Kaur, 2015). Because of its significant role in vascular physiology, genetic mutations may contribute to stroke pathogenesis by altering the expression and enzymatic activity of eNOS.
External influences that affect eNOS cause cancer and stroke through chronic stress. eNOS activity is regulated by adrenaline (Seya et al., 2006; Kou and Michel, 2007; Figueroa et al., 2009; Barbieri et al., 2012). Prolonged stress can act as a contributing factor in the onset and advancement of cancer. Stress is also considered a relevant factor in cancer development (Antoni et al., 1978; Chida et al., 2008; Desaive and Ronson, 2008). eNOS plays an essential role in ensuring vascular homeostasis, which includes regulating vascular integrity, blood flow, cell adhesion, angiogenesis, vascular permeability, immune response, and metabolism. Additionally, chronic stress can elevate the production of specific growth factors that enhance blood supply (Heid, 2014). This can accelerate the progression of cancerous tumors. Furthermore, stress can lead to increased cardiac burden, elevated blood pressure, and raised levels of sugar and fat in the bloodstream (Heart and Stroke, 2023). These factors can elevate the risk of cerebral blood clot formation, resulting in increased susceptibility to stroke.
From for the studies examined for the present meta-analysis, the presence of the eNOS polymorphism 894G>T has been correlated with a raised susceptibility to stroke in individuals of African, Asian, and European ancestry. Research conducted by Anliaçik et al. (2019) indicates there is no significant relationship between eNOS G894T and ischemia stroke among the Anatolia population. Diakite et al. (2014) stated that a significant relationship has been observed between the eNOS polymorphism 894G>T and ischemia stroke found in dominant, recessive, and additive models in the Moroccan population. Furthermore, research conducted by El Gohary, El Azab, and Kamal El-Din (2017) stated that no significant association was found between the eNOS polymorphism G894T and immediate stroke in Egyptian patients.
Research conducted by Kaur, Uppal, and Kaur (2015) stated that the G894T variant has been found to be associated with ischemic stroke and may contribute to ischemic stroke susceptibility in the Northern Indian population Kumar et al. (2016) suggest that the G894T eNOS can be a determinant of ischemic stroke, mainly for the large vessel disease (LVD) subtype, in the Northern Indian population. Additionally, a study by Shyu et al. (2017) reported that genotypic polymorphisms of the eNOS G894T polymorphism were given or used as an optimization of the risk of atherosclerotic stroke in Taiwan.
The role of nitric oxide in stroke can be seen in Figure 5. Based on Figure 5, low levels of NO derived from eNOS may exert neuroprotection in stroke by promoting vasodilatation and increasing cerebral blood flow (Yang et al., 2019). However, at the same time, there is an enhancement in superoxide production due to eNOS uncoupling. This leads to a significant increase in peroxynitrite formation, which damages lipids, proteins, and DNA and can trigger activation of poly adenosine diphosphate rribose (ADP-ribose) polymerase (PARP), which all contribute to neurotoxicity in stroke.
We conducted a meta-analysis considering the association or correlation of nitric oxide, especially NOS 894G>T polymorphism with stroke, and cancer risk. A total of 15 studies included 3,333 controls and 3,019 cases were selected for this meta-analysis. Overall, eNOS polymorphism 894G>T was found to be significantly correlated with increased cancer risk in the GG versus GT+TT genetic model. However, there were no substantial associations in the genetic model as examined in G versus T, T versus G, GT versus GG+TT, and TT versus GG+GT. The polymorphism has a relationship with a substantially higher risk of stroke in the genetic models of both TT versus GG+GT and T versus G.
In conclusion, the recent meta-analysis found that nitric oxide-related polymorphisms with the eNOS 894G>T gene are associated with a substantial risk of cancer in the total population based on the GG vs. GT+TT genetic model and significantly correlated with the manifestation of stroke in the genetic models T vs. G, and TT vs. GG + GT. G vs. T, and GG + GT vs. TT. Considering the conclusion, these results should be reassessed in the coming days through studies with a larger sample population.
All underlying data are available as part of the article and no additional source data are required.
Zenodo: PRISMA Checklist for “Association between nitric oxide and cancer and stroke risk: A meta-analysis”, https://doi.org/10.5281/zenodo.8031323 (Tualeka et al., 2023).
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Yes
Are sufficient details of the methods and analysis provided to allow replication by others?
Yes
Is the statistical analysis and its interpretation appropriate?
Yes
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: NO and cancer
Are the rationale for, and objectives of, the Systematic Review clearly stated?
Partly
Are sufficient details of the methods and analysis provided to allow replication by others?
Partly
Is the statistical analysis and its interpretation appropriate?
Partly
Are the conclusions drawn adequately supported by the results presented in the review?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Gene polymorphisms, meta-analysis, risk association, molecular epidemiology, medical biotechnology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 3 (revision) 10 Jun 24 |
read | read | ||
|
Version 2 (revision) 28 Mar 24 |
read | |||
|
Version 1 13 Nov 23 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)