Keywords
Allium sativa, ajoene, alliin, allicin, diallyl sulphide, diallyl disulphide, diallyl trisulphide, molecular docking, ProTox, SwissADME
This article is included in the Bioinformatics gateway.
Allium sativa, ajoene, alliin, allicin, diallyl sulphide, diallyl disulphide, diallyl trisulphide, molecular docking, ProTox, SwissADME
The rise in use of traditional medicines, mainly due to due to cost implications of conventional medicines and their availability, has led to the clarion call for their integration in treatment and prevention of diseases. The World Health Organization (WHO) estimates that up to 80% of the world’s population in one way or another uses herbal medicines in management and prophylaxis of diseases (2014–2023 WHO Traditional Medicine Strategy, n.d. (https://www.who.int/publications/i/item/9789241506096)). Herbal medicines have been used to manage a myriad of conditions that include and are not limited to cancer, respiratory conditions, diabetes, malaria and heart conditions (Vargas-Mendoza et al., 2019). In fact, the current WHO recommended regimen for acute and severe malaria contains artemisinin derived from Artemisia annua. Use of herbal medicines is thus on the rise due to high embracement among the population at large. The belief is that herbal medicines are cheap, easily accessible, and less toxic than conventional medicines.
It has been estimated that 10% of the human population in the world suffers from diabetes mellitus, since one in every 11 people have diabetes mellitus (Wszola et al., 2021). Projections estimate that, by 2025, 5.4% of human population worldwide will have diabetes mellitus (Wszola et al., 2021). Derangements in metabolism of glucose occurs due to insulin release impairment from the pancreas (Kumar et al., 2011). Natural remedies have been exploited in managing the complications associated with type 2 diabetes mellitus. Allium sativum has been exploited due to its flavonoid and antioxidant activity (Ojo et al., 2021). Moreover, Allium sativum has been exploited for its anticancer properties as well as in management of SARS-CoV-2 infection (Rajagopal et al., 2020). Allium sativum was one of the natural products exploited as an alternative to conventional medicines with an aim to curb the severity of the SARS-CoV-2 infection. Allium sativum modulates secretion of the cytokines and this is vital in regulating immunity. Bioactive agents in Allium sativum such as alliin can therefore be exploited further for management of respiratory infections (Donma & Donma, 2020).
Conventional medicines have been associated with high economic implications and copious adverse effects, which discourage adherence. Patients suffering from chronic illnesses require alternative therapies with less toxicities, which are easily accessible and cheap. There is thus the need to screen novel natural products to elucidate their pharmacological activities in the body in the attempt to provide safer medications. This study, therefore, aimed to screen novel bioactive molecules in Allium sativum for their pharmacological effects in the body, pharmacokinetic as well as toxicity profiles.
I. To identify specific targets for bioactive agents in Allium sativum using SwissTargetPrediction.
II. To identify docking scores of the bioactive agents in Allium sativum to the predicted targets and compare them to the docking scores of the standard drugs.
III. To identify pharmacokinetics properties of bioactive agents in Allium sativum using the SwissADME tool.
IV. To identify toxicity properties of the bioactive agents in Allium sativum.
(https://dx.doi.org/10.17504/protocols.io.j8nlkwm95l5r/v1)
Bioactive agents in Allium sativum, namely z-ajoene, e-ajoene, alliin, allicin, S-allyl-cysteine, diallyl sulphide, diallyl disulphide and diallyl trisulphide, were searched in the PubChem tool and their canonical SMILES were copied. The SwissTargetPrediction (http://www.swisstargetprediction.ch/) tool was opened, and the copied canonical SMILES were pasted, allowing prediction of targets.
Generated results had specific UniProt (RRID:SCR_007071) codes. UniProt was opened using Google Chrome and codes generated in the SwissTarget page were searched in UniProt. UniProt then generated specific Protein Data Bank (PDB) (RRID:SCR_012820) codes. The PDB was searched using Google Chrome, and these specific receptor codes were searched. This generated specific receptors, which were downloaded in PDF format and saved in subfolders. Five receptors of each bioactive agents were downloaded and saved in the subfolders, with each receptor having different subfolders.
The PubChem online tool was used to download the active constituents in SDF format. Standard drugs were accessed from the GeneCards (RRID:SCR_002773) database, which generated specific ligands for specific receptors. The ligands were downloaded from PubChem and saved in SDF format to the subfolders.
Auto-optimization of the ligands was done by using Avogadro (RRID:SCR_015983) Software. The ligands and the bioactives were then saved as optimized mol2 into the folders.
Chimera (RRID:SCR_002959) software was opened and the optimized ligands and bioactives were opened. Minimization of these molecules was done by clicking the structure, editing, then minimizing the structure. The minimized bioactives and ligands were saved as optimized–minimized mol2 compounds to the respective folders. Receptors were opened in Chimera and standardized by removing all the non-standard residues. The receptors were saved to the folders in PDB format and the session was closed.
The standardized receptor was opened first in Chimera followed by optimized–minimized bioactives and ligands, respectively. Surface binding analysis in Chimera was clicked, which brought up the popup allowing the output location to be set. The output location was then saved in PDBQT format to the same folders. The ligands and the receptors were specified. The binding site at the receptor was determined by setting random values at ‘search volumes’, which automatically generated the grid box at the receptor. The box was made to fit the receptor through frequent adjustments. Once the box fitted, the executable location was specified where ‘local’ output file was browsed and AutoDock Vina (RRID:SCR_011958) software present in each of the subfolders was clicked on and the ‘open’ button was tapped. The ‘Ok’ button was clicked, allowing docking of the ligands to specific receptors to occur. The binding results were generated as a popup and the bioactive agents in Allium sativum were compared with the standard compounds.
The SwissADME online tool (http://www.swissadme.ch/) was searched where the canonical SMILES of the bioactives in Allium sativum as well as the standard molecules were pasted. The red button was clicked to allow pharmacokinetics profile generation. The results were downloaded and saved to the folder.
The canonical SMILES of the bioactive agents as well as the reference drugs were copied from PubChem and pasted into the ProTox II (https://tox-new.charite.de/protox_II/index.php?site=compound_search_similarity) (RRID:SCR_018506) server. The predictions were carried out.
Allicin was docked to 11-beta-hydroxysteroid dehydrogenase 1 and was predicted to have a lower docking score of -4.3 kcal/mol compared with tacrolimus -8.5 kcal/mol, as indicated in Table 1. Allicin was as well docked to carbonic anhydrase 9 (CAIX) and 10, carboxylesterase and muscarinic receptor 5 (M5), where allicin showed lower docking scores of -3.3, -4.4, -3.3 and -3.7 kcal/mol, respectively, compared with the reference compound methazolamide (-5.5, -6.4 kcal/mol), irinotecan -8.8 and darifenacin -9.6 kcal/mol, respectively. The docking scores of allicin were lower compared with the known reference compounds.
Alliin showed the highest docking score of 5.6 kcal/mol when docked to nitric oxide synthase compared with docking to farnesoid X receptors (FXR) (-5.0 kcal/mol), gamma-aminobutyric acid (GABA) (-4.3 kcal/mol), glutamine-fructose-6-phosphate transaminase 1 (GFPT1) (-5.4 kcal/mol) and glutamate (-4.2 kcal/mol). All the reference compounds, chenodeoxycholic acid (-9.0 kcal/mol), vigabatrin (-4.6 kcal/mol), famotidine (-6.4 kcal/mol), cyclothiazide (-7.3) and doxorubicin (-9.7 kcal/mol), showed higher docking scores to the named receptors compared with alliin, as illustrated in Table 1.
Diallyl sulphide, diallyl disulphide and diallyl trisulphide were docked to acetylcholinesterase receptor and the docking scores were in close range, with both diallyl disulphide and trisulphide giving a docking score of 3.9 kcal/mol and diallyl sulphide 3.8 kcal/mol. These docking scores were, however, lower than that of galantamine docking to acetylcholinesterase with -7.2 kcal/mol, as shown in Table 1.
Diallyl sulphide was docked to both carbonic anhydrase 1 and CAIX, where the docking scores were 03.5 and -3.0 kcal/mol, respectively, compared with methazolamide that showed double the docking the affinity for these receptors.
Diallyl disulphide and diallyl trisulphide were docked to COX 1 and 2 receptors where they showed lower docking scores to the reference ibuprofen molecule.
Ajoene showed relatively good results but still lower docking scores to ramelteon, when docked to both melatonin 1A and 1B receptors, with -3.6 and -4.2 kcal/mol, respectively. Ramelteon docking scores to melatonin 1A and IB were -5.6 and -5.2, kcal/mol respectively. Ajoene showed some affinity for muscarinic receptors with docking scores of -8.5 kcal/mol for the muscarinic 1 receptor (M1) and -6.6 kcal/mol for the muscarinic 3 receptor (M3), as indicated in Table 1.
Methazolamide was predicted the safest when taken orally, with an LD50 of 1460 mg/kg, as shown in Table 2, compared with allicin (874 mg/kg). Irinotecan was slightly more lethal, with a lower LD50 of 765 mg/kg compared with allicin. Tacrolimus was predicted the most lethal, with an LD50 of 134 mg/kg. Allicin is, therefore, a slightly safer compound compared with the reference compounds with the exception of methazolamide.
Alliin was predicted the safest when taken orally, with an LD50 of 8000 mg/kg, compared with the reference compounds chenodeoxycholic acid (2000 mg/kg), vigabatrin (3000 mg/kg), famotidine (4000 mg/kg) and cyclothiazide (5000 mg/kg), as indicated in Table 2.
Flutamide was predicted to have a higher LD50 of 4000 mg/kg compared with diallyl sulphide with 2980 mg/kg. Diallyl sulphide, however, was predicted to be class VI, which translates to non-toxic, while flutamide was predicted to be class V, which translates to that the compound may be harmful when swallowed.
Diallyl disulphide, diallyl trisulphide, succinylcholine, ibuprofen and phenelzine were predicted to belong to class III of toxicity classification that translates to the compounds being toxic when swallowed.
Ajoene was predicted to have an LD50 of 1600 mg/kg, which is higher than pirenzepine (500) and tiotropium (263). Ajoene belongs to class IV, which is ajoene may be harmful when swallowed, while pirenzepine and tiotropium belongs to class III that translates to they may be toxic when swallowed, as illustrated in Table 2.
Diallyl disulphide was predicted to be carcinogenic active and tumour suppressor p53 active, with probabilities of 0.56 and 1.00, respectively. Diallyl sulphide was shown to be carcinogenic active, with a probability of 0.62. Diallyl trisulphide was predicted to have activity on tumour suppressor P53, with a probability score of 0.65. The rest of the parameters such as hepatoxicity, immunogenicity and cytogenecity were all inactive in all compounds.
All the bioactive agents in Allium sativa showed high gastrointestinal tract (GIT) absorption, as illustrated by Table 3. Allicin, diallyl sulphide, diallyl disulphide and diallyl trisulphide were predicted to permeate the blood–brain barrier (BBB) in the central nervous system (CNS). Ajoene and S-allyl cysteine does not permeate the BBB. None of the bioactive agents were P-glycoprotein efflux pump substrates.
Activities of the bioactive agents to cytochrome enzyme was predicted, as indicated in Table 3. None of the compounds were predicted to be inhibitors of cytochrome P450 1A2 (CYP1A2), CYP2D6, CYP3A4 and CYP2C19. All the compounds showed a lack of activity towards CYP2C9, with the exception of ajoene, as indicated in Table 3.
The interaction of alliin’s pharmacophore and the FXR receptor is due to van der Waals, conventional hydrogen bond, carbon–hydrogen bond, cation–pi as well as pi–sulphur bonds, which indicate possible ligand and protein interactions (see Figure 1).
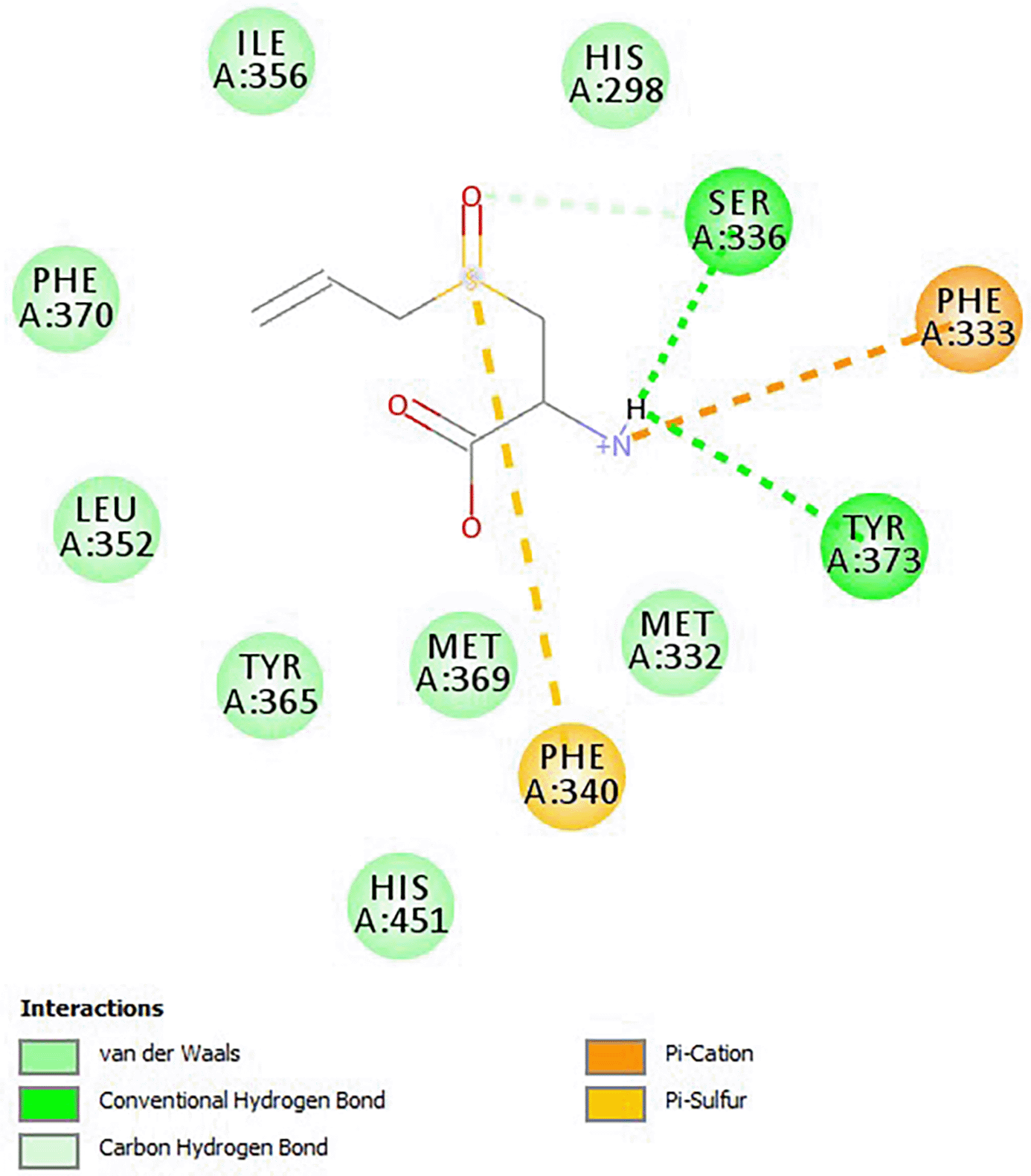
Note: From molecular docking studies on the anti-fungal activity of Allium sativum (garlic) against mucormycosis (black fungus) by BIOVIA discovery studio visualizer 21.1.0.0 by Shaweta, S., et al. (2021), https://doi.org/10.17352/aaa.000013. Annals of Antivirals and Antiretrovirals, 028–032.
Allicin was docked to 11-beta-hydroxysteroid dehydrogenase 1 (11B-HSD), which regulates exposure of glucocorticoids to tissues (Hardy et al., 2018) and showed lower a docking score of 4.3 kcal/mol compared with tacrolimus, with 8.5 kcal/mol. Tacrolimus, however, was predicted to be more lethal than allicin. Tacrolimus showed an oral LD50 of 134 mg/kg compared with allicin, with 874mg/kg. Based on these results, allicin showed some potency in activating 11B-HSD and, therefore, allicin has some anti-inflammatory effects in the body with less oral toxicity than tacrolimus.
Carbonic anhydrase 9 (CAIX) hydrates carbonic dioxide to bicarbonate and protons, and these reactions are vital in maintaining the acid base balance in the plasma and the cells. CAIX is a surface glycoprotein induced by hypoxia expressed mainly in cancerous cells. Expression of CAIX in non-cancerous cells such as the lining of the gallbladder, stomach and intestines is minimal. Deficiency of the CAIX in the stomach causes hyperplasia of the parietal cells, impairing basolateral regulation of pH in the stomach, causing a perforated GIT mucosal barrier and leading to chronic inflammation of the GIT (Pastorekova & Gillies, 2019). CAIX is, therefore, important in the defence mechanism of the stomach from acid overload. Allicin was docked to this enzyme and showed a lower binding score of -3.3 kcal/mol compared with methazolamide (-5.5 kcal/mol). Diallyl sulphide was also docked to this enzyme and showed a lower docking score of -3.0 kcal/mol compared with methazolamide (-6.5 kcal/mol). Based on these results, allicin and diallyl sulphide have potential activity to CAIX and, therefore, have a role in regulation of acidosis in the body. They are potentially protective against the acid and hypoxia in normal as well as in cancerous cells. Methazolamide was predicted to be safer than allicin. Methazolamide showed an oral LD50 of 1460 mg/kg compared with allicin, with 874 mg/kg. Diallyl sulphide was predicted to be safer than methazolamide. Diallyl sulphide showed an oral LD50 of 2980 mg/kg compared with 1460 mg/kg for methazolamide.
The M1 and muscarinic 3 (M3) receptors activation causes production of interleukin 6 and differentiation of B cells into plasma cells. Regulation of the immune system by the muscarinic receptors occurs at the cytokine levels (Chen et al., 2022). Inflammatory reactions in the body, such as along the GIT causing peptic ulcer disease, is mediated by M1 receptors while M3 receptors are expressed along the respiratory system. Pirenzepine and ajoene were docked to M1 receptors and they generated docking scores of -8.5 kcal/mol and -4.4 kcal/mol, respectively. Tiotropium and ajoene were docked to M3 receptors and they generated docking scores of -6.6 kcal/mol and -3.6 kcal/mol, respectively. Ajoene was shown to have considerable affinity to both M1 and M3 receptors, and therefore they have an important role in inflammatory reactions. Ajoene, pirenzepine and tiotropium were predicted for oral toxicity, and they generated an LD50 of 1600 mg/kg,500 mg/kg and 263 mg/kg, respectively. Ajoene was therefore the safest in terms of LD50.
The M5 receptor is widely restricted to the brain and is involved in regulation of dopamine in the substantia nigra as well as tegmental areas of the brain. M5 receptors potentiate accumulation and transmission of dopamine in the nucleus accumbens. M5 receptors are therefore crucial in behavioural adaptations of an individual to external cues (Razidlo et al., 2022). M5 receptors are potential agents in targeting Alzheimer’s diseases and schizophrenia. Darifenacin was docked to this receptor and showed a higher docking score (-6.7 kcal/mol) compared with allicin (-3.7 kcal/mol). Allicin was, however, predicted to be safer than darifenacin, with 874 mg/kg of allicin viz a viz 300 mg/kg of darifenacin. Allicin has potential behavioural and psychological roles in the body.
Carboxylesterases are involved in hydrolysis of the exogenous products in the body such as drugs, chemicals as well as toxins. Carboxylesterases catalyse addition of water to the ester groups to generate carboxylic acids, which are polar enhancing elimination agents (Di Consiglio et al., 2021). Carboxylesterases are potentially targeted for neurodegenerative diseases. Allicin showed a -3.3 mg/kg docking score to this enzyme compared with irinotecan (-8.8 mg/kg). Allicin showed a safer oral toxicity with an LD50 of 874 mg/kg compared with irinotecan (765 mg/kg). Allicin therefore, has potential in elimination of toxins and chemicals from the body as a way of detoxification.
Bile acid synthesis maintains cholesterol homeostasis in the body by synthesis of bile acids from cholesterol. Bile acids interact with nuclear FXR to prevent hyperglycaemia, dyslipidaemia, obesity and diabetes. This helps in reduction of metabolic syndrome. FXR receptors are highly expressed in the GIT to maintain bile acid homeostasis by regulation of bile acid synthesis through feedback inhibition, preventing cholestasis and liver injury. The gut–liver–brain axis in bile acid synthesis and circadian rhythms are important in ensuring homeostasis as well as prevention of metabolic diseases and dysbiosis, which leads to a fatty liver and obesity. Activation of FXR receptors is important in glycaemic control through glucose metabolism as well as lipid metabolism, reducing inflammation (Chiang & Ferrell, 2020). Chenodeoxycholic acid and alliin were docked to the FXR receptor and showed docking scores of 9.0 and 5.0 kcal/mol, respectively. Alliin showed a lower docking score to the FXR receptor than chenodeoxycholic acid. Alliin and chenodeoxycholic acid were predicted for their oral toxicity, with 8000 mg/kg and 2000 mg/kg as LD50, respectively. Alliin, therefore, showed little affinity for the FXR receptor.
Nitric oxide (NO) is important in vasodilation, relaxation of smooth muscles as well as regulation of immune responses. NO is a free radical generated by nitric oxide synthases from oxidation of l-arginine to l-citrulline. The considerable amount of NO produced is important in defending the body against pathogens and is important in regulation of inflammatory responses. Overexpression of NO causes detrimental effects such as septic shock, pain, diabetes and cancers. Regulation of the synthesis of NO is therefore important in preventing the deleterious effects of NO (Cinelli et al., 2019). Doxorubicin and alliin were docked to NO synthase and they generated docking scores of 9.7 kcal/mol and 5.6 kcal/mol, respectively. Alliin showed substantial activity on NO synthase, and therefore has potential in decreasing oxidative stress in the body, decreasing inflammatory conditions.
Acetylcholinesterase enzymes inhibit metabolism of acetylcholine in the CNS, increasing the acetylcholine in the CNS. This is essential in management of the Alzheimer’s, decreasing the chances of dementia and improving cognitive impairment (Santos et al., 2018). Galantamine, diallyl sulphide, diallyl disulphide and diallyl trisulphides were docked to the acetylcholinesterase enzyme and generated docking scores of 7.2 kcal/mol, 3.8 kcal/mol, 3.9 kcal/mol and 3.9 kcal/mol, respectively. Diallyl sulphide, disulphide and trisulphide showed lower docking scores to galantamine. Diallyl sulphide, diallyl disulphide and diallyl trisulphide were predicted for toxicity with an oral LD50 of 2980 mg/kg, 260 mg/kg and 100 mg/kg, respectively. Diallyl trisulphide was the most toxic. Galantamine oral toxicity results were not available. The butyrylcholinesterase enzyme is important in advanced Alzheimer’s disease by acting as a compensating enzyme. Succinylcholine and diallyl trisulphide were docked to the butyrylcholinesterase enzyme generating -5.3 kcal/mol and -4.1 kcal/mol, respectively. Succinylcholine was predicted to be more lethal, with 299 mg/kg oral toxicity compared with alliin, with 8000 mg/kg.
Melatonin is a neurohormone produced by the pineal gland. Melatonin has physiological effects in the body such as anti-inflammatory, hypnotic, anticonvulsant, analgesic as well as sedative effects (Zhang et al., 2019). Melatonin binds to melatonin 1 and 2 receptors to cause these physiological effects. Ramelteon was docked to melatonin 1A and melatonin 1B receptors, generating docking scores of -5.6 kcal/mol and -5.2 kcal/mol, respectively. Ajoene showed lower docking scores to melatonin 1A and 1B, with docking scores of -3.6 kcal/mol and -4.2 kcal/mol, respectively. Ajoene has some affinity for melatonin 1 receptors, therefore they regulate the circadian rhythm.
Cyclooxygenase enzymes cause production of prostaglandins in the body. The two isoforms of cyclooxygenase include COX-1 and COX-2 enzymes. Gastroprotectivity activity is due to COX-1 enzymes (Lee et al., 2020). Ibuprofen, diallyl trisulphide and diallyl disulphide were docked to COX-1 receptors, generating docking scores of 7.5 kcal/mol, 4.0 kcal/mol and 4.2 kcal/mol, respectively. Diallyl disulphide and trisulphide showed some activity to the COX-1 receptor and therefore could have gastroprotective activity. Ibuprofen, diallyl trisulphide and diallyl disulphide were as well docked to COX-2 receptors, generating docking scores of 7.6 kcal/mol, 3.8 kcal/mol and 4.0 kcal/mol, respectively. Ibuprofen was predicted to be safer than diallyl trisulphide and diallyl disulphide. Ibuprofen was predicted to have an LD50 of 299 mg/kg compared with diallyl disulphide with 260 mg/kg and diallyl trisulphide predicted to have 100 mg/kg.
Androgen receptors repress transcription of testosterone and dehydroepiandrosterone in prostate cancer. In normal cells, the androgen receptor supplies the secretory proteins to the prostate gland. Androgen receptors are used to mediate the inflammatory processes in prostate cancer. Flutamide and diallyl sulphide were docked to the androgen receptor and generated docking scores of -8.8 kcal/mol and -3.7 kcal/mol, respectively. Diallyl sulphide was predicted to be less safe, with an oral LD50 of 2980 mg/kg compared with flutamide with 4000mg/kg.
All compounds were predicted to have high GIT absorption. All bioactives in Allium sativum were predicted to have higher BBB permeation except E-ajoene, Z-ajoene, alliin and s-allylcysteine. All compounds were predicted to lack inhibition of CYP2C9 except E-and Z-ajoene. E- and Z-ajoene when used concomitantly with warfarin and omeprazole could cause inhibition of metabolism of these drugs. All compounds complied with Lipinski’s rule of five.
None of the bioactives in Allium sativum had better docking scores than the standard drugs. However, all the bioactives showed considerable amounts of activity to the docked receptors. Activity of the bioactives in Allium sativum could be due to synergistic effects of the bioactives compared with the single pharmacological activity of the standard drugs.
Toxicity profiles indicated that allicin was less toxic than all the standard dugs except methazolamide. Alliin, E- and Z-ajoene were safer than all the standard drugs. Diallyl trisulphide was considered more lethal than the standard drugs.
In conclusion, bioactives in Allium sativum have potential physiological effects, which could be due to synergistic effects, and they are less toxic compared with the standard drugs.
Harvard Dataverse: Underlying data for ‘In silico study of Allium sativa bioactives. https://doi.org/10.7910/DVN/F7FONP (Ouma et al., 2023)
This project contains the following underlying data:
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The researchers of this study would like to express gratitude to the developers of the software and webservers, which include Chimera, Avogadro, SwissADME, SwissTargetPrediction, GeneCards and ProTox II.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Plant bioactives
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 1 09 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)