Keywords
malnutrition, ovarian failure, stem cells, good health and well being
This article is included in the Cell & Molecular Biology gateway.
malnutrition, ovarian failure, stem cells, good health and well being
Stem cell transplantation has been explored as a therapy for various diseases such as stroke, 1 Alzheimer’s disease, 2 diabetes mellitus, 3 Parkinson’s, 4 myocardial infarction, 5 HIV, 6 testicular failure, 7 , 8 and other degenerative diseases such as ovarian failure. 9 As an important organ in female reproduction, the ovary produces ovum and female hormones. 10 MSC transplantation derived from rabbit bone marrow, 11 rat bone marrow, 7 , 9 rat adipose tissue, 12 or umbilical cord blood 13 has shown promising results for tissue repair via the proliferation and development of endogenous stem cells into germ cells. 7 , 9 , 14
However, the lack of viability and differentiation of implanted stem cells results in poor adaptivity and a low survival rate, indicating that the therapy is ineffective. The low survivability and function of MSCs might be related to a higher incidence of apoptosis during culture and after transplantation. This finding indicates that the milieu of damaged tissues is not conducive to stem cell adaptation survival; hence, stem cell transplantation is not a feasible option. Recently, researchers cultivating stem cells under low oxygen tension have observed cell stress, although one study has found that the stress situation activates HSP27 as a function of anti-apoptosis via caspase-9 suppression. 15
The success of stem cell transplantations is limited by their weak adaptivity and differentiation. The low efficacy of MSC transplantation might be due to apoptosis occurring during cell growth. 15 , 16 Consequently, high doses of stem cells via regular boosters are necessary for effective therapy, which increases the therapy cost. 17 In vitro stem cell cultivation must be adapted to a niche environment, such as the microenvironment of bone marrow, to avoid apoptosis. Low O2 tension is an example of a niche habitat for stem cells in bone marrow. 18 Low O2 tension caused by in vitro culture reduces apoptosis, viability, and differentiation of stem cells, all of which promote ovarian function in ovulation production via folliculogenesis and oogenesis. The expression of HSP70 19 and caspase-3 20 in ovarian tissue inhibits apoptosis, whereas that of VEGF-1 and GDF-9 promotes viability and differentiation. 21
This study aimed to determine the role of HSP70 and caspase-3 as apoptotic inhibitors, as well as VEGF-1 and GDF-9 as viability and differentiation markers, respectively, in MSCs cultivated under low O2 tension for malnutrition-induced ovarian failure in female rats.
This research, including experiments with animals, was approved by the ethical clearance committee of the Animal Care and Use Committee of the Veterinary Medicine Faculty, Universitas Airlangga with number 239-KE. Experiments were performed in adherence with the guideline manual by the ethical committee. All efforts were undertaken to ameliorate animal suffering.
Bone marrow stem cells were extracted by aspirating the iliac crest 7 , 9 of three-month-old male rats. 22 The aspirate was placed in heparinized tubes (Z181099, Sigma Aldrich®, Burlington, Massachusetts, USA), which was then transported to the lab and kept at 4°C. 7 , 9 , 23 The aspirate was transferred to 15-mL sterile tubes (SIAL0790-500EA, Sigma Centrifuge tubes, Sigma Aldrich, Burlington, Massachusetts, USA), cleaned two times with 5 mL of sterile phosphate-buffered saline (PBS; MFCD00131855, Sigma Aldrich, Burlington, Massachusetts, USA), and then filled to a final volume of 10 mL. The same amount of Ficoll (F9378, Sigma Aldrich, Burlington, MA, USA) was added after diluting the sample in a separate 15-mL tube. Centrifugation (Sorvall MX Series Floor Model Micro-Ultracentrifuge, Thermo Fisher, Grand Island, NY, USA) was performed at 1,600 rpm for 15 min at room temperature. The mononucleated cells were collected in a 15-mL tube after centrifugation in a form of a “buffy coat” that had accumulated on the Ficoll–PBS surface. In PBS, the cells were resuspended in a 15-mL total volume. The tube (CLS6791 Sigma, Corning LSE Benchtop Shaking Incubator with Platform, Sigma Aldrich, Burlington, MA, USA) was inverted gently and shaken five times to homogenize the suspension.
For another 10 min, the suspension was centrifuged. The cell pellet was resuspended in 6 mL of an alpha-modified essential medium after the supernatant and floating cells were removed from the experiment (α-MEM; M0894; Sigma Aldrich, Burlington, MA, USA). Mononucleated cells were plated in a 10 cm2 plate (Falcon™, Thermo Fisher Scientific, Pittsburgh, PA, USA) with approximately 2×107 cells and incubated at 37°C in a humidified atmosphere containing 5% CO2 (BioSpherix, Canada/USA) for 24 h to allow cell adherence. Medium and non-adherent cells were removed after 24 h. The plate was returned to the incubator after the adhering cells had been rinsed two times with 5 mL of PBS and 10 mL of fresh α-MEM media. An inverted microscope was used to monitor the culture every day. The medium was replaced every four days, followed by washing with 10 mL of PBS before adding 10 mL of fresh α-MEM media. The culture was maintained until a convergence of about 75%–80% confluence was achieved. The cells were moved to various dishes after confluence to grow subcultures. 7 The cells were separated into two low-O2-tension treatments of 1% after three rounds in a hypoxic chamber (BioSpherix, Canada/USA) inside a 5% CO2 incubator. A separate treatment was the use of 21% O2 concentration (normoxic chamber) over four days. MSCs were observed and characterized by using a microscope before being transferred into an ovary.
MSC characterization between low O2 and normoxic culture conditions was performed before being transferred into the ovary based on morphological analysis of cells using an inverted microscope (Phase Contrast, MXD-400, Nanjing BW Optics & Instrument Co., Ltd). HSP70 and caspase-3 were identified via real-time polymerase chain reaction (PCR; MxPro software, PCR, [Cat No. 401513]; Brilliant II QPCR Low ROX Master Mix [Cat No. 600806], Agilent 2100 Bioanalyzer system [Cat No. G2939AA], Macherey-Nagel GmbH & Co [Düren, Germany]). Apoptosis was detected using immunofluorescence (Apoptag Fluorescein In Situ Apoptosis Detection Kit, S7110, Sigma Aldrich, Burlington, MA, USA). 15
Real-time PCR was performed using the following method. The MSC-containing media were aspirated, rinsed two times with 1 mL of PBS, and then added with 500 μL of RNAiso/well. Afterward, 100 μL of chloroform was poured into a small tube containing scraped-off cells. Then, the tube was repeatedly shaken. Centrifugation was performed to separate the supernatant (250–300 μL) for 15 min at 12,000 rpm and 4°C. Only RNA molecules remained in the tube after the supernatant was removed; thus, 500 μL of 75% ethanol vortex was added until the pellet floated. At 12,000 rpm and 4°C, the samples were centrifuged for 5 min. After the supernatant was removed from the tube, it was dried for 5–10 min before adding 25 μL of Aqua bidest. The RNA concentration suitable for spectrophotometric measurement and purification was 1.7–2.1 μL in 1 μL of the tested sample. 15
The RNA sample was then added to 11 μL of Aqua bidest, and 9 μL of the combined component was placed into a new tube and mixed with 11 μL of RNA samples. Thereafter, the mixture was incubated in a PCR machine followed by pre-denaturation at 37°C for 15 min, denaturation at 65°C for 10 min, annealing at 65°C for 10 min, and final extension at 65°C for 2 h. All stages were repeated for 40 cycles. This identification was used a primary sequence template CD44f (5′-TCC CAG TAT GAC ACA TAT TGC-3′) and CD44r (5′-CAC CTT CTT CGA CTG TTG AC-3′) (Oligo Macrogen, Seoul, Korea). Real-time PCR identification was prepared for 20 e-DNA samples for 30 min.
Female rats used in this study were fasted for five days but given unlimited access to water, creating a rat model with ovarian failure. 21 , 24 Female rats aged 12 to 14 weeks and weighing 250 to 300 g served as the study's model animals. In the experimental animal facility of the Faculty of Veterinary Medicine, Universitas Airlanggas, the rats were housed in separate plastic cages with sufficient ventilation.
The transplanted MSCs were evaluated against negative and positive control rats, as well as female rats with ovarian failure. The T1 group included 15 infertile female rats that received 200 million stem cells per rat from a four-day normoxic culture (21% O2 concentration). 7 , 9
The T2 group included 15 infertile female rats that received 200 million stem cells per rat from a four-day low O2 culture (1% O2 concentration). 7 , 9 0.1 mL of PBS injection was given to 15 healthy female rats in the fertile positive control group. A total of 15 infertile female rats were injected with 0.1 mL of PBS as part of the negative control group (infertile rat).
Ovarian tissue was collected from the ovaries of female rats 10 days after surgical excision (allowing for two estrous cycles). 21
Histopathological preparations using hematoxylin and eosin (HE) stain (B8438, Sigma Aldrich, Burlington, MA, USA) allowed researchers to detect an improvement in ovarian tissue. HSP70 expression (with monoclonal antibody HSP 70, catalog number MA3-007, Thermo Fisher Scientific, Waltham, MA USA) as anti-apoptosis was assessed by immunohistochemistry (IHC). Caspase-3 expression (with caspase-3 monoclonal antibody, E-8 7272 for IHC staining, Santa Cruz Biotechnology, LI-COR, Bioscience, Santa Cruz, CA, USA), as a pro-apoptotic factor, was inhibited. In addition, VEGF-1 expression (with a mouse monoclonal antibody, cone OTI4E3 [formerly 4E3], True MAB, OriGene, Beijing, China) served as a marker of the viability of stem cells, whereas GDF-9 (with a GDF-9 monoclonal antibody, sc-514933, Santa Cruz Biotechnology, LI-COR, Bioscience, Santa Cruz, CA, USA) served as a marker of the differentiation of stem cells into progenitor germ cells to improve ovarian failure and infertility.
Histopathological observation of ovarian tissue in the presence of a Graafian follicle and regeneration of tissue were performed through light microscopy. 25
A 10% formalin solution was used to fix the tissues of the ovaries. Subsequently, the ovaries were dehydrated using a series of progressively increasing alcohol concentrations, cleaned with xylol, and fixed in paraffin. Routine staining was performed on thin sections and placed on slides. 26
A light microscope with 400× magnification was used to conduct histopathological evaluation. Each slide’s five fields of view were evaluated. Based on an existing histological description, a Graafian follicle was observed and identified, and seminiferous tubular regeneration was performed. 21
The expression of HSP70, caspase-3, VEGF-1, and GDF-9 was examined by IHC. Samples were prepared for histological examination by making an incision transversely from the paraffin blocks of ovarian tissue. IHC used monoclonal antibodies to analyze the expression of HSP70 (catalog number MA3-007, HSP 70 monoclonal antibody, Thermo Fisher Scientific, Waltham, MA, USA), caspase-3 (caspase-3 E-8 7272 monoclonal antibody for IHC staining, Santa Cruz Biotechnology, LI-COR, Bioscience, Santa Cruz, CA, USA), VEGF-1 (mouse monoclonal antibody, cone OTI4E3 [formerly 4E3], True MAB, OriGene, Beijing, China), and GDF-9 (sc-514933, GDF-9 monoclonal antibody, Santa Cruz Biotechnology, LI-COR, Bioscience, Santa Cruz, CA, USA). Observations were performed using a light microscope with 200× magnification. Each slide’s five fields of view were evaluated to determine the score of tissue with brownish chromogen as a result of HSP70, caspase-3, VEGF-1, and GDF9-9. The IHC scoring system 27 assigned an IHC score of A×B (A=width of expression percentage; B=intensities of chromogen color; Table 1).
The expression of HSP70, caspase-3, VEGF-1, and GDF-9 and the Graafian follicle count with a 99% confidence level (= 0.01) and 0.05 significance threshold of difference (p<0.05) were statistically analyzed using SPSS (v. 17 for Windows XP; SPSS Inc, Chicago, IL, USA). The comparative steps for hypothesis testing included normality data test, Kolmogorov–Smirnov test, homogeneity of variance test, analyses of variance, and Tukey’s significant difference post hoc test with 5% least significant difference.
Data were collected from 60 female rats. The data were divided into four groups of treatment: (1) fertile female (normal rat) as the positive control group; (2) infertile female treated with PBS as the negative control group; (3) infertile female with stem cells transplanted from the four-day normoxic culture (21% O2 concentration) as the T1 group (first treatment); (4) infertile female with stem cells transplanted from the four-day low-O2 culture (1% O2 concentration) as the T2 group (second treatment). The study results revealed that MSC transplantation from the hypoxic precondition culture improved ovarian function by decreasing the damage level and increasing fertility score. The expression level of HSP70, VEGF-1, and GDF-9 was upregulated, whereas the expression level of caspase-3 was decreased; the regeneration of ovarian tissue achieved a normal histopathological figure as evidenced by the development of a follicle into a Graafian follicle.
Characteristics of MSCs between low-O2 and normoxic cultures were observed before being transferred into the ovaries to assess cell morphology, HSP27 and caspase-3 expression, and apoptosis. MSC characterization was performed using an inverted microscope after the third passage of normoxic (O2 21%) and low-O2 (O2 1%) cultures on the second day. The cells exhibited a larger cell size, fewer cell deaths, and slower proliferation rate in the low-O2 culture, whereas the cells showed a higher rate of proliferation, more cell deaths, and smaller cell sizes in the normoxic culture (Figure 1).
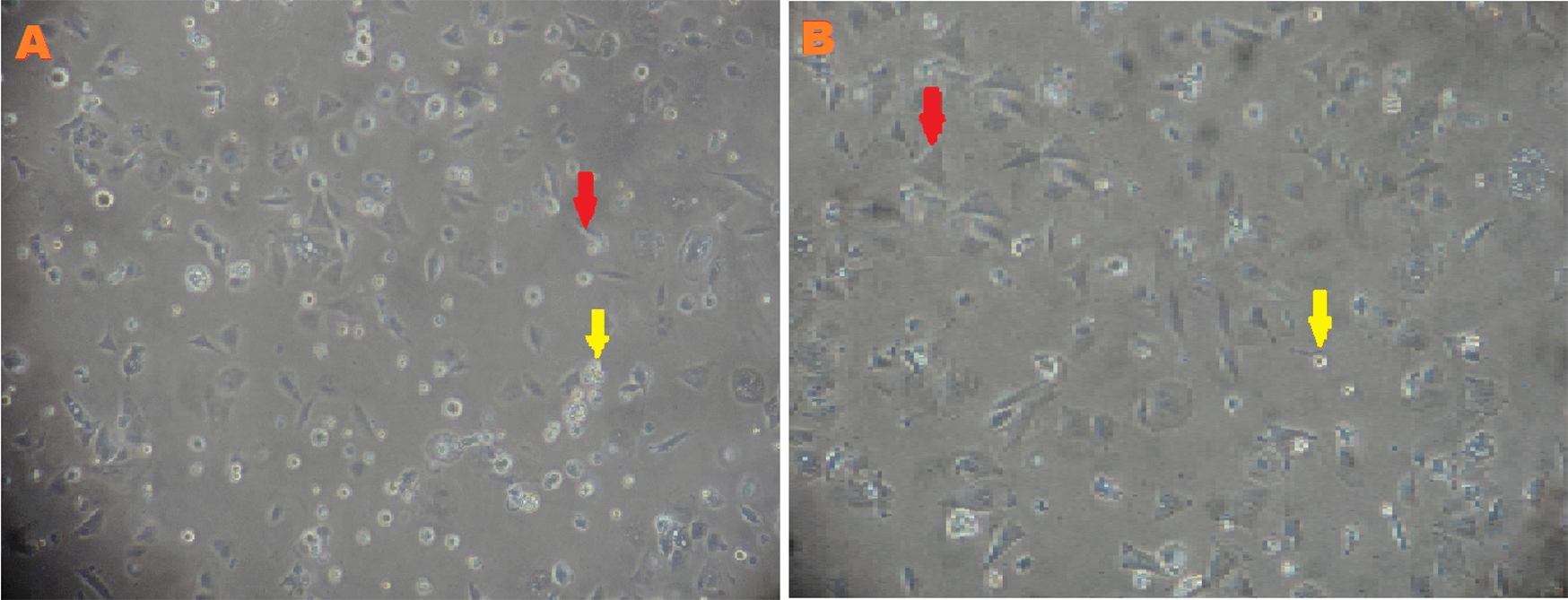
MSCs (red arrow) and cell death (yellow arrow). Magnification at 400×.
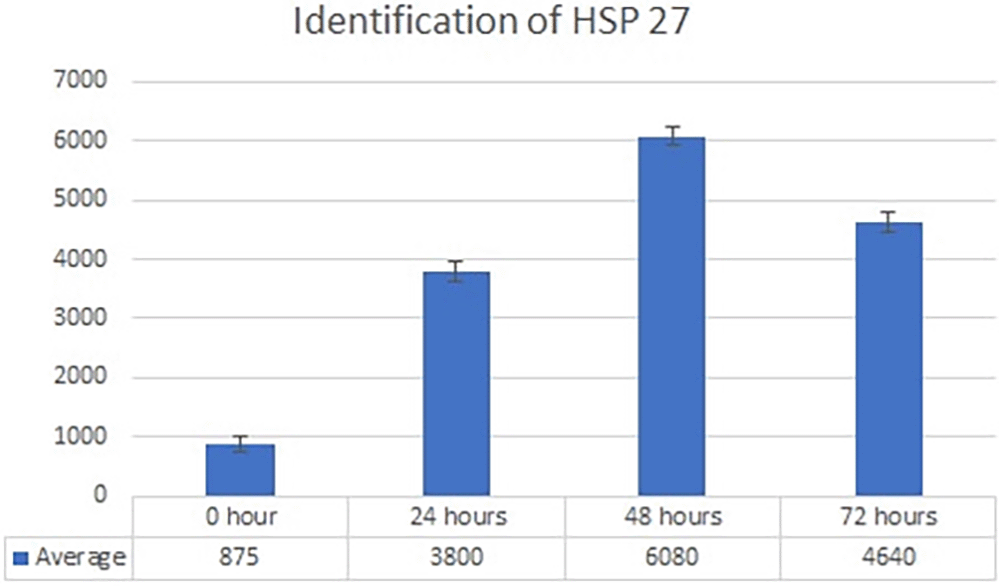
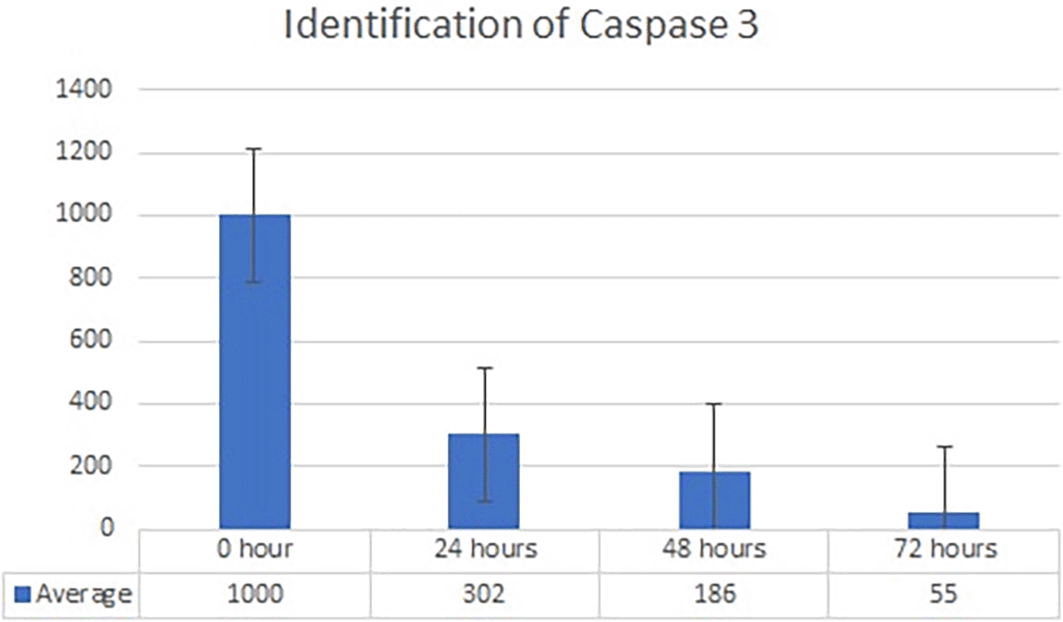
Real-time PCR analysis of HSP27 and caspase-3 expression revealed considerably and significantly increased HSP27 and decreased caspase-3 under low oxygen tension (O2 1%; p<0.01) compared with those under the normoxic culture as a control (Figures 2 and 3).
Apoptosis was detected through IHC under normoxic and low-O2 conditions. The positive expression of apoptosis reached 50% of cells cultured under a normoxic condition, whereas only 5% of cells cultured under low O2 culture in vitro until 72 h exhibited apoptotic cells (Figure 4).
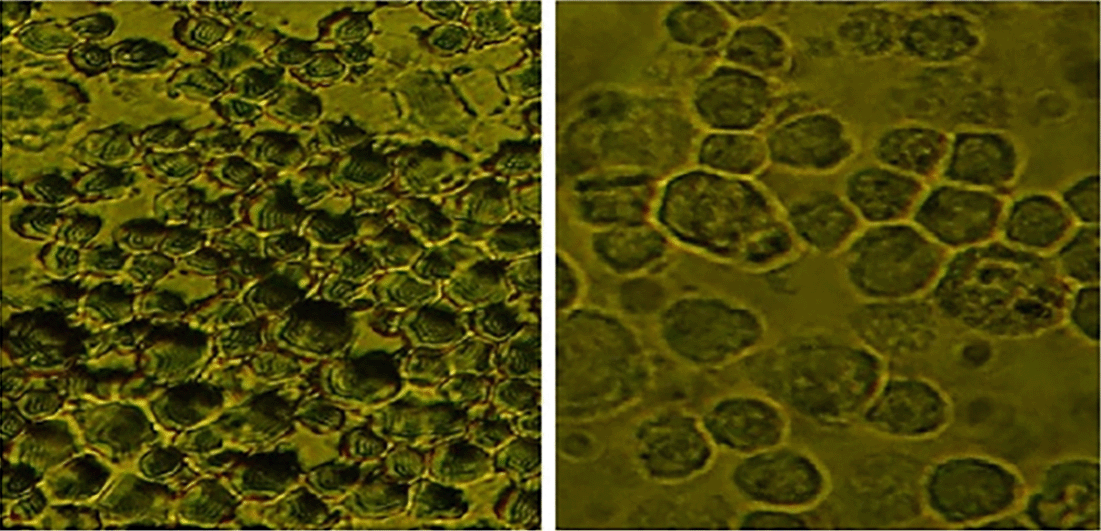
(A) Positive expression of apoptosis in normoxia (red arrow) and number of apoptotic cells reaching 25%. (B). Low-O2 culture in vitro for 72 h show a small number of apoptotic cells (5%, red arrow). Magnification at 400×.
The IHC score for HSP70 expression in ovarian tissue from four treatments is shown in Figures 2, 5 and Table 2. As shown in Table 2, the average score of HSP70 expression (brown) was 0.5a ± 0.53 for the positive (fertile) control group, 1.7a±0.82 for the T2 group, 6.2b±1.5 for the T1 group, and 9.6c±1.3 for the negative (infertile) control group.
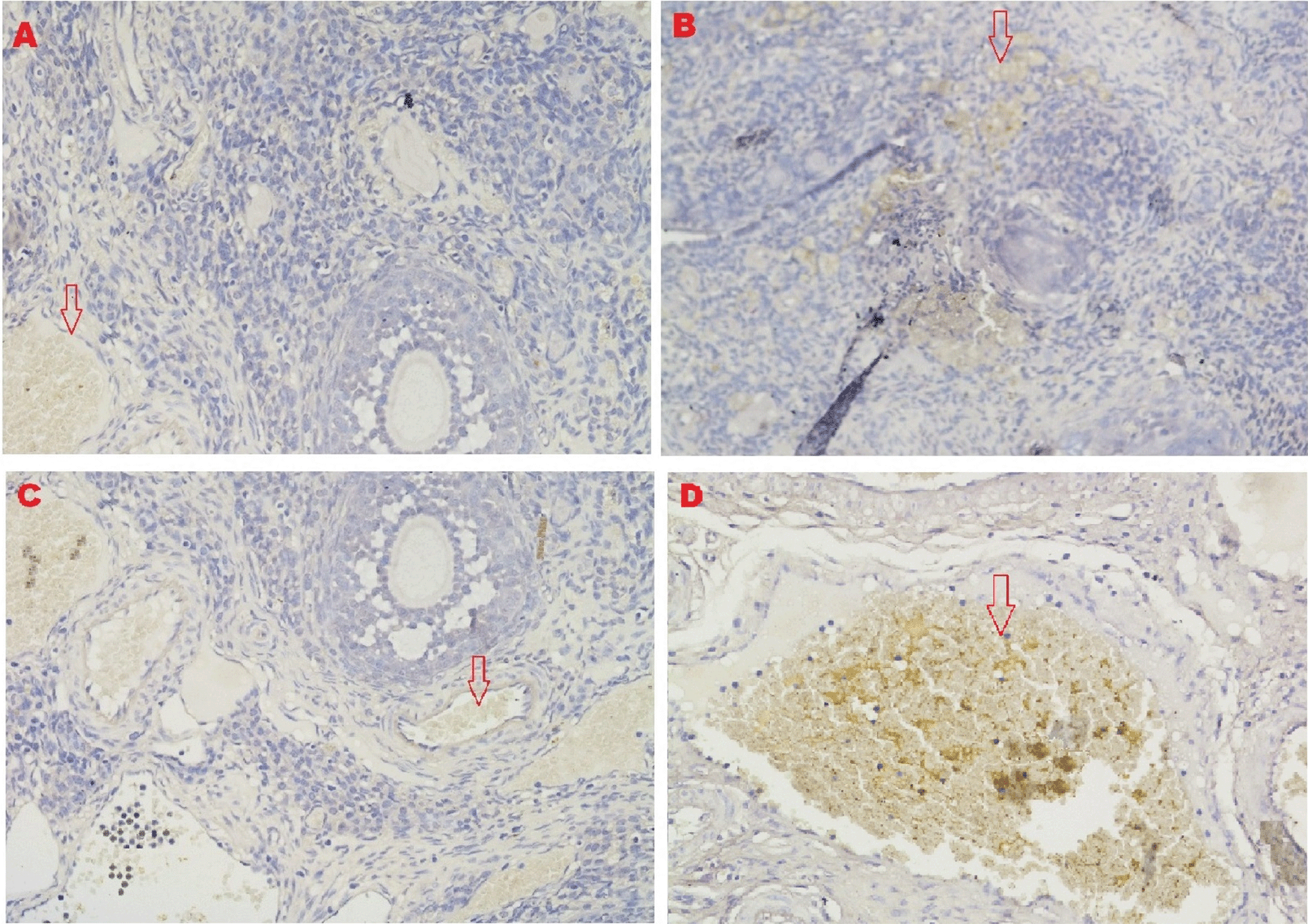
The IHC score for caspase-3 expression in ovarian tissue from four treatments is shown in Figures 3, 6 and Table 2. As shown in Table 2, the average score of caspase-3 expression (brown) was 0.2a±0.42 for the positive (fertile) control group, 0.6a±0.52 for the T2 group, 4.8b±1.03 for the T1 group, and 7.3c±1.42 for the negative (infertile) control group.
The IHC score of VEGF-1 expression in ovarian tissue from four treatments is shown in Figure 7 and Table 2. As shown in Table 2, the average score of VEGF-1 expression (brown) was 10.8c±1.55 for the positive (fertile) control group, 8.7b±0.48 for the T2 group, 0.4a±0.52 for the T1 group, and 0.2a±0.42 for the negative (infertile) control group.
The IHC score of the GDF-9 expression in ovarian tissue from four treatments is shown in Figure 8 and Table 2. As shown in Table 2, the average score of the GDF-9 expression (brown) was 5.8c±1.47 for the positive control group (fertile rats), 4.6b±0.97 for the T2 group, 0.5a±0.53 for the T1 group, and 0.3a±0.48 for the negative control group (infertile rats).
The microscopic examination from five different angles showed that the T2 group had a repaired tissue ovary. As shown in Figure 9, an improvement in ovarian tissue regeneration and Graafian follicle count was detected via microscopic examination with 400× magnification using HE staining of rat ovarian tissue from the four treatment groups. The positive (fertile) control group exhibited a Graafian follicle without hemorrhage, congestion, hemosiderin, or deposition of fibrin in the ovaries. The T2 group exhibited the regeneration of intact ovaries despite hemorrhage in some areas; however, a Graafian follicle had developed. The T1 group showed ovarian congestion and severe hemorrhage, although follicles were developed. Moreover, no Graafian follicle was observed. The negative (infertile) control group exhibited ovarian congestion, severe hemorrhage, and hemosiderin because of blood cell lysis (brownish yellow) with fibrin deposition, which indicated the presence of chronic congestion (pink colour); no follicles or Graafian follicle had developed.
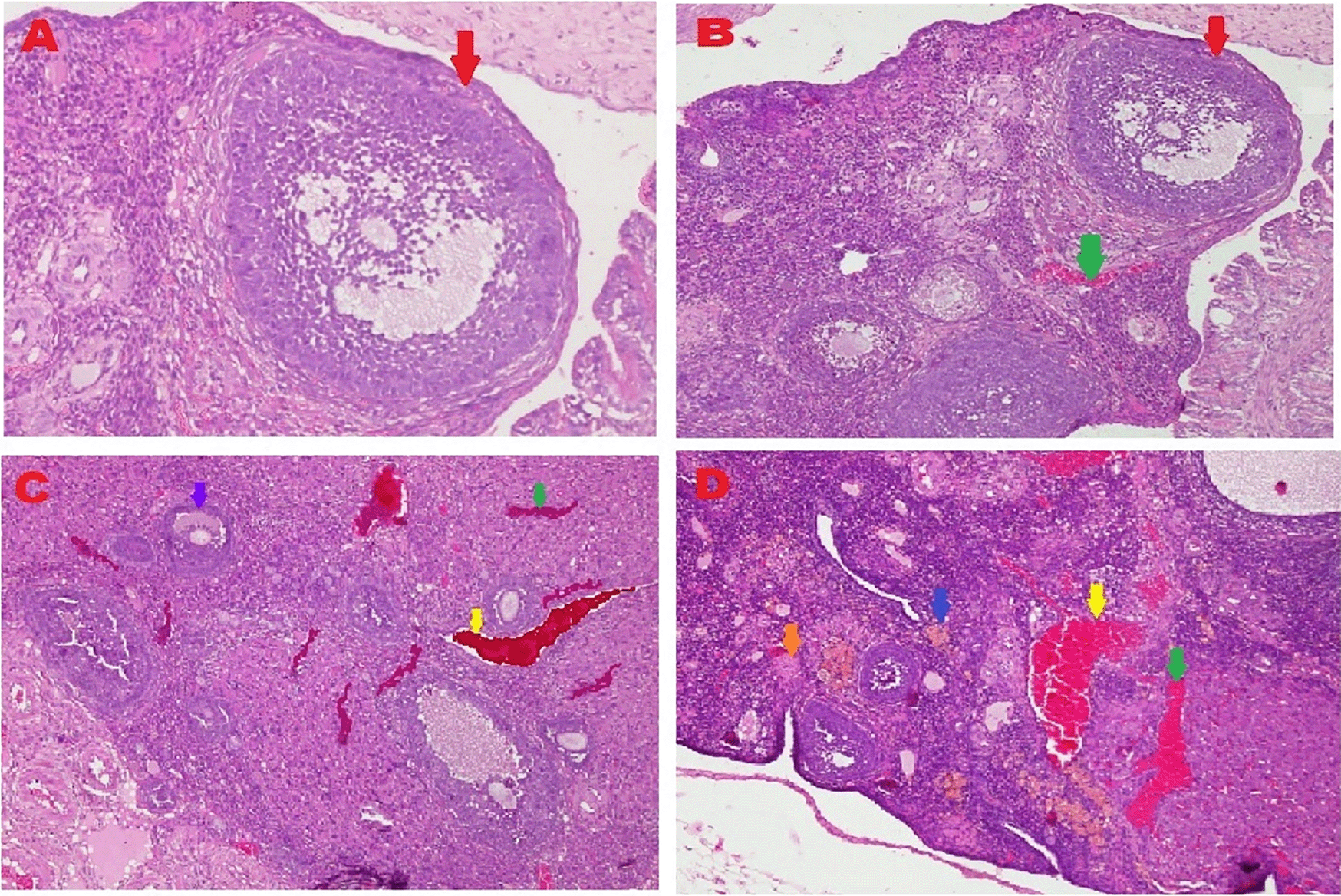
A. Positive (fertile) control group showed Graafian follicle ( ), with an average count of 8.9c±0.74; B. T2 group, ovaries begin to regenerate and become intact despite the presence of hemorrhage (
), with an average count of 8.9c±0.74; B. T2 group, ovaries begin to regenerate and become intact despite the presence of hemorrhage ( ) in some areas, but a Graafian follicle (
) in some areas, but a Graafian follicle ( ) was observed with an average count of 4.5b±0.71; C. T1 group, ovarian congestion (
) was observed with an average count of 4.5b±0.71; C. T1 group, ovarian congestion ( ), severe hemorrhage (
), severe hemorrhage ( ), and growing follicles (
), and growing follicles ( ) were observed, but no Graafian follicle was found (0.5a±0.53); D. Negative (infertile) control group, ovarian congestion (
) were observed, but no Graafian follicle was found (0.5a±0.53); D. Negative (infertile) control group, ovarian congestion ( ), severe hemorrhage (
), severe hemorrhage ( ), and visible hemosiderin (
), and visible hemosiderin ( ) caused by blood cell lysis (brownish yellow) with fibrin deposition (
) caused by blood cell lysis (brownish yellow) with fibrin deposition ( ) were observed, indicating that chronic congestion occurred (pink) without growing follicles or Graafian follicle (0.4a±0.52).
) were observed, indicating that chronic congestion occurred (pink) without growing follicles or Graafian follicle (0.4a±0.52).
A Graafian follicle count for ovarian tissue from each of the four treatments was conducted using HE staining (Figure 9 and Table 2). As shown in Figure 9, the Graafian follicle count obtained by microscopic examination at 400× magnification using HE staining of rat ovarian tissue from each of the four treatments were as follows: the positive (fertile) control group displayed an average count of 8.9c±0.74; the T2 group displayed an average count of 4.5b±0.71; the T1 group showed growing follicles but no Graafian follicle (=0.5a±0.53); the negative (infertile) control group showed no growing follicles nor Graafian follicle (=0.4a±0.52).
Low O2 tension is a crucial element in the stem cell microenvironment, 15 which is important for stem cells self-renewal, viability, and proliferation stability. In this research, the effect of low O2 on cell proliferation was observed on the basis of morphological analysis of MSCs. After the third passage on the second day of culturing under low O2, cells exhibited a larger cell size, fewer cell deaths, and a slower proliferation rate; meanwhile, cells in the normoxic culture exhibited a smaller size, higher cell deaths, and faster proliferation rate (Figure 1). This research was consistent with a previous study, 28 which reported that the slow proliferation of stem cells is necessary for the inhibition of senescence cells or rapidly aging cells. Another study has reported that rapid proliferation is needed to achieve cell confluence and prevent cell deaths. 15
The results of this research indicated that low O2 can inhibit apoptosis. Based on real-time PCR, the expression of HSP27 and caspase-3 was detected. This result indicated significantly increased HSP27 and decreased caspase-3 expressions in cells in vitro cultured with low oxygen tension (O2 1%, p<0.05) compared with those in cells cultured under normoxic conditions, which served as the control (Figures 2 and 3). This research was consistent with a previous study, 15 which reported that low O2 had a significant effect on apoptotic resistance, as indicated by the expression of HSP27. The study indicated that low O2 could reduce apoptosis by increasing HSP27 expression and decreasing caspase-3 expression. Caspase-3 is a marker of apoptosis; thus, a decrease in caspase-3 indicated that low O2 could reduce apoptosis. 20
Furthermore, IHC of cells cultured under low-O2 (O2 1%) conditions until 72 h exhibited a small number of apoptotic cells (5%) compared with those cultured under a normoxic condition (O2 21%), which showed an apoptotic cell number of 25% (Figure 4). The study results were supported by several studies that stated that under low-O2 (O2 1%) conditions, the stem cells decreased the level of apoptosis. 21 , 22 The incidence of apoptosis was between 2.45% and 2.55% under low O2 for 24, 48, and 72 h compared with a number of cells that were cultured under normoxic (O2 21%) conditions, in which the apoptotic cell number reached 12.5%. 23 , 24 In vitro culture of stem cells required the same treatment as those maintained in vivo because stem cells require a hypoxic condition (a low concentration of O2) to maintain self-renewal, 25 viability, 26 and slow proliferation based on the location and type of the stem cells. 27 , 28
A low-O2 culture of stem cells maintained the proliferation rate 25 and viability 26 and achieved self-renewal because stem cells require a low-O2 concentration. 29 – 31 Hematopoietic stem cells require an O2 concentration of 0%–5%; 24 adipose stem cells require an O2 concentration of 5%; 32 neural stem cells require an O2 concentration of 1%–5%; 33 cord blood requires an O2 concentration of 3%, 34 and MSCs require an O2 concentration of 0.5%–3%. 35
In nearly every type of tissue that makes up the body of multicellular creatures, stem cells may be considered as an undifferentiated cell. 36 However, in this study, endogen stem cells cannot repair ovarian tissues under chronic conditions because of severe malnutrition. Therefore, stem cell transplantation from an in vitro culture that simulates endogenous stem cells under low oxygen conditions is a potential therapy option. This research investigated an in vitro culture of stem cells under 1% oxygen. 37
Stem cells have two unique characteristics: first, stem cells can renew or regenerate themselves by replicating identically through cell division. Secondly, stem cells can differentiate from other cells. Stem cells can develop into various types of mature cells, such as nerve, heart muscle, skeletal muscle, and pancreatic cells; 38 female gametes; 39 and male gametes. 17 This research focuses on female gamete cells. Low O2 is important for stem cells to maintain their differentiation ability, and plasticity. 40 , 41 Therefore, a low-O2 culture can promote the survival, strong attachment, and integration of stem cells into the microenvironment of original cells to achieve successful therapy.
This study reported that MSCs culture can improve the fertility of female rats with malnutrition-induced. The success of the therapy was determined on the basis of six parameters: (1) increased expression of HSP70 as a chaperone molecule for the repair of cell protein and inhibition of apoptosis, (2) decreased expression of caspase-3 for the inhibition of apoptosis, (3) increased expression of VEGF-1 as a marker of viability, (4) increased expression of GDF-9 as a marker of differentiation, (5) regeneration of ovarian tissue as evidenced by the intact structure of ovarian tissue and the development of follicles, and (6) improvement of the Graafian follicle count.
Based on the result of this study, the first parameter for successful therapy was the IHC average score of the HSP70 expression in ovarian tissue from the four treatment groups (Figure 5 and Table 2). HSP70 expression increased under T2 treatment (O2 1%). Although this increase was inconsistent with that of the positive control group (fertile rats), this increase improved ovarian failure caused by malnutrition, as evidenced by the increase of follicle growth and Graafian follicle count. The chaperone protein HSP70 must be produced at optimal levels to heal injured cells (not exceeding the levels necessary for cell repair)
HSP70 is a protein that is strongly expressed when exposed to oxidative stressors such reactive oxygen species. This study found that malnutrition caused oxidative stress. Overexpressed HSP70 could be found in damaged cells. 42 The expression of HSP70 as a chaperone molecule promotes the activity of endogenous stem cells to improve the development of follicles and Graafian follicle. Both follicle types contain the ovum and estrogen hormone which are important for female reproduction. Previous studies on male rats have shown that male gametes, also known as spermatogonia stem cells, which are found in mouse testicles, can develop into progenitor cells after transplantation. 17 In this study, the expression of HSP70 in T2 (O2 1%) improved the development of follicles and Graafian follicle of the ovaries, which was significantly different from the result of the negative control (infertile rats), but these advancements were still not on par with the positive control (fertile rats).
The second parameter for successful therapy was based on the average IHC score of caspase-3 expression (Figure 6 and Table 2). The caspase-3 expression decreased under T2 treatment (O2 1%). The decrease was consistent with that of the positive (fertile) control group, which led to an improvement in ovarian damage caused by malnutrition, as evidenced by an enhancement in follicular growth and Graafian follicle count. Oxidative stress caused by malnutrition triggers apoptosis via an inherent route in the mitochondrial component of cells. The intrinsic route requires mitochondrial activity because of the activation and release of the caspase protein into the cytosol caused by oxidative stress. A protease, namely caspase, can disassemble protein chains. In this study, malnutrition-induced oxidative stress could be due to cytochrome binding to apoptotic protease-activating factor-1 (APAF-1) released from the mitochondria; then procaspase-9 can activate caspase-9. 43 Caspase-9, which serves as an apoptosis initiator, is dimerized, which triggers feedback by inhibiting BCL-2 release and then binds to procaspase-3 to activate caspase-3. Then, as an executor, caspase-3 aids in the activation of cytoplasmic and endonuclease, which may fragment nuclear DNA and break down cytosol proteins in cells. The production of apoptotic bodies, which contain intracellular organelles, express phosphatidylserine, and cause phagocytosis, is the ultimate stage of fragmentation, thereby causing cell failure as a constituent of the tissue. 44
The third parameter for successful therapy is based on the viability of transplanted stem cells, which is assessed on the basis of VEGF-1 expression (Figure 7 and Table 2). VEGF-1 expression increased under T2 treatment (O2 1%). Although this increase was not consistent with the positive control group (fertile rat), it could improve ovarian damage caused by malnutrition, as evidenced by the increase in follicular growth and Graafian follicle count. VEGF-1 as a marker of viability of transplanted stem cells is expressed in ovarian tissue. 21
The fourth parameter for successful therapy is based on the expression of GDF-9 (Figure 8 and Table 2). GDF-9 expression increased under T2 treatment (O2 1%). Although this increase was not consistent with the positive control group (fertile rats), it could improve ovarian damage caused by malnutrition. The growth of ovarian cortical cells is induced by the progenitor cell marker GDF-9, which is produced from germline stem cells. 21 , 45 Stem cells rapidly develop into cells that are required to respond to damage and strengthen the immune response. 46 The oocyte produces GDF-9, a growth factor that is a member of the TGF-ß family. GDF-9 is essential for folliculogenesis and fertility. 47 In addition, progenitor germ cells can address issues with folliculogenesis caused by malnutrition by repairing damaged follicles and can restore molecular communication in ovarian follicles by increasing the synthesis of SCF and GDF-9. 21 Oogenesis can activate homing directly through the activation of cells that have been repressed and indirectly through the stimulation of the microenvironment (niche) of injured cells. 48
The fifth and sixth parameters for determining the success of therapy are based on the regeneration of ovarian tissue and improvement of the Graafian follicle count (Figure 9 and Table 2). In the positive (fertile) control group, the Graafian follicle developed. In the T2 group (O2 1%), the ovaries began to regenerate and appeared intact, and a Graafian follicle was observed despite hemorrhage in some areas. In the T1 group (O2 1%), ovarian congestion and severe hemorrhage were observed, and no Graafian follicle was found despite the presence of follicles. In the negative (infertile) control group, ovarian congestion and severe hemorrhage were observed, and blood cell lysis produced hemosiderin, which is brownish-yellow and deposits fibrin, indicating the occurrence of chronic congestion (pink colour), with no development of follicles or the Graafian follicle.
Ovarian tissue regeneration showed an intact ovarian tissue structure with the development of follicles and a Graafian follicle. This result indicated the effectiveness of the therapy using stem cells cultured under low O2 tension (1%). In this study, the regeneration of the ovarium was observed microscopically with HE staining. 49 Microscopic observation showed stem cell therapy cultured under low-O2 tension (1%, T2) achieved ovarian tissue repair. Improvements were found on the basis of the formation of follicles and a Graafian follicle and the regeneration of ovarian tissue. The positive control group (fertile rat), which did not experience ovarian failure and maintained a normal condition with developing follicles and a Graafian follicle, was contrasted with these advances (Figure 9). The degenerative ovarian tissue of the negative (infertile) control group of rats was compared with the aberrant characteristics of the damaged ovarian tissue. The latter demonstrated chronic congestion with ovarian congestion, severe hemorrhage, and hemosiderosis (yellow brown) caused by hemolysis of red blood cells with fibrin deposition (Figure 9).
Ovarian treatment with MSCs cultured under low-O2 tension could improve ovarian failure caused by malnutrition in female rats based on increased HSP70 expression and decreased caspase-3 expression as apoptotic inhibitors, increased VEGF1 and GDF-9 expression as markers of viability and differentiation, regeneration of ovarian tissue, and improved count of Graafian follicle.
Figshare: Graafian follicle count data sets, as well as HSP70, Caspase-3, VEGF-1, and GDF-9 results. https://doi.org/10.6084/m9.figshare.20440575. 50
This project contains the following underlying data:
Figshare: ARRIVE Checklist MSCs therapy. https://doi.org/10.6084/m9.figshare.20440509. 51
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The Faculty of Veterinary Medicine, Universitas Airlangga, provided the research facilities, and the chairman and staff of LIPJPHKI helped with the grammar check and Prof. Dr. R. Heru Prasetyo, dr., MS., SpParK, for his assistance in this research. The author would like to express his gratitude to all of them.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Female reproduction
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Bhartiya D, Singh P, Sharma D, Kaushik A: Very small embryonic-like stem cells (VSELs) regenerate whereas mesenchymal stromal cells (MSCs) rejuvenate diseased reproductive tissues.Stem Cell Rev Rep. 2022; 18 (5): 1718-1727 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: I have worked on ovarian stem cells, MSCs , oogenesis from stem cells in vivo
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 3 (revision) 16 Apr 24 |
read | ||
|
Version 2 (revision) 04 Dec 23 |
read | read | |
|
Version 1 09 Jan 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)